Abstract
Objective
Echocardiographic recording of the tricuspid annular plane systolic excursion (TAPSE) has been recommended for assessing right ventricular function in cardiac patients. The ability of TAPSE to reflect right ventricular function at baseline and to monitor acute changes in right ventricular function was tested in critically ill patients.
Design
Prospective study.
Setting
A 24-bed medical intensive care unit.
Patients
Eighty-six patients admitted for acute respiratory failure, circulatory failure, or coma.
Interventions
In 40 patients, the examination was repeated after volume expansion (n = 15), passive leg raising (n = 5), or dobutamine infusion (n = 20).
Measurements and results
The right ventricular fractional area change, TAPSE, the left ventricular ejection fraction, and the ratio of right to left ventricular end-diastolic area were measured using Doppler echocardiography. In the overall population, TAPSE (19 ± 5 mm) was positively related to left ventricular ejection fraction (r 2 = 0.31, p < 0.001) and right ventricular fractional area change and was negatively related to age and to the ratio of right to left ventricular end-diastolic area. Multivariate analysis indicated that only left ventricular ejection fraction and agewere independently related toTAPSE (multiple r 2 = 0.36, p < 0.001). Following dynamic interventions, the changes in TAPSE were linearly related to changes in left ventricular ejection fraction (r 2 = 0.65, p < 0.01) but notto changes in the right ventricular fractional area change.
Conclusions
Unexpectedly, TAPSE was more strongly related to left ventricular ejection fraction than to indices of right ventricular function in critically ill patients. The potential interest of TAPSE as a dynamic marker of left ventricular systolic function deserves further study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In critically ill patients, abnormal function of the right ventricle may contribute to hemodynamic instability, and various echocardiographic indices quantifying right ventricular dimensions have been proposed to diagnose right ventricular dysfunction [1–5]. Recently, American and European guidelines for chamber quantification have also recommended that the displacement of the tricuspid annular plane be observed in an attempt to quantify right ventricular function [4]. Indeed, right ventricular function critically depends not only on active right ventricular free-wall shortening but also on the base-to-apex piston-like motion of the atrioventricular valve [6, 7]. Numerous studies have documented that the tricuspid annular plane systolic excursion (TAPSE) is linearly related to right ventricular ejection fraction and/or fractional area change in various forms of cardiac disease and clinical settings, including ischemia, congestive heart failure, cardiomyopathy, and pulmonary hypertension [8–18]. Furthermore, decreased TAPSE is associated with poor prognosis in patients with ischemic heart disease [10, 12], pulmonary hypertension [15], and heart failure [18]. Finally, measurement of TAPSE has been shown to be highly reproducible, and it has been stressed that it is fast and easy to obtain [4, 8, 10, 15].
The ability of TAPSE to reflect right ventricular function at baseline and to monitor acute changes in right ventricular function remains to be established in intensive care unit (ICU) patients. In the present study performed in ICU patients, two hypotheses were tested: 1) TAPSE is related to right ventricular function at baseline, and 2) coordinated increases in TAPSE and indices of right ventricular function can be observed following positive inotropic therapy, while TAPSE and indices of right ventricular function remain unchanged following acute changes in preload.
Patients and methods
Patients
We prospectively evaluated a total of 117 patients. Only patients with good acoustic windows with clear visualization of all cardiac chambers and valves were included in the study. In addition, patients with significant valvular regurgitation, ventricular rhythm disturbances, or atrial fibrillation were excluded from the study. A total of 86 patients met study criteria. Reasons for admission to our ICU were acute respiratory failure, circulatory failure, or coma. All procedures were in accordance with the recommendations found in the Helsinki Declaration of 1975. This study was approved by the Ethics Committee of the Société de Réanimation de Langue Française. All patients or relatives gave their informed consent. Preliminary results have been published elsewhere [19].
Methods
Echocardiography
The echocardiographic examinations were performed by the same operator (B.L.) using a transthoracic ultrasound device (EnVisor; Philips, France) equipped with a phased-array transducer of 2.5 MHz. Conventional echocardiography, including M-mode, two-dimensional (2D), and pulsed and color Doppler measurement, was performed. Echocardiographic images were recorded together with electrocardiograms. All measurement were recorded on paper at a speed of 100 mm/s and were stored digitally for later playback and analysis.
TAPSE
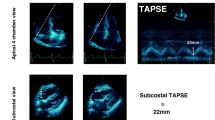
Using the 2D four-chamber view, the right ventricular base-to-apex shortening during systole was measured as the systolic displacement of the lateral portion of the tricuspid annulus. TAPSE was recorded on the M-mode format under 2D echocardiographic guidance. The cursor was oriented to the junction of the tricuspid valve plane with the free wall of the right ventricle using the apical four-chamber view as previously recommended [10, 17] (Fig. 1). Data were averaged over five beats, as previously recommended [10].
RVEDA/LVEDA ratio, RVFAC, and LVEF
Using the apical four-chamber view, the right ventricular end-diastolic area (RVEDA), right ventricular end-systolic area (RVESA), and left ventricular end-diastolic area (LVEDA) were measured. We calculated the RVEDA/LVEDA ratio. The right ventricular fractional area change (RVFAC) was calculated as follows:RVFAC = [(RVEDA – RVESA) / RVEDA] × 100The left ventricular ejection fraction (LVEF) was measured using the biplane Simpson's method from the apical two-chamber and four-chamber views [1, 20, 21]. Although dependent on loading conditions (mainly afterload), at least in part, LVEF is a classic parameter of global left ventricular function [20, 21].
Hemodynamic measurements
In all patients, heart rate and systolic, diastolic, and mean arterial pressures (oscillometric method, n = 44; femoral arterial catheter, n = 42) were measured. Forty patients with acute circulatory failure were also equipped with a pulmonary artery catheter (n = 9; Edwards Lifesciences, Maurepas, France) or a pulse-induced contour cardiac output device that uses transpulmonary thermodilution (n = 31; Pulsion Medical Systems, Munich, Germany), thus allowing the measurement of thermodilution cardiac output in this patient subgroup. The thermodilution stroke volume index (SVi) was calculated as cardiac index divided by heart rate.
Study design
All data for the 86 patients were obtained at admission. Measurement were also obtained after various interventions in the subgroup of patients equipped with an invasive hemodynamic monitoring device and in whom the attending physician decided to perform a fluid challenge, a passive leg raising (PLR) maneuver, or a dobutamine infusion. Fluid challenge (500 ml saline; n = 15) was decided upon if clinical signs of inadequate global perfusion together with LVEF ≥ 45% were present. PLR was performed (n = 5) to test preload responsiveness [22]. Finally, dobutamine (n = 20) was given if clinical signs of inadequate global perfusion together with normal preload and depressed contractility (i.e., LVEF < 45%) were present. Patients with an increase in stroke volume index ≥ 15% or < 15% following fluid infusion, PLR, or dobutamine, were deemed responders and nonresponders, respectively [23].
Intraobserver and interobserver variability were tested for TAPSE, RVFAC, RVEDA/LVEDA, and LVEF on 10 random patients in sinus rhythm. Different beats from the same recordings were used for testing the intraobserver reproducibility, and the interobserver reproducibility was calculated from different recordings obtained by a second, blinded operator (X.M.) just after the first examination.
Statistical analysis
The values of continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and those of variables with nonnormal distribution as percentages. Linear regression analysis was used to examine the relationships between TAPSE and echocardiographic and hemodynamic variables. A stepwise regression analysis was performed to look for independent variables related to TAPSE. Following univariate analysis, a p-value < 0.20 was necessary for a variable to enter forward stepwise multiple regression analysis. The influence of preload changes (fluid challenge and PLR) and dobutamine infusion on hemodynamic and echocardiographic variables was tested using analysis of variance. A linear regression analysis was used to examine the relationships between the change in TAPSE and the change in hemodynamic variables following these three interventions (fluid challenge, PLR, dobutamine infusion). Finally, intraobserver and interobserver reproducibility of TAPSE, RVEDA/LVEDA, RVFAC, and LVEF were estimated by using mean measurement bias ± SD and coefficient of variation(Cvar; 100 × (measurement 1 – measurement 2) / (measurement 1 + measurement 2) / 2). Box and whisker diagrams were drawn to show the spread of TAPSE in patients depending on the threshold values of the indices of ventricular function. Receiver operating characteristic (ROC) curves were constructed to determine the cut-off values of TAPSE with optimal sensitivity and specificity that could predict decreased ventricular function A p-value < 0.05 was considered statistically significant.
Results
Complete transthoracic echocardiography with adequate endocardial border resolution for determining end-diastolic and end-systolic right ventricular area as well as tricuspid annular motion was obtained in 86 patients. Characteristics of the study population are listed in Table 1. The reproducibility of RVFAC, LVEF, and TAPSE measurement is indicated on Table 2.
TAPSE at baseline
The TAPSE value ranged from 7 mm to 35 mm (mean ± SD = 19 ± 5 mm; median = 20 mm). TAPSE was similar in women (20 ± 5 mm) and in men (19 ± 5 mm). Using univariate analysis, TAPSE was shown to be negatively related to age (r 2 = 0.18; p < 0.001) and to the RVEDA/LVEDA ratio (r 2 = 0.05; p < 0.05), and it was positively related to LVEF (r 2 = 0.31; p = 0.001), diastolic arterial pressure (r 2 = 0.04; p < 0.05), and RVFAC (r 2 = 0.03; p < 0.05). There was no linear relationship between TAPSE and body weight, body height, body surface area, heart rate, systolic arterial pressure, or mean arterial pressure. Patients with depressed right ventricular systolic function (RVFAC < 25%; n = 26) had lower TAPSE than patients with normal RVFAC (n = 60): 17 ± 5 mm vs. 20 ± 5 mm; p = 0.02. Patients with depressed left ventricular systolic function (LVEF < 45%, n = 9) had lower TAPSE than patients with normal LVEF (n = 47): 17 ± 5 mm vs. 22 ± 4 mm; p < 0.01.
Figure 2 presents box and whisker plots according to various combinations of right and left ventricular function. In the subgroup of 25 patients with reduced left ventricular systolic function and normal right ventricular systolic function, TAPSE was higher than in the subgroup of 14 patients with both reduced left ventricular systolic function and reduced right ventricular systolic function (TAPSE = 18 ± 5 mm vs. 13 ± 3 mm, p < 0.001). The LVEF was similar in these two subgroups (36 ± 7% vs. 33 ± 8%). Patients with both reduced left ventricular systolic function and reduced right ventricular systolic function had lower TAPSE than patients with preserved left ventricular function and either preserved right ventricular function (n = 36; TAPSE = 21 ± 5 mm) or impaired right ventricular function (n = 11; TAPSE = 23 ± 2 mm). Finally, patients with impaired left ventricular function and preserved right ventricular function had a lower TAPSE than patients with preserved left ventricular function and right ventricular function (p < 0.05).
Box and whisker plots of tricuspid annular plane systolic excursion (TAPSE) values according to various combinations of right ventricular and left ventricular function. * p < 0.01 when compared with TAPSE of the three other subgroups; # p < 0.05 when compared with TAPSE of patients with preserved left ventricular systolic function and preserved right ventricular systolic function
In the overall population, multivariate analysis indicated that TAPSE was related only to LVEF and age (multiple r 2 = 0.36; p < 0.001). Using ROC curve analysis, TAPSE < 22 mm identified patients with LVEF < 45% with an 85% sensitivity and 62% specificity.
Effects of preload manipulations and dobutamine infusion
The effects of fluid infusion (n = 15) and PLR (n = 5) are presented in Table 3. On average, the thermodilution SVi significantly increased, whereas LVEF, RVFAC, and TAPSE remained unchanged. The effects of dobutamine infusion (n = 20) are presented in Table 4. On average, RVFAC remained unchanged, whereas SVi, LVEF, and TAPSE increased.
Overall, in the 40 patients for whom a dynamic maneuver was performed, there was no relationship between the changes in TAPSE and the changes in RVFAC. Conversely, the changes in TAPSE were positively related to changes in LVEF (r 2 = 0.65, p < 0.01; see Fig. 3). In the subgroup of 20 patients who received dobutamine, there was no relationship between the changes in TAPSE and the changes in RVFAC. Conversely, the changes in TAPSE were positively related to changes in LVEF (r 2 = 0.62, p < 0.01).
Discussion
Contrary to our initial study hypothesis, TAPSE was either weakly related to or unrelated to the echocardiographic indices quantifying right ventricular function in the critically ill population studied, both at baseline and following dynamic interventions. Conversely, it was observed that TAPSE was significantly related to echocardiographic indices of left ventricular function at baseline. Furthermore, following dynamic interventions (namely contractility and preload manipulations), the changes in LVEF and in TAPSE were significantly related. Although preliminary, the present study thus suggests that TAPSE may be included in the battery of echocardiographic markers of static and dynamic left ventricular function in critically ill patients.
The observed TAPSE values (mean, 95% confidence interval) were consistent with values previously published for controls and for patients with various forms of cardiac disease [8–18]. The high reproducibility of TAPSE measurement has been previously documented in ischemic heart disease [8], congestive heart failure [10], and pulmonary hypertension [15], and this was confirmed in our ICU population. Recently, it has been stressed that TAPSE measurement is far more reproducible than other echocardiographic indices of heart function, especially right ventricular function [4, 10, 15], as also observed in our study.
In our study, the patients with decreased RVFAC or increased RVEDA/LVEDA had a lower TAPSE than patients with preserved right ventricular function. This result is consistent with previous studies showing decreased TAPSE in cardiac patients with low right ventricular ejection fraction or low RVFAC [8–17]. This result is also in agreement with previous studies showing that right ventricular function critically depends on the base-to-apex piston-like motion of the tricuspid valve and not only on active free-wall shortening [6, 7].
Our study indicates that TAPSE is more strongly related to echocardiographic indices of left ventricular function than to echocardiographic indices of right ventricular function in critically ill patients. In an attempt to explain this finding, one hypothesis could be that the indices of right ventricular function we used were not sensitive enough to precisely stratify the overall spectrum of right ventricular function at baseline and to precisely monitor mild-to-moderate changes in right ventricular function during dynamic maneuvers [1–4]. Indeed, assessment of the end-systolic and end-diastolic dimensions of the right ventricle is notoriously difficult given the complex right ventricular anatomy, suboptimal endocardial border definition, and lesser right ventricular free-wall contractile function as compared with the left ventricle [4–6]. This explains why the indices quantifying right ventricular diameter, area, or volume are not always reproducible and are highly operator- and model-dependent. This also explains why 2D echocardiographic calculations of right ventricular volumes must be discouraged [1–5, 24]. However, it must be noted that the average baseline values of RVFAC and RVEDA/LVEDA in our study were comparable to the values previously documented in populations similar to ours [25].
The physiological biventricular interdependence [5, 26] could help explain the link between left ventricular function and TAPSE. As recently reviewed [5], the right ventricle is linked to the left ventricle in several ways: by a shared wall (the septum), by mutually encircling epicardial fibers, by attachment of the right ventricular free wall to the anterior and posterior septum, and by sharing of the pericardial space. It must also be noted that a recent study has documented a positive relationship between TAPSE and left ventricular function in cardiomyopathic patients [17]. Indeed, Lopez-Candales et al. have documented that TAPSE depends on left ventricular function in patients undergoing routine echocardiographic examination [17].
Besides the positive relationship between LVEF and TAPSE, there was a weak negative relationship between age and TAPSE, both with univariate analysis (r 2 = 0.18) and by the use of multivariate analysis, with age accounting for an extra 5% variability in TAPSE. Further studies are needed to explain the influences of age on TAPSE.
In our study, positive inotropic stimulation (dobutamine) increased LVEF, and preload manipulations did not modify LVEF, as expected [27]. One of the strengths of our study was documentation of coordinated changes in both TAPSE and left ventricular systolic function under positive inotropic stimulation. Indeed, dobutamine induced coordinated increases in LVEF and TAPSE. Another tested hypothesis was that TAPSE would remain unchanged following acute changes in preload. Consistent with our study hypothesis, TAPSE (and LVEF as well) appeared unaltered by acute changes in preload induced by fluid challenge or PLR. Overall, it was thus suggested that the dynamic changes in TAPSE may parallel the changes in LVEF and may be considered preload-independent.
The limitations of our study must be discussed. First, only 86 out of 117 screened patients (74%) were included in our study. The results may not apply to individuals with significant valvular regurgitation, ventricular rhythm disturbances, or atrial fibrillation, as patients with these conditions were excluded from the study. Second, we cannot exclude the possibility that the range of right ventricular function under study was not large enough to document a strong relationship between RVFAC and TAPSE, as has been previously documented in cardiac patients with a wide range of right ventricular function [17]. However, our prospective study included a reasonably high number of patients (n = 86) who were representative of the “real-life” ICU population. Third, given the above-discussed limitations of RVFAC, we cannot exclude the possibility that a more precise evaluation of right ventricular function (e.g., invasive pressure–volume loops with conductance catheters or invasive pressure–flow relationships) would have detected a stronger link between TAPSE and right ventricular function. However, such tools are not routinely usable in the ICU population. Fourth, the potential correlation between TAPSE and pulsed tissue Doppler measurement was not documented, and it certainly deserves further study. Finally, because dobutamine is also a pulmonary vasodilator, further studies are needed to test the potential relationship between changes in pulmonary vascular resistance and TAPSE.
Taking such limitations into account, the implications of our study need to be discussed. Given that TAPSE may be rapidly, easily, and reproducibly obtained at the bedside, one implication of our preliminary study is that the diagnostic and prognostic value of TAPSE deserves particular attention in further echocardiographic studies done in the ICU. The inclusion of TAPSE measurement in the battery of echocardiographic indices of risk stratification of ICU patients has been proposed in cardiac patients. Indeed, recent reports have indicated that decreased TAPSE is associated with a significantly increased risk of death in myocardial infarction [12], pulmonary hypertension [15], heart failure [18], and congestive heart failure with depressed systolic function [10]. It was not the aim of our preliminary study to test such a hypothesis in ICU patients, but this point deserves further investigation. Importantly, we wish to emphasize the fact that the present study cannot be used to support the proposal that TAPSE may replace LVEF in the noninvasive assessment of cardiac systolic function in critically ill patients. Indeed, although baseline measurement of TAPSE < 22 mm identified patients with LVEF < 45% with 85% sensitivity, the 62% specificity makes this proposal unsuitable for current practice.
In conclusion, contrary to our initial study hypothesis, TAPSE was weakly related to right ventricular function at baseline and following dynamic interventions in critically ill patients. Interestingly, TAPSE was related to left ventricular function at baseline, and TAPSE increased following dobutamine infusion (whereas it remained unchanged following preload manipulations), with changes in TAPSE and LVEF being linearly related. Because measurement of TAPSE is highly reproducible, fast, and easy to obtain, the present preliminary study suggests that the diagnostic and prognostic value of TAPSE certainly deserves particular attention in further echocardiographic studies performed in the ICU.
References
Beaulieu Y, Marik PE (2005) Bedside ultrasonography in the ICU: part 1. Chest 128:881–895
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis. Bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Jardin F, Dubourg O, Bourdarias JP (1997) Echocardiographic pattern of acute cor pulmonale. Chest 111:209–217
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Galdwin M, Denholm EM, Gail DD (2006) Right ventricular function and failure. Report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114:1883–1891
Rushmer RF, Crystal DK (1951) Changes in configuration of the ventricular chambers during the cardiac cycle. Circulation 4:211–218
Smiley I, Rich S, McLaughlin VV (1992) Cardiology clinics: the right ventricle. WB Saunders, Philadelphia, pp 1–196
Kaul S, Tei C, Hopkins JM, Shah PM (1984) Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 107:526–531
Di Salvo TG, Mathier M, Semigran MJ, Dec GW (1995) Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 25:1143–1153
Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L (2000) Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol 85:837–842
Shah AR, Grodman R, Salazar MF, Rehman NU, Coppola J, Braff R (2000) Assessment of right ventricular dysfunction induced by right coronary artery occlusion using echocardiographic atrioventricular plane displacement. Echocardiography 17:513–519
Samad BA, Alam M, Jensen-Urstad K (2002) Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol 90:778–781
Hébert JL, Chemla D, Gérard O, Zamani K, Quillar J, Azarine A, Frank R, Lecarpentier Y, Fontaine G (2004) Angiographic right and left ventricular function in arrhythmogenic right ventricular dysplasia. Am J Cardiol 93:728–733
Miller D, Farah MG, Liner A, Fox K, Schluchter M, Hoit BD (2004) The relation between quantitative right ventricular ejection fraction and indices of tricuspid annular motion and myocardial performance. J Am Soc Echocardiogr 17:443–447
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM (2006). Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 174:1034–1041
Saxena N, Rajagopalan N, Edelman K, Lopez-Candales A (2006) Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography 23:750–755
Lopez-Candales A, Rajagopalan N, Saxena N, Gulyasy B, Edelman K, Bazaz R (2006) Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol 98:973–977
Kjaergaard J, Akkan D, Iversen KK, Kober L, Torp-Pedersen C, Hassager C (2007) Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur J Heart Fail 9:610–616
Lamia B, Teboul JL, Monnet X, Richard C, Chemla D (2006) Relation between the tricuspid annulus plane systolic excursion and heart function in ICU patients. Intensive Care Medicine. Abstracts of the 19th Annual Congress of the European Society of Intensive Care Med 32:S35
Slama M, Maizel J (2006) Echocardiographic measurement of ventricular function. Curr Opin Crit Care Med 12:241–248
Poelaert J, Schüpfer G (2005) Hemodynamic monitoring utilizing transesophageal echocardiography. The relationships among pressure, flow and function. Chest 127:379–390
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky M, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky M, Teboul JL (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162:134–138
Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S (2002) Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 20:1314–1331
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F (2001) Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 29:1551–1555
Santamore WP, Dell'Italia LJ (1998) Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 40:289–308
Robotham JL, Takata M, Berman M, Harasawa Y (1991) Ejection fraction revisited. Anesthesiology 74:172–183
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lamia, B., Teboul, JL., Monnet, X. et al. Relationship between the tricuspid annular plane systolic excursion and right and left ventricular function in critically ill patients. Intensive Care Med 33, 2143–2149 (2007). https://doi.org/10.1007/s00134-007-0881-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0881-y