Abstract
Objective
This review aims to help critical care clinicians maintain a high level of suspicion regarding the diagnosis of Hemophagocytic Histiolymphocytosis (HLH). It describes the clinical and laboratory features of HLH, outlines its pathophysiology and reviews the most frequent etiologies related to HLH. Prognostic factors and therapeutic options are also reported.
Data sources
Review of the literature.
Results
The diagnosis of HLH relies on the association of clinical abnormalities and hemophagocytosis in bone marrow, spleen, or lymph node specimens. Liver, pulmonary, renal, cardiac and skin involvement may occur at various degrees possibly leading to multiple organ failure. Three main etiologies can be found, namely infections, lymphoproliferative diseases, or connective tissue diseases. Immune deficiency is often retrieved. Mortality can be as high as 50%. Although clinically mimicking severe sepsis, HLH has a distinct pathophysiology on which specific therapy is based. Early diagnosis and treatment is mandatory to increase the chances of survival.
Conclusion
The comprehensive management of severe HLH requires the involvement of a multidisciplinary team in order to determine the best therapeutic strategy and to identify the underlying cause.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemophagocytosis describes the pathological finding of activated macrophages engulfing erythrocytes, leukocytes, platelets, and their precursor cells. This phenomenon is an important finding in patients with hemophagocytic syndrome, more properly referred to as hemophagocytic lymphohistiocytosis (HLH).
Hemophagocytic lymphohistiocytosis is a distinct clinical entity characterized by fever, pancytopenia, splenomegaly, and the pathological finding of hemophagocytosis in bone marrow and other tissues. The syndrome, also referred to as “histiocytic medullary reticulosis,” was first described in 1939 as a condition characterized by a fever, a rapid decline in general health, peripheral lymph node enlargement, pancytopenia, and histiocyte proliferation in the bone marrow with a fatal outcome [1]. Forty years later, Risdall et al. used the term “reactive hemophagocytic syndrome” to designate an inappropriate immune response to viral infection leading to uncontrolled proliferation of benign histiocytes with hemophagocytosis and symptoms matching those described by Scott and Robb-Smith [1]. Subsequently, additional cases related to viral infection were reported [3–5], and hemophagocytic syndrome was described in association with other diseases, including malignancies and systemic connective tissue diseases [6–8].
Hemophagocytic lymphohistiocytosis is a life-threatening condition which may be difficult to distinguish from severe sepsis [9]. A simple clinical approach may be helpful to appraise the diagnosis of HLH. Along this line, autopsy studies suggest that HLH may be underrecognized in intensive care unit (ICU) patients [10, 11]. On the other hand, incidence of HLH may be overestimated. Indeed, studies in critically ill septic patients with cytopenia report an incidence of HLH in marrow smears between 0.8 and 4% [10, 12]; however, these studies are often difficult to interpret as no cytological results from relevant control populations are available. Moreover, criteria for the definite diagnosis of HLH were not met, suggesting that although hemophagocytosis was identified, diagnosis of HLH remained doubtful [13].
Several criteria sets have recently been developed for the diagnosis of HLH. In the latest HLH-2004 protocol, a recent revision of diagnostic criteria suggests that HLH diagnosis can be established if five of the eight following diagnostic criteria are fulfilled: (a) fever; (b) splenomegaly; (c) bicytopenia; (d) hypertriglyceridemia (> 3.0 mmol/l fasting value), and/or hypofibrinogenemia (< 1.5 g/l); (e) hemophagocytosis; (f) low or absent natural killer (NK) cell activity; (g) hyperferritinemia (> 500 ug/l); and (h) and increased soluble CD-25 levels (> 2400 IU/ml; Table 1).
This review aims to help critical care clinicians maintain a high level of suspicion regarding the diagnosis of HLH. It describes the clinical and laboratory features of HLH, outlines its pathophysiology, and reviews the most frequent etiologies related to HLH. Prognostic factors and therapeutic options are also reported.
Clinical and laboratory features
The clinical and laboratory features of HLH are nonspecific and may be difficult to separate from those of the underlying disease; however, HLH should be considered routinely in patients with unexplained and atypical multiple organ failure [12]. Diagnosis of HLH relies on clinical, laboratory, and histological findings. Clinical and laboratory manifestations were proposed by the Histiocyte Society and are listed in Table 1. Acute onset with high-grade fever is the rule. Rapid weight loss may occur [14, 15]. Overall, macrophage and T-lymphocyte proliferation and activation in the reticuloendothelial system manifest as peripheral lymphadenopathy (35% of patients) and as enlargement of the liver and spleen (50% of patients) [16, 17]. Clotting disorders may lead to bleeding, and liver involvement may manifest as jaundice and portal hypertension. Pulmonary infiltrates are found in 20–30% of patients [18]. Cardiac or renal involvement may occur. Skin abnormalities are noted in 20% of patients [17], with the most common patterns being rash, erythema, and purpura. Of central nervous system manifestations, encephalopathy, meningitis, and seizures are the most commonly reported [19]. In severe cases, mechanical ventilation is required because of alterations in consciousness. Multiple organ failures may occur. Table 2 reports usual causes in connective-tissue diseases associated with HLH.
Laboratory tests can assist in the diagnosis of HLH. The most prominent laboratory abnormalities noted are cytopenia, which may be profound. Cytopenia results from both hemophagocytosis in the bone marrow and depression of hematopoiesis by cytokines such as interferon-γ (IFN-γ), tumoral necrosis factor-α (TNF-α), and interleukin 1-β (IL-1β) [20]. All patients have anemia, which is usually nonregenerative. Serum chemistry findings may suggest hemolysis, with hyperbilirubinemia and elevation of lactate dehydrogenase. Thrombocytopenia is almost consistently present, occurs early in the course of the disease, and is usually profound. Leukopenia is less common, less severe, and occurs later in the course of the syndrome. Overall, three of every four patients have pancytopenia and all have bicytopenia. Serum ferritin elevation is the rule [21, 22], the most likely mechanism being IL-1β elevation [23]. Serum ferritin levels may correlate with disease activity and outcome under treatment. Hypertriglyceridemia is an extremely common finding that is ascribable to lipoprotein lipase inhibition by TNF-α [24, 25].
Coagulation disorders are present in most patients. The most common pattern is isolated fibrin deficiency due to liver dysfunction and, above all, plasminogen and factor-X activation by IL-1β [26, 27]. More rarely, disseminated intravascular coagulation (DIC) develops as a result of IFN-γ and TNF-α overproduction. The DIC is associated with high mortality [2, 24]. Liver dysfunction (cytolysis and cholestasis) is frequently reported [28, 29]. IFN-γ contributes to the development of cholestasis [30]. In addition, colony-stimulating factor (CSF), together with Fas/Fas-ligand interaction in response to IFN-γ overproduction, contribute to cause apoptosis and liver damage. IFN-γ elevation also leads to hypoalbuminemia [31].
Renal failure is often reported at the advanced stage of HLH and is related to abnormally high concentrations of nephrotoxic interleukin-6 (IL-6) in serum [32]. Renal biopsy usually shows tiny glomerular lesions [33, 34]. Markers for inflammation are markedly elevated. Many other nonspecific laboratory abnormalities may be found, such as hypo- or hypergammaglobulinemia, a positive Coombs test, or hyponatremia due to syndrome of inappropriate antidiuretic hormone secretion [35].
Cytology and histology
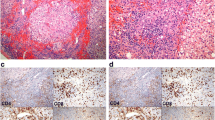
The pathological hallmark of HLH is a proliferation of activated macrophages (histiocytes) engulfing blood cells and their precursors (Fig. 1). This proliferation is found in the reticuloendothelial system (bone marrow, lymph nodes, spleen, and liver) and occasionally affects other sites, such as the skin. Cytological examination of bone marrow smears is the best investigation for confirming HLH, although normal findings, do not rule out the diagnosis. Cellularity is usually normal for all three lines at an early stage. Hypocellularity with reduced granulopoiesis and erythropoiesis may be present [2]. Hyperplasia of the megacaryocyte line with good maturation initially is the rule. Hemophagocytosis in bone marrow occurs not only in HLH, but also in hemolytic diseases and other hematological disorders; therefore, hemophagocytosis does not indicate a diagnosis of HLH unless other clinical and laboratory features of the syndrome are present also [10].
Evidence of hemophagocytosis on histological samples. Hematoxylin-eosin stain of bone marrow sample shows histiocytes, phagocytosing erythroblasts, and lymphocytes. a Hematoxylin-eosin stain of bone marrow sample shows phagocytic cells with engulfed erythrocytes and platelets. b Hematoxylin-eosin stain of bone marrow sample shows phagocytic cells with engulfed erythrocytes and platelets
Histological examination of bone marrow biopsies may be less effective in establishing the diagnosis of HLH than examination of bone marrow smears. Nevertheless, bone marrow biopsy may show an underlying hematological disorder or infectious process, as in tuberculosis for example [16]. Liver histology is abnormal in 50% of patients with HLH. Findings may consist of nonspecific histiocytic infiltration of the sinusoid capillaries and portal tracts and/or hepatocyte necrosis [36]. In a study of 30 patients with HLH and liver dysfunction, de Kerguenec and coworkers consistently found sinusoid dilation and hemophagocytosis, with liver biopsy identifying the underlying disease in 50% of cases [28]. Examination of spleen specimens may show red pulp expansion with hemophagocytosis, as well as lymphocyte depletion in white pulp. In HLH, histological examination of spleen sections may also identify the etiology of the process. In lymph node specimens, histiocytic infiltration is more meaningful when found in the sinusoids than in the cortical or paracortical area. Lymphocyte depletion with atrophic germinal centers is an extremely rare pattern. When lymph node architecture is not invaded by a tumoral proliferation, its structure is usually normal, although vessel proliferation may be present.
Etiologies
Viral infections, other infections, autoimmune disorders, and underlying malignancy are the most common triggers for reactive HLH (Table 2). In adults, acquired (reactive) HLH is commonly associated with immune deficiency, which should be looked for routinely.
Viral infections
Although many viruses can trigger HLH, herpes viruses account for more than 50% of cases of virus-associated HLH [37]. Epstein–Barr virus (EBV) is the most common triggering agent for HLH. The HLH associated with primary EBV infection is more common in young children than in other age groups and may be fatal, most notably in immunocompromised individuals [37]. The diagnosis rests on serology, MNI test, and PCR detection of viral DNA in serum. Cytomegalovirus (CMV) contributes 30–50% of all cases of virus-associated HLH and should be sought routinely, as specific treatment is available [38]. Herpes simplex virus (HSV) [39], and parvovirus [40] are common triggers of HLH. Cases associated with adenovirus [41], hepatitis viruses [42], rubella, respiratory syncytial virus, and coxsackie [43] have been reported. Post-mortem analyses in patients dying after severe avian influenza A (H5N1) infection have also revealed hemophagocytosis [44]. Human immunodeficiency virus (HIV) alone or in the presence of other opportunistic or nonopportunistic infections, or malignancies (e.g., Hodgkin's lymphoma and Castleman's disease), has been associated with hemophagocytic syndrome [45].
Bacterial infections
Although pyogenic infections have been reported in association with HLH, the link is poorly documented. In contrast, stronger evidence exists to support a relation with intracellular bacteria (mycobacteria, mycoplasma, Rickettsia sp., Legionella sp., Chlamydia sp., Brucella sp., and Borrellia sp.) [46, 47].
Fungal and parasitic infections
Histoplasmosis is the most common fungal infection found in association with HLH [48, 49]. Leishmaniasis is akin to an animal model of hemophagocytosis [50, 51]. HLH has been reported during malaria attacks due to Plasmodium falciparum and in Babesia-related infections. More rarely, disseminated strongyloidiasis, Pneumocystis jiroveci infection [52], aspergillosis [53], toxoplasmosis, cryptococcosis, and candidiasis [54] have been described in association with HLH.
Lymphoproliferative diseases
In non-immunocompromised patients, the first malignancy to be found associated with HLH is T-cell lymphoma [55], above all when the trigger is identified as EBV [56]. Hodgkin's disease is the second malignancy associated with HLH [37, 57, 58]. B-cell lymphoma and intravascular lymphoma may also be associated with HLH, more particularly in Asians [59]. The EBV-induced lymphomas, transplant-recipient lymphomas, and lymphomas in HIV-infected patients are associated with a higher risk of HLH [60]. Human herpes virus 8 (HHV-8) is associated with several distinct lymphoproliferative disorders [61–63]. The HLH triggered by HHV-8 is extremely rare but has been reported in associated lymphoproliferative disorders as well as in immunocompromised patients. Conditions rarely reported in association with HLH include acute T-cell or NK leukemia [64, 65].
Systemic diseases
Occurrence of HLH during connective disease course may be related to the systemic disease activity, to infection, or rarely to lymphoma [66–69]. In a study by Dhote et al. among 26 patients with systemic diseases and HLH, the diagnoses were systemic lupus erythematosus (n = 14), Still's disease (n = 4), rheumatoid arthritis (n = 2), polyarteritis nodosa (n = 2), Kawasaki disease (n = 1), mixed connective tissue disease (n = 1), sarcoidosis (n = 1), and Sjögren syndrome (n = 1). In 15 patients, HLH was triggered by active infection (viral, n = 3; bacterial, n = 10; mycobacterial, n = 1; and Aspergillus, n = 1), which required a reduction in the immunosuppressive regimen. Only Lupus or Still's disease were directly responsible for HLH (in 9 patients), which required intensification of immunosuppressive regimens [70].
Pathophysiology
Genetic defects in familial HLH: keys to HLH pathophysiology
Studies of genetic HLH have provided valuable insight into the mechanisms of host defense and the pathophysiology of acquired (reactive) HLH. The clinical and laboratory features of primary HLH are identical to those of reactive HLH, except for occurrence in childhood, greater frequency, and severity of neurological involvement, and higher rates of fatal primary viral infections.
Genetic (primary) HLH is inherited in an autosomal or X-linked manner and can be divided into two subgroups: familial HLH (FHLH), in which the clinical syndrome of HLH is the only manifestation; and the immune deficiencies Chédiak–Higashi syndrome (CHS1), Griscelli syndrome (GS2), and X-linked proliferative syndrome (XLP), which have distinctive clinical features besides the sporadic, though frequent, development of HLH [37].
Since 1999, several genetic loci related to the activity of perforin and granzyme granule have been associated with genetic hemophagocytic syndrome, thus explaining the impaired or absent function of NK cells and cytotoxic T cells characteristic of the disease. The cytotoxic activity of NK cells and of CD8+ T-cell lymphocytes (CTL) is mediated by the release of cytolytic granules (containing large amounts of perforin, granzymes, and other serin-like proteases) via the immunological synapse to the target cell [71]. In genetic HLH, mutations impair the cytotoxic activity of CTL and NK cells without modifying their activation capacity or cytokine secretion. Most of the mutations affect the cytoplasmic granules in cytotoxic cells, altering either the effectors they contain (perforin) or their ability to migrate to the cell membrane [71]. This impairment may remain asymptomatic until the cytokine system is stimulated, when paradoxical inefficient overactivation reveals the illness.
Uncontrolled TH1 response and defective cytotoxic function: key points to reactive HLH pathophysiology
In reactive HLH, there is an overwhelming activation of normal T cells and macrophage which cause clinical and biological alterations: cooperation among triggered histiocytes, macrophages, CTL, and NK cells is at the hub of HLH, where evidence of a cytotoxic response, including Th1 response and cytotoxic cell overactivation, soon becomes apparent (Fig. 2) [72].
Immunopathological mechanisms in hemophagocytic lymphohistiocytosis: clinical effects of Th1 activation loop and cytokines production. Activation of CD-8 T lymphocytes results in clonal proliferation and activation of NK cells, with production of high levels of activating cytokines. Elaboration of TNF-α and other cytokines causes fever and systemic illness. TNF-α and IFN-γ production contribute to macrophage activation with resulting hemophagocytosis. TNF-α, tumor necrosis factor alpha; IFN-γ, interferon-gamma; IL1-β, interleukin-1 beta; IL-2, interleukin-2; IL-6, interleukin-6; IL-8, interleukin-8; IL-12, interleukin-12; sCD-8, soluble cluster of differentiation 8; NK cell, natural killer cell
Infection with a virus or intracellular pathogen normally induces a Th1 response in which cytotoxic Th1 cells and macrophage cooperate to increase the efficiency of the CTL system and the capacity of macrophage to proliferate. The antigen-presenting cells promote CTL and NK cells expansion and activation via the secretion of interleukin-12 (IL-12) and TNF-α. In turn, the cytotoxic cells release increased amounts of IFN-γ, TNF-α, and macrophage colony-stimulating factor (M-CSF). In HLH, this loop is amplified continuously, leading to the lymphohistiocytic proliferation responsible for the tumoral syndrome, and to the cytokine storm responsible for the other clinical and laboratory features (Fig. 2).
Activation manifests predominantly as a Th1 cytotoxic response with elevated serum levels of IFN-γ, IL-12, IL-2, M-CSF [73, 74], and Fas ligand [75], reflecting the Th1/Th2 imbalance (Fig. 2). The CTL upregulates activation markers such as CD-25 (alpha-chain of the IL-2 receptor), HLA-DR, and Fas [76]. The serum also contains high levels of the macrophage-produced monokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), TNF-α, and granulocyte colony-stimulating factor (G-CSF) [77, 78], as well as of coagulation factors (V, VII, IX, and X) and transferrin. Paradoxically, there is a tendency to peripheral CD8+ lymphocytopenia as a result of tissue infiltration by these cells [74, 79].
Linkage between HLH and infection
The linkage between HLH and infection is complex, as an infection may trigger the development of HLH or complicate the course of HLH. Infection accounts directly for half the deaths in patients with HLH [37]. Clinical immune deficiency complicating HLH results not only from neutropenia, but also from anergy in Th1 cells associated with increased levels of cytokines such as IFN-γ. Infection complicating HLH probably reflects acquired impairments of similar nature. Immune deficiency has been reported in 40–60% of cases of HLH: The main causes of acquired immune deficiency were HIV infection [80] and immunosuppressive treatment for systemic diseases [81] or transplantation [82].
Linkage between HLH and lymphoproliferation
Reactive HLH secondary to EBV-related or T-cell lymphoproliferative disease seems to be independent from a triggering factor. Indeed, uncontrolled transcription of messenger RNA for INF-γ in lymphoid T-cells [83] or of TNF-α in EBV+ lymphoid cells [84] has been documented in such cases. In addition, supernatant from T-EBV cell cultures induce macrophagic differentiation of monocyte lines [56]; thus, some lymphoid proliferations can trigger and perpetuate the Th1 activation and loop via a paracrine effect.
Prognostic factors and mortality
The overall mortality rate from HLH ranges across studies from 22 to 59% (Table 3). The HLH related to hematological malignancies or EBV infection carries a higher mortality rate than cases related to viruses or intracellular bacteria. In fatal HLH, death usually occurs during the first 4–8 weeks, from multiple organ failure, bleeding, or sepsis. In a retrospective study of 34 cases of HLH, Kaito et al. found that factors predicting death were: (a) age older than 30 years; (b) nature of the underlying disease; (c) hemoglobin level < 10 g/dl; (d) platelet count < 100,000/mm3; (e) ferritin level > 500 μg/l; or (f) bilirubin or alkaline phosphatase elevation [85]. In adults with active systemic disease and HLH, Dhote et al. did identify the following factors as being associated with death: absence of lymphadenopathy at diagnosis; corticosteroid treatment at diagnosis; and thrombocytopenia [70]; however, some of the prognostic factors identified in both studies are actually considered to be diagnostic criteria. It is thus likely that the outcome of patients with proper HLH was affected in these studies. In some studies, the time of etoposide administration was the main determinant of long-term survival. This effect was particularly marked for EBV-associated HLH. Imashuku et al. reported that survival was 90% in patients given etoposide within the first 4 weeks compared with only 56% in those treated later [86–88].
Therapeutic options
Supportive care
Comprehensive ICU management is needed to support organ function, to apply specific measures aiming to control the symptoms, to identify and treat the underlying cause of HLH, to prevent its recurrence, and also to manage infectious complications. Special attention should be given to correcting coagulation disorders, by transfusing platelets, plasma, and fibrinogen, as appropriate. Fluid and electrolyte balance must be restored and renal replacement therapy given, if needed. Vasoactive drugs may be needed to maintain cardiac function and hemodynamics and assisted ventilation to treat acute respiratory insufficiency. Anemia and neurological disorders may require additional treatment. Antibiotic and antifungal agents should be given as needed to treat infectious complications.
The underlying cause should be treated as soon as it is identified. Antiviral agents have been reported as beneficial in patients with herpes simplex virus, varicella zoster virus, or cytomegalovirus infection [89, 90], but not in HLH associated with EBV, herpes human virus 8, or herpes human virus 6. As soon as infection is ruled out, immediate treatment of lymphoproliferative or systemic disease, along with empiric or prophylactic anti-infectious agents, is essential to control both HLH and its trigger; however, lymphoma may be difficult to detect, as severe hemophagocytosis may develop despite a small tumor burden. The diagnosis may require invasive procedures such as bone marrow or lymph node biopsy, liver biopsy, or splenectomy. In the absence of specific etiological treatment, hemophagocytosis relapses a few days or weeks after the symptomatic treatment.
Measures targeted specifically at HLH
Hemophagocytic lymphohistiocytosis is a highly fatal disease if untreated. Severe HLH should be treated promptly after symptom onset. In less severe forms, investigations for a cause can be performed first, albeit rapidly, as sudden worsening may occur at any time. Life-threatening hyperinflammation, caused by excessive levels of cytokines, can be treated by corticosteroids.
In patients without underlying systemic diseases, etoposide combined with corticosteroid therapy is now the treatment of reference for HLH [86]. Etoposide (VP-16) is a cytotoxic drug that targets the enzyme topoisomerase-2. Although nonspecific, etoposide selectively targets the monocyte line. Etoposide was reported to benefit patients with HLH nearly 10 years ago and was subsequently proven effective in several studies [37, 86]. In patients with severe HLH, etoposide should be administered immediately and acts rapidly, within 24–48 h. Its efficacy far outweighs the risk of secondary leukemia and transient worsening of the neutropenia. Etoposide has been proved superior over intravenous immunoglobulins and cyclosporine in patients with EBV-induced HLH [88, 91]. Moreover, times to treatment was associated with outcome [86]. Once HLH control is achieved, the appropriateness of continuing etoposide therapy must be determined according to the underlying cause.
In patients with infection-related HLH, intravenous immunoglobulin has some chance of success, only if used early [72]; however, intravenous immunoglobulin combined with steroids is thought to be inferior to an etoposide-containing regimen [73].
In case of HLH secondary to lymphoproliferative diseases, treatment should target malignant lymphocytes using combined chemotherapy regimens (which all include corticosteroids). Addition of etoposide in this setting is questionable as it may add some medullar or mucosal toxicity.
In patients with systemic diseases, such as lupus or Still's disease, corticosteroid therapy is the reference [92]. When complementary immunosuppressive treatment is needed, cyclosporine is often the best choice [81, 93, 94]. In patients with Still's disease, TNF-α antagonists (etanercept and infliximab) have generated interest because TNF-α plays a key role in the pathophysiology of both HLH and Still's disease [24, 95].
Conclusion
In conclusion, the diagnosis of HLH relies on the association of clinical abnormalities (fever, splenomegaly, pancytopenia) and hemophagocytosis in bone marrow, spleen, or lymph node specimens. Liver, pulmonary, renal, cardiac, and skin involvement may occur at various degrees possibly leading to multiple organ failure. Three main associated etiologies can be found, namely infections (viral, bacterial, fungal, or parasitic), lymphoproliferative diseases, or connective tissue diseases. Immune deficiency is often retrieved. Although clinically mimicking severe sepsis, HLH has a distinct pathophysiology on which specific therapy is based. The comprehensive management of severe HLH requires the involvement of a multidisciplinary team in order to determine the best therapeutic strategy and to identify the underlying cause. The high mortality in patients with no etiological diagnosis warrants aggressive investigations and treatment. Studies are needed to identify whether early administration of etoposide reverses organ failure and decreases mortality in critically ill patients with HLH.
References
Scott R, Robb-Smith A (1939) Histiocytic medullary reticulosis. Lancet 2:194–198
Risdall RJ, McKenna RW, Nesbit ME, Krivit W, Balfour HH Jr, Simmons RL, Brunning RD (1979) Virus-associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 44:993–1002
Cohen RA, Hutter JJ Jr, Boxer MA, Goldman DS (1980) Histiocytic medullary reticulosis associated with acute Epstein–Barr (EB) virus infection. Am J Pediatr Hematol Oncol 2:245–248
McKenna RW, Risdall RJ, Brunning RD (1981) Virus associated hemophagocytic syndrome. Hum Pathol 12:395–398
Wilson ER, Malluh A, Stagno S, Crist WM (1981) Fatal Epstein–Barr virus-associated hemophagocytic syndrome. J Pediatr 98:260–262
Ezdinli EZ, Kucuk O, Chedid A, Sinclair TF, Thomas K, Singh S, Sarpel S, Jovanovic L (1986) Hypogammaglobulinemia and hemophagocytic syndrome associated with lymphoproliferative disorders. Cancer 57:1024–1037
Liang DC, Chu ML, Shih CC (1986) Reactive histiocytosis in acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Cancer 58:1289–1284
March LM, Webb J, Eckstein RP (1986) Cytophagic panniculitis. Aust N Z J Med 16:397–401
Gauvin F, Toledano B, Champagne J, Lacroix J (2000) Reactive hemophagocytic syndrome presenting as a component of multiple organ dysfunction syndrome. Crit Care Med 28:3341–3345
Strauss R, Neureiter D, Westenburger B, Wehler M, Kirchner T, Hahn EG (2004) Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients: a postmortem clinicopathologic analysis. Crit Care Med 32:1316–1321
Nahum E, Ben-Ari J, Stain J, Schonfeld T (2000) Hemophagocytic lymphohistiocytic syndrome: unrecognized cause of multiple organ failure. Pediatr Crit Care Med 1:51–54
Stephan F, Thioliere B, Verdy E, Tulliez M (1997) Role of hemophagocytic histiocytosis in the etiology of thrombocytopenia in patients with sepsis syndrome or septic shock. Clin Infect Dis 25:1159–1164
Grom AA (2003) Macrophage activation syndrome and reactive hemophagocytic lymphohistiocytosis: The same entities? Curr Opin Rheumatol 15:587–590
Dinarello CA, Wolff SM (1993) The role of interleukin-1 in disease. N Engl J Med 328:106–113
Dinarello CA, Cannon JG (1993) Cytokine measurements in septic shock. Ann Intern Med 119:853–854
Reiner AP, Spivak JL (1988) Hematophagic histiocytosis. A report of 23 new patients and a review of the literature. Medicine (Baltimore) 67:369–388
Shirono K, Tsuda H (1995) Virus-associated haemophagocytic syndrome in previously healthy adults. Eur J Haematol 55:240–244
Ohta H, Yumara-Yagi K, Sakata N, Inoue M, Kawa-Ha K (1994) Capillary leak syndrome in patients with hemophagocytic lymphohistiocytosis. Acta Paediatr 83:1113–1114
Honig LS, Snipes GJ, Vogel H, Horoupian DS (1991) Sensorimotor neuropathy in hemophagocytosis syndrome. Acta Neurol Scand 84:316–320
Hanada T, Ono I, Iinuma S, Nagai Y (1989) Pure red cell aplasia in association with virus associated haemophagocytic syndrome (VAHS). Br J Haematol 73:570–571
Koduri PR, Carandang G, DeMarais P, Patel AR (1995) Hyperferritinemia in reactive hemophagocytic syndrome report of four adult cases. Am J Hematol 49:247–249
Esumi N, Ikushima S, Todo S, Imashuku S (1987) Hyperferritinemia in malignant histiocytosis and virus-associated hemophagocytic syndrome. N Engl J Med 316:346–347
Esumi N, Ikushima S, Hibi S, Todo S, Imashuku S (1988) High serum ferritin level as a marker of malignant histiocytosis and virus-associated hemophagocytic syndrome. Cancer 61:2071–2076
Dinarello CA, Gelfand JA, Wolff SM (1993) Anticytokine strategies in the treatment of the systemic inflammatory response syndrome. J Am Med Assoc 269:1829–1835
Henter JI, Carlson LA, Soder O, Nilsson-Ehle P, Elinder G (1991) Lipoprotein alterations and plasma lipoprotein lipase reduction in familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand 80:675–681
Ooe K (1992) Pathogenesis and clinical significance of hemophagocytic syndrome: hypothesis. Pediatr Pathol 12:309–312
McClure PD, Strachan P, Saunders EF (1974) Hypofibrinogenemia and thrombocytopenia in familial hemophagocytic reticulosis. J Pediatr 85:67–70
de Kerguenec C, Hillaire S, Molinie V, Gardin C, Degott C, Erlinger S, Valla D (2001) Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol 96:852–857
Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C (2005) Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood 105:1648–1651
Whiting JF, Green RM, Rosenbluth AB, Gollan JL (1995) Tumor necrosis factor-alpha decreases hepatocyte bile salt uptake and mediates endotoxin-induced cholestasis. Hepatology 22:1273–1278
Iso ON, Hashimoto N, Tanaka A, Sunaga S, Oka T, Kurokawa K, Watanabe T (1998) Cytokine-induced hypoalbuminemia in a patient with hemophagocytic syndrome: direct in vitro evidence for the role of tumor necrosis factor-alpha. Dig Dis Sci 43:67–73
Weber J, Yang JC, Topalian SL, Parkinson DR, Schwartzentruber DS, Ettinghausen SE, Gunn H, Mixon A, Kim H, Cole D et al. (1993) Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J Clin Oncol 11:499–506
Braun MC, Cohn RA, Kletzel M (1996) Nephrotic syndrome accompanying familial hemophagocytic syndrome. J Pediatr Hematol Oncol 18:195–197
Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan JL, Noel LH, Karras A (2006) Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 69:1892–1898
Chubachi A, Miura I, Hatano Y, Ohshima A, Nishinari T, Miura AB (1995) Syndrome of inappropriate secretion of antidiuretic hormone in patients with lymphoma-associated hemophagocytic syndrome. Ann Hematol 70:53–55
Favara BE (1996) Histopathology of the liver in histiocytosis syndromes. Pediatr Pathol Lab Med 16:413–433
Janka GE (2007) Hemophagocytic syndromes. Blood Rev 21:245–253
Danish EH, Dahms BB, Kumar ML (1985) Cytomegalovirus-associated hemophagocytic syndrome. Pediatrics 75:280–283
Lasserre M, Huguet C, Terno O (1993) Acute severe herpes simplex hepatitis with virus-associated hemophagocytic syndrome in an immunocompetent adult. J Hepatol 18:256–257
Boruchoff SE, Woda BA, Pihan GA, Durbin WA, Burstein D, Blacklow NR (1990) Parvovirus B19-associated hemophagocytic syndrome. Arch Intern Med 150:897–899
Morimoto A, Teramura T, Asazuma Y, Mukoyama A, Imashuku S (2003) Hemophagocytic syndrome associated with severe adenoviral pneumonia: usefulness of real-time polymerase chain reaction for diagnosis. Int J Hematol 77:295–298
Kyoda K, Nakamura S, Machi T, Kitagawa S, Ohtake S, Matsuda T (1998) Acute hepatitis A virus infection-associated hemophagocytic syndrome. Am J Gastroenterol 93:1187–1188
Guerin C, Pozzetto B, Berthoux F (1989) Hemophagocytic syndrome associated with coxsackie virus A 9 infection in a non-immunosuppressed adult. Intensive Care Med 15:547–548
Henter JI, Chow CB, Leung CW, Lau YL (2006) Cytotoxic therapy for severe avian influenza A (H5N1) infection. Lancet 367:870–873
Chen TL, Wong WW, Chiou TJ (2003) Hemophagocytic syndrome: an unusual manifestation of acute human immunodeficiency virus infection. Int J Hematol 78:450–452
Baraldes MA, Domingo P, Gonzalez MJ, Aventin A, Coll P (1998) Tuberculosis-associated hemophagocytic syndrome in patients with acquired immunodeficiency syndrome. Arch Intern Med 158:194–195
Yang WK, Fu LS, Lan JL, Shen GH, Chou G, Tseng CF, Chi CS (2003) Mycobacterium avium complex-associated hemophagocytic syndrome in systemic lupus erythematosus patient: report of one case. Lupus 12:312–316
Masri K, Mahon N, Rosario A, Mirza I, Keys TF, Ratliff NB, Starling RC (2003) Reactive hemophagocytic syndrome associated with disseminated histoplasmosis in a heart transplant recipient. J Heart Lung Transplant 22:487–491
Kumar N, Jain S, Singh ZN (2000) Disseminated histoplasmosis with reactive hemophagocytosis: aspiration cytology findings in two cases. Diagn Cytopathol 23:422–424
Kontopoulou T, Tsaousis G, Vaidakis E, Fanourgiakis P, Michalakeas E, Trigoni E, Samarkos M (2002) Hemophagocytic syndrome in association with visceral leishmaniasis. Am J Med 113:439–440
Tapisiz A, Belet N, Ciftci E, Ince E, Dogru U (2007) Hemophagocytic lymphohistiocytosis associated with visceral leishmaniasis. J Trop Pediatr 11:11
Taillan B, Ferrari E, Heudier P, Fuzibet JG, Dujardin P (1991) Hemophagocytic syndrome in pneumocystosis. Presse Med 20:1456–1457 [in French]
Garcia Escudero A, Benitez Moya JM, Lag Asturiano E (2000) Hemophagocytic syndrome and invasive aspergillosis in a patient with Churg-Strauss vasculitis. Med Clin (Barc) 115:598 [in Spanish]
Bhatia S, Bauer F, Bilgrami SA (2003) Candidiasis-associated hemophagocytic lymphohistiocytosis in a patient infected with human immunodeficiency virus. Clin Infect Dis 37:e161–e166
Jaffe ES, Costa J, Fauci AS, Cossman J, Tsokos M (1983) Malignant lymphoma and erythrophagocytosis simulating malignant histiocytosis. Am J Med 75:741–749
Chuang HC, Lay JD, Hsieh WC, Su IJ (2007) Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to Epstein–Barr virus-associated T-cell lymphoma: nuclear factor-kappa B pathway as a potential therapeutic target. Cancer Sci 98:1281–1287
Janka G, Imashuku S, Elinder G, Schneider M, Henter JI (1998) Infection- and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am 12:435–444
Kojima H, Takei N, Mukai Y, Hasegawa Y, Suzukawa K, Nagata M, Noguchi M, Mori N, Nagasawa T (2003) Hemophagocytic syndrome as the primary clinical symptom of Hodgkin's disease. Ann Hematol 82:53–56
Aouba A, Lambotte O, Vasiliu V, Divine M, Valensi F, Varet B, Bazarbachi A, Hermine O (2004) Hemophagocytic syndrome as a presenting sign of transformation of smoldering to acute adult T-cell leukemia/lymphoma: efficacy of anti-retroviral and interferon therapy. Am J Hematol 76:187–189
Srichaikul T, Punyagupta S, Mongkolsritrakul W, Jidpugdeebodin S (2004) EBV and hemophagocytic syndrome: analysis of 3 cases, with speculation on clinical features, therapy and role of EBV. J Med Assoc Thai 87:974–983
Fardet L, Blum L, Kerob D, Agbalika F, Galicier L, Dupuy A, Lafaurie M, Meignin V, Morel P, Lebbe C (2003) Human herpesvirus 8-associated hemophagocytic lymphohistiocytosis in human immunodeficiency virus-infected patients. Clin Infect Dis 37:285–291
Seliem RM, Griffith RC, Harris NL, Beheshti J, Schiffman FJ, Longtine J, Kutok J, Ferry JA (2007) HHV-8+, EBV+ multicentric plasmablastic microlymphoma in an HIV+ Man: the spectrum of HHV-8+ lymphoproliferative disorders expands. Am J Surg Pathol 31:1439–1445
Li CF, Ye H, Liu H, Du MQ, Chuang SS (2006) Fatal HHV-8-associated hemophagocytic syndrome in an HIV-negative immunocompetent patient with plasmablastic variant of multicentric Castleman disease (plasmablastic microlymphoma). Am J Surg Pathol 30:123–127
Okuda T, Sakamoto S, Deguchi T, Misawa S, Kashima K, Yoshihara T, Ikushima S, Hibi S, Imashuku S (1991) Hemophagocytic syndrome associated with aggressive natural killer cell leukemia. Am J Hematol 38:321–323
Ma L, Bandarchi B, Glusac EJ (2005) Fatal subcutaneous panniculitis-like T-cell lymphoma with interface change and dermal mucin, a dead ringer for lupus erythematosus. J Cutan Pathol 32:360–365
Palazzi DL, McClain KL, Kaplan SL (2003) Hemophagocytic syndrome after Kawasaki disease. Pediatr Infect Dis J 22:663–666
al-Eid W, al-Jefri A, Bahabri S, al-Mayouf S (2000) Hemophagocytosis complicating Kawasaki disease. Pediatr Hematol Oncol 17:323–329
Okada M, Suzuki K, Hidaka T, Shinohara T, Kataharada K, Matsumoto M, Takada K, Ohsuzu F (2001) Hemophagocytic syndrome in systemic lupus erythematosus. Intern Med 40:1263–1264
Sekigawa I, Suzuki J, Nawata M, Ikeda K, Koike M, Iida N, Hashimoto H, Oshimi K (2001) Hemophagocytosis in autoimmune disease. Clin Exp Rheumatol 19:333–338
Dhote R, Simon J, Papo T, Detournay B, Sailler L, Andre MH, Dupond JL, Larroche C, Piette AM, Mechenstock D, Ziza JM, Arlaud J, Labussiere AS, Desvaux A, Baty V, Blanche P, Schaeffer A, Piette JC, Guillevin L, Boissonnas A, Christoforov B (2003) Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum 49:633–639
Menasche G, Feldmann J, Fischer A, Basile Gde S (2005) Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev 203:165–179
Egeler RM, Shapiro R, Loechelt B, Filipovich A (1996) Characteristic immune abnormalities in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol 18:340–345
Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, Ohta H, Fujisaki H, Kobayashi M, Sakata N, Kawa-Ha K, Okada S, Tawa A (1997) Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood 89:4100–4103
Akashi K, Hayashi S, Gondo H, Mizuno S, Harada M, Tamura K, Yamasaki K, Shibuya T, Uike N, Okamura T et al. (1994) Involvement of interferon-gamma and macrophage colony-stimulating factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol 87:243–250
Hasegawa D, Kojima S, Tatsumi E, Hayakawa A, Kosaka Y, Nakamura H, Sako M, Osugi Y, Nagata S, Sano K (1998) Elevation of the serum Fas ligand in patients with hemophagocytic syndrome and Diamond-Blackfan anemia. Blood 91:2793–2799
Imashuku S, Hibi S, Sako M, Ishida Y, Mugishima H, Chen J, Tsunematsu Y (1995) Soluble interleukin-2 receptor: a useful prognostic factor for patients with hemophagocytic lymphohistiocytosis. Blood 86:4706–4707
Imashuku S, Hibi S (1991) Cytokines in hemophagocytic syndrome. Br J Haematol 77:438–440
Henter JI, Elinder G, Soder O, Hansson M, Andersson B, Andersson U (1991) Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood 78:2918–2922
Fujiwara F, Hibi S, Imashuku S (1993) Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol 15:92–98
Castilletti C, Preziosi R, Bernardini G, Caterini A, Gomes V, Calcaterra S, Carletti F, Capobianchi MR, Armignacco O (2004) Hemophagocytic syndrome in a patient with acute human immunodeficiency virus infection. Clin Infect Dis 38:1792–1793
Lambotte O, Khellaf M, Harmouche H, Bader-Meunier B, Manceron V, Goujard C, Amoura Z, Godeau B, Piette JC, Delfraissy JF (2006) Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosus-associated hemophagocytic syndrome. Medicine (Baltimore) 85:169–182
Karras A, Thervet E, Legendre C (2004) Hemophagocytic syndrome in renal transplant recipients: report of 17 cases and review of literature. Transplantation 77:238–243
Lin MT, Chang HM, Huang CJ, Chen WL, Lin CY, Lin CY, Chuang SS (2007) Massive expansion of EBV+ monoclonal T cells with CD5 down regulation in EBV-associated haemophagocytic lymphohistiocytosis. J Clin Pathol 60:101–103
Chuang HC, Lay JD, Chuang SE, Hsieh WC, Chang Y, Su IJ (2007) Epstein–Barr virus (EBV) latent membrane protein-1 down-regulates tumor necrosis factor-alpha (TNF-alpha) receptor-1 and confers resistance to TNF-alpha-induced apoptosis in T cells: implication for the progression to T-cell lymphoma in EBV-associated hemophagocytic syndrome. Am J Pathol 170:1607–1617
Kaito K, Kobayashi M, Katayama T, Otsubo H, Ogasawara Y, Sekita T, Saeki A, Sakamoto M, Nishiwaki K, Masuoka H, Shimada T, Yoshida M, Hosoya T (1997) Prognostic factors of hemophagocytic syndrome in adults: analysis of 34 cases. Eur J Haematol 59:247–253
Imashuku S (2000) Advances in the management of hemophagocytic lymphohistiocytosis. Int J Hematol 72:1–11
Imashuku S, Hibi S, Tabata Y, Todo S (1999) Hemophagocytic syndrome in five patients with Epstein–Barr virus negative B-cell lymphoma. Cancer 85:2298–2300
Imashuku S, Kuriyama K, Sakai R, Nakao Y, Masuda S, Yasuda N, Kawano F, Yakushijin K, Miyagawa A, Nakao T, Teramura T, Tabata Y, Morimoto A, Hibi S (2003) Treatment of Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in young adults: a report from the HLH study center. Med Pediatr Oncol 41:103–109
Hardikar W, Pang K, Al-Hebbi H, Curtis N, Couper R (2006) Successful treatment of cytomegalovirus-associated haemophagocytic syndrome following paediatric orthotopic liver transplantation. J Paediatr Child Health 42:389–391
Ramasamy K, Lim ZY, Savvas M, Salisbury JR, Dokal I, Mufti GJ, Pagliuca A (2006) Disseminated herpes virus (HSV-2) infection with rhabdomyolysis and hemophagocytic lymphohistiocytosis in a patient with bone marrow failure syndrome. Ann Hematol 85:629–630
Imashuku S, Hibi S, Ohara T, Iwai A, Sako M, Kato M, Arakawa H, Sotomatsu M, Kataoka S, Asami K, Hasegawa D, Kosaka Y, Sano K, Igarashi N, Maruhashi K, Ichimi R, Kawasaki H, Maeda N, Tanizawa A, Arai K, Abe T, Hisakawa H, Miyashita H, Henter JI (1999) Effective control of Epstein–Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society. Blood 93:1869–1874
Papo T, Andre MH, Amoura Z, Lortholary O, Tribout B, Guillevin L, Piette JC (1999) The spectrum of reactive hemophagocytic syndrome in systemic lupus erythematosus. J Rheumatol 26:927–930
Ravelli A, Viola S, Benedetti F de, Magni-Manzoni S, Tzialla C, Martini A (2001) Dramatic efficacy of cyclosporine A in macrophage activation syndrome. Clin Exp Rheumatol 19:108
Ravelli A (2002) Macrophage activation syndrome. Curr Opin Rheumatol 14:548–552
Henzan T, Nagafuji K, Tsukamoto H, Miyamoto T, Gondo H, Imashuku S, Harada M (2006) Success with infliximab in treating refractory hemophagocytic lymphohistiocytosis. Am J Hematol 81:59–61
Acknowledgements
The authors thank M.T. Daniel for her contribution to iconography preparation and A. Wolfe for her help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Créput, C., Galicier, L., Buyse, S. et al. Understanding organ dysfunction in hemophagocytic lymphohistiocytosis. Intensive Care Med 34, 1177–1187 (2008). https://doi.org/10.1007/s00134-008-1111-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1111-y