Abstract
Purpose
The Risk, Injury, Failure, Loss and ESRD (RIFLE) classification has been widely accepted for the definition of acute kidney injury (AKI); however, no study has described in detail the last two stages of the classification: “Loss” and “ESRD”. We aim to describe and evaluate the development of “Loss” and “ESRD” in a group of critically ill patients.
Methods
We conducted a retrospective analysis of cases prospectively collected from the Acute Physiology and Chronic Health Assessment (APACHE III) database. Subjects were consecutive critically ill patients >18 years of age admitted to three ICUs of two tertiary care academic hospitals, from January 2003 through August 2006, excluding those who denied research authorization, chronic hemodialysis therapy, kidney transplant recipients, readmissions, and admissions for less than 12 h for low risk monitoring.
Results
11,644 patients were included in the study. The median age was 66 (interquartile range, 52–76), 90% were Caucasians and 54% of the patients were male. Half of the patients developed AKI, and most of the patients were in the Risk and Injury stages. From the patients that developed AKI, a total of 1,065 (19%) patients required renal replacement therapy (RRT), 415 (39%) underwent continuous renal replacement therapy (CRRT) and 650 (61%) underwent intermittent hemodialysis. A total of 281 patients on RRT did not survive hospital discharge, 97 patients progressed to “Loss”, and 282 patients progressed to “ESRD”. After multivariable adjustment, the progression to “ESRD” was associated with higher baseline creatinine, odds ratio (OR) 1.19 per every increase in creatinine of 0.1 mg/dl (95% CI, 1.11–1.29) P < 0.001; and less frequent use of CRRT, OR 0.18 (95% CI, 0.11–0.29) P < 0.001.
Conclusion
In this large retrospective study we found that almost 50% developed some form of AKI as defined by the RIFLE classification. Of these, 19% required RRT, and 4.9% progressed to “ESRD”. “ESRD” was more likely in patients with elevated baseline creatinine and those treated with intermittent hemodialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a common and highly lethal problem faced in the intensive care unit (ICU) [1], with a reported incidence of 1–31% [2–4], and a mortality that ranges from 28 to 90% [3, 5–7]. This wide range in the incidence and the mortality is in part due to the near 35 different definitions of AKI [8]. To solve this problem, the RIFLE classification (acronym indicating Risk of renal dysfunction; Injury to the kidney; Failure of kidney function, Loss of kidney function and End-stage renal disease [ESRD]) was designed in 2004 by the Acute Dialysis Quality Initiative Group (ADQIG) [9] in order to standardize the diagnosis of AKI in the ICU (Table 1). Since then, 24 studies of AKI in different populations have been reported in the literature [10–24]. However, most of the studies have relied the diagnosis of AKI based only on the creatinine (glomerular filtration rate [GFR]) criterion and not taking into account the urine output (UO) criterion which is part of the classification and increases the sensitivity of the diagnosis[9, 10]. Moreover, none of these studies reported the incidence of the two outcome-stages, “Loss” and “ESRD”, after the development of AKI; consequently, there is scant information available regarding the outcome of AKI beyond the “Failure” stage utilizing the RIFLE classification. Such information is vital in order to accurately understand the impact of AKI in health costs, patient survival and quality of life, and also in providing information to patients and their families. In addition, the risk factors involved in the development of “ESRD” after an episode of acute kidney injury are important for prognostic and preventive strategies. Therefore, we carried out this retrospective cohort study of more than 10,000 patients admitted to medical and surgical ICUs in two tertiary care hospitals. Our primary objectives were to (a) describe the incidence and main outcomes of AKI in our patient cohort, as defined by the RIFLE classification utilizing both creatinine (GFR) and urine output criteria, (b) to examine the frequency with which these patients progressed to “Loss” and “ESRD”, and (c) to identify risk factors that correlated with progression to “ESRD”.
Methods
The Institutional Review Board of our institution approved the study protocol and waived the informed consent because this study was considered a minimal risk observational study. We performed a retrospective analysis of prospectively collected data in the Acute Physiology and Chronic Health Evaluation (APACHE) database, including consecutive critically ill patients (>18 years old of age) admitted from January of 2003 until August 2006 to three medical and surgical ICUs at the two Mayo Clinic hospitals in Rochester, MN. Patients on chronic renal replacement therapy (RRT), kidney transplant recipients, readmissions, admissions for less than 12 h for low risk monitoring, and those who denied research authorization for their medical records to be reviewed were excluded from the study. Patients (including patients that required RRT) were classified according to the maximum RIFLE class reached during their ICU stay [9, 25]. Patients who developed “Failure” or were started on RRT during the hospitalization were followed for at least 3 months to evaluate the progression to either “Loss” or “ESRD”. The RIFLE class was determined based on the worst of either creatinine criterion or UO criterion (based on the hourly UO). The main outcomes measured were hospital mortality, renal recovery (defined as liberation from renal replacement therapy), and hospital and ICU length of stay (LOS).
In order to compare the characteristics between the patients that required RRT but did not progress to ESRD, we performed two different analyses: first, we excluded from the comparison all patients that did not survive hospital discharge because they did not have the possibility to progress to “ESRD” due to timing; secondly, we analyzed all RRT patients to evaluate for possible biases of not including the sickest patients that died during their hospitalization and were not able to develop ESRD. Risk factors identified in the development of “ESRD” were compared and analyzed in a multivariate analysis.
Survival analysis is initially reported for 28 days after admission for the following RIFLE categories: No AKI, Risk, Injury and Failure. Because “Loss” and “ESRD” contain only patients who at least survived 4 weeks, we also reported the long term survival (until May of 2009) for all RIFLE classes including only patients that at least survived 4 weeks after ICU admission.
Statistical analysis
All continuous data are summarized as medians (interquartile range, IQR) or means (standard deviation, SD). Categorical data are summarized as percentages. Difference in medians between groups was tested with the Wilcoxon sum rank test or the Kruskal–Wallis one-way analysis of variance where appropriate. Differences in proportions were compared utilizing the chi-squared test or the Fisher exact test. Standardized mortality ratios (SMRs) were calculated by dividing the number of observed deaths per group by the number of expected deaths per group (predicted by the APACHE III score).
The predictive accuracy of the multivariate models is reported as the area under the curve (AUC). Odds ratio (OR) and 95% confidence intervals (CI) were calculated and P-values of <0.05 were considered statistically significant. Survival analysis was performed with a Kaplan–Meier Curve and the log-rank test. JMP statistical software (version 8.0, SAS, Cary, NC) was used for all analyses.
Additional detailed information regarding the methodology of the study is provided in the Electronic supplementary material of the journal.
Results
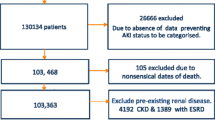
A total of 16,009 consecutive patients were admitted during the study period. After exclusion of 4,365 patients (254 denied research authorization, 110 were readmissions, 550 were on chronic RRT, 27 were kidney transplant recipients, and 3,425 were admitted for less than 12 h for low risk monitoring), a total of 11,644 patients were included in the study. Baseline creatinine was available in 11,316 patients (97.2%). Half of the patients developed AKI, and most of the patients were in the Risk and Injury stages. A total of 326 patients (2.8% of the cohort) had a baseline creatinine ≥4 mg/dl. The diagnosis of acute kidney injury was based in 26% of the cases on the urine output criteria (59.3% of Risk patients, 74.8% of Injury patients and 10.3% of Failure patients). RRT was used in 9.1% of the total critically ill patients (19% of all patients that developed AKI), of whom 41 patients were in the Injury group, and the remainder (1,024 patients) were in the Failure group. Forty-one patients in the failure group did not require RRT for several reasons: patients were given comfort care only, not clinically indicated, or patients died before initiation of RRT. None of the patients in the Injury group progressed to “Loss” or “ESRD” because they either died or recovered their renal function.
The general characteristics and main outcomes of the patients according to the RIFLE classification group are described in detail in Table 2. A total of 1,065 patients required RRT, 415 (39%) underwent continuous renal replacement therapy (CRRT) and 650 (61%) underwent intermittent hemodialysis (IHD) (Fig. 1). A total of 281 patients that required RRT died before hospital discharge. From hospital survivors, 97 patients progressed to “Loss”, and 282 patients progressed to “ESRD” (Fig. 1). Most of the patients that progressed to ESRD had advanced chronic kidney disease (CKD) according to their hospital admission stage of the CKD classification [26] (Fig. 2). A total of 74 patients in the Failure group did not progress and remained in the failure category because they died within 4 weeks (27 patients), their renal function improved and dialysis was withdrawn (26 patients), or because they were recipients of renal or liver transplant and their renal function subsequently improved (21 patients). A total of 453 patients were discharged on RRT which is only 7.9% of all the patients that developed AKI.
As seen in the univariate analysis (Table 3), patients in the “ESRD” group were slightly younger, presented significantly higher baseline creatinine levels and higher worst creatinine levels during their hospitalization, were less acutely ill as measured by the APACHE III predicted hospital mortality, were less likely to be treated with CRRT as opposed to IHD; and presented less oliguria upon presentation than the patients with renal recovery.
In the multivariate analysis of the risk factors for the development of “ESRD”, we included the following variables in the two models: age, APACHE III ICU predicted mortality, baseline creatinine levels, CRRT actual utilization, and oliguria. We excluded worst creatinine during the hospitalization due to strong collinearity with baseline creatinine. We also excluded APS and mechanical ventilation due to strong collinearity with the predicted mortality. After adjustment, the progression to “ESRD” was associated with higher baseline creatinine, lower predicted mortality as predicted by APACHE III, and less frequent use of CRRT in both analyses. The results of the two models with their respective AUC are presented in Table 4.
The 28-day survival was significantly affected in the “Failure” group as compared to No AKI, Risk or Injury (Fig. 3). We were also able to follow the patients that survived at least 4 weeks after admission for 6 years and the survival according to the different categories of the RIFLE classification is described in Fig. 4. There was an early and sustained drop in survival in both the “Failure” and “Loss” groups during the long term follow-up period.
Discussion
Our study reports that AKI defined by the RIFLE classification complicated almost 50% of 11,644 consecutive critically ill patients admitted during a 4 year period to three different ICUs in the two Mayo Clinic hospitals in Rochester, MN. In this large cohort of ICU patients, we provide significant new information regarding the progression of AKI to “Loss” and “ESRD”. Among all patients that developed AKI and after excluding 281 patients that died before hospital discharge, we found that nearly 8% of the survivors required prolonged RRT or permanent hemodialysis. This information is novel and represents an important outcome beyond survival, given the significant burden that this represents for the healthcare system, and the quality of life of the patients and their families. In addition, our study is one of the largest studies reporting the incidence of AKI in the ICU utilizing both the creatinine criterion (GFR) and the UO criterion of the RIFLE classification; moreover, the measured baseline creatinine was available in >97% of the patients.
The incidence of AKI utilizing the RIFLE classification varies in the literature depending on the population studied. In ICU patients, our cohort reports similar incidence (49.3%) as compared to the incidence reported in a study of 85 critically ill patients by Herget-Rosenthal et al. [27]. However, the incidence found in our study differs from the 67% AKI incidence found by Hoste et al. [12] in one of the largest studies on AKI in ICU patients. Similar to our study, the latter study utilized both the UO and the GFR criteria; however, our lower incidence could be explained by the fact that we included only three ICUs with fewer surgical patients (38%), whereas the study by Hoste et al. included a more diverse ICU population with close to 60% of surgical patients. The higher risk of AKI in the surgical population is well known [18, 28], which was also found in the same study [12]. Recently, in a large cohort study by Ostermann and colleagues in more than 40,000 critically ill patients, the reported incidence of AKI was 36% [15]. This lower incidence is likely an underestimation explained by the fact that the UO criteria were not used in the determination of AKI in the aforementioned study.
The hospital mortality follows an escalating pattern when patients develop AKI and advance from Risk to Failure in most of the studies reported in the literature [12, 13, 19, 21]. Our study followed a similar pattern for all-cause hospital mortality with the exemption of Injury that presented a lower actual mortality than Risk. Bell and colleagues found a similar response for 30 day mortality [14]. This discrepancy found in our study is likely explained by a lower severity of disease in this group as we can see from the lower predicted mortality calculated by their APACHE III scores. Furthermore, the diagnosis of AKI was based on the urine UO in 74.8% of Injury patients, and it has been shown that mortality rates of AKI stages defined by worst UO are consistently lower than for worst serum creatinine [10, 24, 29]. The actual mortality follows the predicted mortality in all groups of AKI defined by the RIFLE classification but does not match it very well. This is understandable because one would not expect that a measure of one organ system will provide accurate information on mortality, and the RIFLE classification was never intended to be a scoring system such as APACHE or SAPS [30, 31]. When comparing the SMRs, there is almost a linear increment in the different RIFLE stages in a similar trend as recently reported by Joannidis and collegues [29].
Our report of the incidence of Loss and ESRD after the development of AKI in the ICU is novel and significant. Bell et al. reported preexistent “Loss” and “ESRD” on ICU admission [14] and then compared their outcomes with patients that underwent RRT after the development of AKI. To the best of our knowledge, however, no prior study has reported the incidence of these two outcome stages of the RIFLE classification after the development of AKI in the ICU. In our study, 97 hospital survivors that required RRT remained on dialysis for at least 4 weeks and 282 patients became dialysis-dependent (progressed to “ESRD”). Variable results regarding renal recovery have been reported, but these studies are difficult to directly compare because different definitions of AKI and different definitions of renal recovery have been used [32]. Most of the studies have reported results similar to ours; that approximately 85–90% of surviving AKI patients are dialysis-independent upon hospital discharge [28, 32–36]. It is interesting to note that one of these studies, Bagshaw et al. [34], not only found renal recovery rates similar to those we found in our study, but they also found that pre-RRT creatinine levels were higher in the patients that remained dialysis-dependent after 90 days, similar to our findings of significantly higher baseline creatinine in the group of patients that progressed to ESRD. Similar results were also found by Ali et al., where renal recovery was more frequent in patients without underlying renal dysfunction as evidenced by normal baseline creatinine levels [23]. Together these studies suggest that the degree of preexisting renal impairment is a strong predictor of renal recovery.
There is an ongoing controversy as to which RRT modality is better suited for patients with AKI in the ICU. Historically, most of these patients were treated with IHD. However, IHD has various limitations which include a higher risk of hemodynamic instability and the possibility that this may induce further renal injury [37, 38]. Indeed, Ronco et al. [39] performed direct measurements of blood volume during IHD showing significant drops in circulatory blood volume and perfusion, which is known to be deleterious to the recovering kidney. To circumvent these limitations, many ICUs have adopted CRRT, which provides for a gentle yet effective “clearance” of solute and excess fluid. Despite the physiologic advantages of CRRT over IHD, it has been difficult to demonstrate that CRRT improves outcomes (survival or renal recovery). A large systematic review of 15 randomized clinical trials comparing these two methods of RRT reported no differences in hospital mortality, ICU mortality, hypotension or hemodynamic instability [40]. Our study, like previous ones (as described in a meta-analysis by Kellum et al. [41]) found that our sickest patients were usually started on CRRT. Even after including in the multivariate analysis all patients who died during their hospital stays, the patients who progressed to “ESRD” were less likely to have been treated with CRRT. While we can not prove a possible advantage of CRRT on renal recovery [28, 42–45], our observations suggest different case mix and different etiologies of AKI as a more likely explanation. Further prospective studies that address this important issue are necessary. In addition, our study also showed that the patients who were treated with IHD in general had higher serum creatinine at baseline; consequently, the difference in renal recovery may have been simply due to the possibility that renal regeneration is less likely in patients with CKD. In addition, there might be significant selection bias in these studies. If the clinician sees a patient with CKD and feels that the injury has now transitioned them to stage 5 CKD, they may be treated differently. Recent published data from a small cohort of patients with AKI in the ICU of our institution revealed that patients who underwent CRRT were younger, had greater APACHE II and were less likely to have chronic renal insufficiency; results that are mirrored in our study [46]. Our study also provided a 28-day survival follow up showing an early and sustained drop in survival in the “Failure group”. In addition, we also presented a long term survival follow up where it was interesting to observe a sustained drop in survival in both the Loss and Failure groups.
We acknowledge several limitations. First, our study has a retrospective observational design with its inherent biases; however, our prospectively collected APACHE III database and our electronic medical records provide accurate information with urine output validated by the bedside nurse, and we have included a large sample size. Also, our study is limited by the predominantly Caucasian population seen at our institution. The study was performed in a tertiary referral center; therefore the results are difficult to generalize; moreover, our institution's practices may differ from other institutions. Finally, we included only baseline characteristics of the patients and we understand that other variables not collected during the ICU stay might have influenced the final outcomes.
In conclusion, in this retrospective study of 11,644 consecutive critically ill patients, we found that almost 50% developed some form of AKI as defined by the RIFLE classification. Of these, 19% required RRT, and 4.9% progressed to end-stage renal disease. “ESRD” was more likely in patients with elevated baseline creatinine and those treated with IHD.
References
Uchino S (2006) The epidemiology of acute renal failure in the world. Curr Opin Crit Care 12:538–543
Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J (1997) Preoperative renal risk stratification. Circulation 95:878–884
Vivino G, Antonelli M, Moro ML, Cottini F, Conti G, Bufi M, Cannata F, Gasparetto A (1998) Risk factors for acute renal failure in trauma patients. Intens Care Med 24:808–814
de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F (2000) Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intens Care Med 26:915–921
Schiffl H, Lang SM, Fischer R (2002) Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346:305–310
Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W (2002) Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30:2051–2058
Cosentino F, Chaff C, Piedmonte M (1994) Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant 9(Suppl 4):179–182
Kellum JA, Levin N, Bouman C, Lameire N (2002) Developing a consensus classification system for acute renal failure. Curr Opin Crit Care 8:509–514
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Ricci Z, Cruz D, Ronco C (2008) The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73:538–546
Maccariello E, Soares M, Valente C, Nogueira L, Valenca RV, Machado JE, Rocha E (2007) RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intens Care Med 33:597–605
Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10:R73
Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM (2005) The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis 46:1038–1048
Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR (2005) Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant 20:354–360
Ostermann M, Chang RW (2007) Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 35:1837–1843 quiz 1852
Lopes JA, Jorge S, Neves FC, Caneira M, da Costa AG, Ferreira AC, Prata MM (2007) An assessment of the RIFLE criteria for acute renal failure in severely burned patients. Nephrol Dial Transplant 22:285
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ Jr, Klodell CT, Ejaz AA, Garvan C, Tribble CG, Beaver TM (2007) RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg 134:1554–1560 discussion 1560–1551
Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V (2006) Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg 81:542–546
O’Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ (2007) Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant 7:168–176
Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C (2006) An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34:1913–1917
Bagshaw SM, George C, Dinu I, Bellomo R (2007) A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 23:1203–1210
Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18:1292–1298
Cruz DN, Bolgan I, Perazella MA, Bonello M, de Cal M, Corradi V, Polanco N, Ocampo C, Nalesso F, Piccinni P, Ronco C (2007) North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol 2:418–425
Kellum JA, Ronco C, Mehta R, Bellomo R (2005) Consensus development in acute renal failure: the acute dialysis quality initiative. Curr Opin Crit Care 11:527–532
Steinberg EP, Eknoyan G, Levin NW, Eschbach JW, Golper TA, Owen WF, Schwab S (2000) Methods used to evaluate the quality of evidence underlying the National Kidney Foundation-Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: description, findings, and implications. Am J Kidney Dis 36:1–11
Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A (2004) Early detection of acute renal failure by serum cystatin C. Kidney Int 66:1115–1122
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. Jama 294:813–818
Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG (2009) Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intens Care Med [Epub ahead of print]
Bellomo R, Kellum JA, Ronco C (2007) Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intens Care Med 33:409–413
Bellomo R, Kellum JA, Ronco C (2007) Comment on “RIFLE classification in patients with acute kidney injury in need of renal replacement therapy” by Maccariello et al. Intens Care Med 33:1850 author reply 1851–1852
Macedo E, Bouchard J, Mehta RL (2008) Renal recovery following acute kidney injury. Curr Opin Crit Care 14:660–665
Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J (2002) Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 30:2205–2211
Bagshaw SM, Mortis G, Godinez-Luna T, Doig CJ, Laupland KB (2006) Renal recovery after severe acute renal failure. Int J Artif Organs 29:1023–1030
Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM (1995) Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155:1505–1511
Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF Jr, Teno JM, Wenger N, Lynn J, Wu AW, Fulkerson W, Tsevat J (1997) Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med 127:195–202
Schiffl H, Lang SM, Konig A, Strasser T, Haider MC, Held E (1994) Biocompatible membranes in acute renal failure: prospective case-controlled study. Lancet 344:570–572
Van der Schueren G, Diltoer M, Laureys M, Huyghens L (1996) Intermittent hemodialysis in critically ill patients with multiple organ dysfunction syndrome is associated with intestinal intramucosal acidosis. Intens Care Med 22:747–751
Ronco C, Brendolan A, Bellomo R (1999) Online monitoring in continuous renal replacement therapies. Kidney Int 72:S8–S14
Rabindranath K, Adams J, Macleod AM, Muirhead N (2007) Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev: CD003773
Kellum JA, Angus DC, Johnson JP, Leblanc M, Griffin M, Ramakrishnan N, Linde-Zwirble WT (2002) Continuous versus intermittent renal replacement therapy: a meta-analysis. Intens Care Med 28:29–37
Jacka MJ, Ivancinova X, Gibney RT (2005) Continuous renal replacement therapy improves renal recovery from acute renal failure. Can J Anaesth 52:327–332
Bell M, Granath F, Schon S, Ekbom A, Martling CR (2007) Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intens Care Med 33:773–780
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60:1154–1163
Manns B, Doig CJ, Lee H, Dean S, Tonelli M, Johnson D, Donaldson C (2003) Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med 31:449–455
Rauf AA, Long KH, Gajic O, Anderson SS, Swaminathan L, Albright RC (2008) Intermittent hemodialysis versus continuous renal replacement therapy for acute renal failure in the intensive care unit: an observational outcomes analysis. J Intens Care Med 23:195–203
Acknowledgment
LAJ is supported in part by NIH DK0294 from the National Institute of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cartin-Ceba, R., Haugen, E.N., Iscimen, R. et al. Evaluation of “Loss” and “End stage renal disease” after acute kidney injury defined by the Risk, Injury, Failure, Loss and ESRD classification in critically ill patients. Intensive Care Med 35, 2087–2095 (2009). https://doi.org/10.1007/s00134-009-1635-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1635-9