Abstract
Objective
We examined whether guiding therapy by an algorithm based on optimizing the global end-diastolic volume index (GEDVI) reduces the need for vasopressor and inotropic support and helps to shorten ICU stay in cardiac surgery patients.
Design and setting
Single-center clinical study with a historical control group at an university hospital.
Patients
Forty cardiac bypass surgery patients were included prospectively and compared with a control group.
Interventions
In the goal-directed therapy (GDT) group hemodynamic management was guided by an algorithm based on GEDVI. Hemodynamic goals were: GEDVI above 640 ml/m2, cardiac index above 2.5 l/min/m2, and mean arterial pressure above 70 mmHg. The control group was treated at the discretion of the attending physician based on central venous pressure, mean arterial pressure, and clinical evaluation.
Results
In the GDT group duration of catecholamine and vasopressor dependence was shorter (187 ± 70 vs. 1458 ± 197 min), and fewer vasopressors (0.73 ± 0.32 vs. 6.67 ± 1.21 mg) and catecholamines (0.01 ± 0.01 vs. 0.83 ± 0.27 mg) were administered. They received more colloids (6918 ± 242 vs. 5514 ± 171 ml). Duration of mechanical ventilation (12.6 ± 3.6 vs. 15.4 ± 4.3 h) and time until achieving status of fit for ICU discharge (25 ± 13 vs. 33 ± 17 h) was shorter in the GDT group.
Conclusions
Guiding therapy by an algorithm based on GEDVI leads to a shortened and reduced need for vasopressors, catecholamines, mechanical ventilation, and ICU therapy in patients undergoing cardiac surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemodynamic management and appropriate fluid therapy remains a challenge in critically ill patients [1, 2]. Inadequate cardiac output and reduced organ perfusion may lead to impaired microcirculation and multiorgan dysfunction. This has been shown for patients undergoing coronary artery bypass grafting (CABG) [3, 4]. According to the Frank-Starling mechanism, cardiac preload is a major determinant of cardiac performance. Optimizing cardiac preload is therefore essential specifically in this group of patients who frequently present with a reduced cardiac reserve [5].
Monitoring of cardiac filling pressures, such as central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP) is unreliable for assessing cardiac preload in mechanically ventilated patients [1, 2, 6, 7, 8]. In contrast, volumetric monitoring using a transcardiopulmonary thermodilution technique has been shown to be useful for reliably assessing preload, particularly the measurement of global end-diastolic volume index (GEDVI) reflecting central blood volume [9, 10], although these parameters do not allow the additional prediction of fluid responsiveness [3, 11]. Further measuring extravascular lung water index (EVLWI) seems to offer a reliable tool for assessing potential pulmonary edema [12, 13, 14, 15].
Recently evidence has been provided that septic and surgical patients benefit from hemodynamic goal-directed therapy (GDT) optimizing stroke volume (SV) and cardiac output [16, 17, 18]. No study has yet investigated the usefulness of GDT focusing on preload optimization using GEDVI as guiding parameter in patients undergoing cardiac surgery. Therefore we developed an algorithm for intra- and postoperative hemodynamic management in CABG patients. This algorithm is based on the measurement of GEDVI reflecting cardiac preload volume, cardiac index (CI) as surrogate for cardiac performance, EVLWI reflecting potential volume overloading, mean arterial pressure (MAP), and heart rate (HR). The goal of the study was to assess whether guiding hemodynamic therapy during and after cardiac surgery strictly using this algorithm reduces the need for vasopressors and catecholamines and shortens the duration of ICU therapy compared to conventional hemodynamic monitoring using CVP and MAP. Parts of this manuscript were presented at the annual meeting of the European Society of Intensive Care Medicine 2004 in Berlin and have been published in abstract form [19].
Methods
Following approval of the institutional review board and obtaining written informed consent, 40 patients scheduled for elective CABG surgery were included prospectively (GDT group). Inclusion criteria were: body mass index below 30 kg/m2, left ventricular ejection fraction higher than 30%, and estimated number of three or more coronary bypass grafts. Excluded were patients with any significant valvular dysfunction, preoperative hemoglobin concentration less than 10 g/dl, platelet count below 1.5 × 105/ml, or signs of relevant renal insufficiency (creatinine > 1.5 mg/dl). Of the 40 patients one was excluded because of a relevant mitral valve regurgitation. Another 40 patients who had undergone elective CABG surgery at our institution without volumetric monitoring served as controls. Patients were selected as matched pairs by an independent investigator using the same inclusion and exclusion criteria as for the GDT group. Data on the control group were retrieved from written patient records. The two groups did not differ significantly in demographic or surgical data (Table 1). More detailed data about hemodynamic parameters, fluids, and administered medications are provided as in the Electronic Supplementary Material.
Anesthetic technique and hemodynamic monitoring
In the GDT group a 5-F thermistor-tipped catheter (PV2025 L20, Pulsiocath, Pulsion Medical Systems, Munich, Germany) was inserted prior to anesthesia into a femoral artery and connected to a hemodynamics monitor (PiCCO, V 5.1, Pulsion Medical Systems). Anesthesia was induced with 0.15–0.25 mg/kg midazolam and 0.8–1.2 μg/kg sufentanil. After induction an 8-F triple-lumen central venous catheter (Arrow, Reading, Pa., USA) was inserted into a internal jugular vein. Both pressure transducers were positioned at the level of midaxillary line and zeroed to atmospheric pressure. Anesthesia was then maintained by continuous administration of 0.5–1.0 μg/kg sufentanil per hour and 0.8–1.1 vol% isoflurane. Values of cardiac output, global end-diastolic volume, and extravascular lung water, all measured by transcardiopulmonary thermodilution were indexed with body surface area to receive CI, GEDVI, and EVLWI [20, 21].
Patients in the control group received a 20-G radial artery catheter (Abbocath-T G718-A01, Abbott, Ireland) prior to induction of anesthesia. Further, the same anesthetic technique as described for the GDT group was used.
Hemodynamic management during surgery
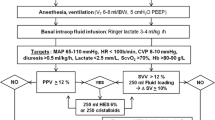
In the GDT group hemodynamic status was assessed by measurement of GEDVI, EVLWI, CI, and MAP after anesthesia induction, immediately following termination of cardiopulmonary bypass (CPB) and at the end of surgery (defined as end of scin suture, EOS). Preload (defined as GEDVI) and the other goal-directed parameters were optimized immediately according to the treatment algorithm after each assessment or in the case of hemodynamic instability (see Fig. 1). If GEDV, reflecting preload volume, was lower than 640 ml/m2, defined as the lower limit normovolemia [20], stepwise fluid loading in 500-ml aliquots was performed until GEDVI was higher than 640 ml/m2. EVLWI served as indicator of pulmonary edema and fluid overloading. If EVLWI was higher 10 ml/kg, which has been reported as an early sign for pulmonary edema [13, 22], no further fluid loading was performed. Then CI was judged. If CI was below 2.5 l/min/m2, GEDVI was reassessed. If GEDVI was below 800 ml/m2, reported to be the upper limit of normovolemia [20], further fluid loading was performed until GEDVI reached 800 ml/m2. During volume loading procedures data were reassessed every 500 ml infusion volume. Time between assessments was about 15–30 min, the period needed to complete the infusion of 500 ml. Catecholamine support with epinephrine was initiated only if CI remained below 2.5 l/min/m2. MAP was also measured. If MAP was less than 70 mmHg, again, GEDVI was reassessed and increased to 800 ml/m2 by fluid loading. If MAP remained below 70 mmHg, norepinephrine was used. Heart rate was kept between 80 and 110 bpm by epicardial pacing, pharmacological intervention, elevation in hemoglobin concentration (transfusion until hematocrit > 26%) or deepening of anesthesia. In the case of rapid hemodynamic instability, norepinephrine was used to bridge until fluid loading was performed.
In the control group hemodynamic management was performed according to routine clinical practice in our service, primarily based on CVP, MAP and clinical evaluation of the attending physician. Epinephrine and norepinephrine were used at the discretion of the attending physician.
Cardiopulmonary bypass
Surgery was performed using CPB under mild hypothermia (31–32 °C). Transfusion trigger during CPB was a hematocrit value less than 23%.
ICU treatment
Patients were ventilated using intermittent positive pressure support to achieve normocapnia and normoxia. If necessary, patients were sedated using single doses of propofol or midazolam. All patients received a continuous infusion of 80 ml/h cristalloids for the first 24 h, followed by 40 ml/h for the rest of the ICU treatment. In both groups we recorded the period required to achieve the “fit for ICU discharge” criteria (modified from [23]): cooperative patient, SpO2 higher than 90% at FIO2 less than 0.5, no ventricular arrhythmia, chest tube drainage below 50 ml/h, urine output above 0.5 ml/kg per hour, no inotropes or vasopressors, no signs of ischemia on electrocardiography, all to be achieved within 3 successive hours.
In the GDT group preload was evaluated according to the algorithm after ICU admission and then every 8 h until 48 h after EOS or, if earlier, until ICU discharge, and in any instance of hemodynamic instability. Preload optimization and other procedures to achieve goal-directed thresholds were performed as described during surgery. For fluid loading 6% 130/0.4 hydroxyethylstarch (Fresenius Kabi, Bad Homburg, Germany) up to a maximum daily dose of 120 g and, if necessary, urea linked polygelin (Aventis Pharma, Frankfurt, Germany) was used. Transfusion trigger was a hematocrit value lower than 26%.
In control patients hemodynamic and fluid loading management was performed according to attending physician's decision, based principally on CVP, MAP, and subjective evaluation. The same transfusion triggers were used.
Statistics
Documentation of data began directly after induction of anesthesia. A sample size of 36 patients was calculated based on values of the preexisting institutional database. We hypothesized a difference in means of 3.35 mg norepinephrine (according to a 50% reduction in dose) between the two groups, a standard deviation of ± 5.0 mg, allowing a type I error of 5% and a type II error of 20% in an unpaired t test. No interim analysis was performed. Data are expressed as mean ± standard error of the mean. For comparisons between groups we used the t test if data were distributed normally; otherwise the Mann–Whitney rank sum test was used. Statistics were performed using SAS version 8.2 (SAS Institute, Heidelberg, Germany).
Results
Vasopressors and catecholamines
Catecholamine and vasopressor support was significantly higher in the control group, during both surgery and ICU therapy (Table 2, Figs. 2, 3).
Fluid therapy, urine output, and fluid balance
Overall (from anesthesia induction until 48 h after EOS) patients of the GDT group received more intravenous fluids. Until EOS they received 1515 ± 60 ml colloids vs. 1327 ± 50 ml in the control group (p < 0.05). During ICU treatment 5403 ± 222 ml colloids was given in the GDT group vs. 4187 ± 167 ml in the control group (p < 0.001). The total amount of cristalloids administered until EOS (GDT 2260 ± 92 ml, control 2044 ± 113 ml) did not differ significantly. Also during ICU treatment there were no significant differences between the groups (GDT 2912 ± 107 ml, control 3208 ± 133 ml). In the GDT group a total of 28 U packed red blood cells was transfused vs. 33 U in the control group (p = 0.47). No other blood products were used in either group. During ICU treatment urine output in GDT group patients was significantly higher (data not shown). In neither group was dialysis or hemofiltration necessary. Intravenous fluid balance during surgery was more positive in the GDT group (5663 ± 172 vs. 5052 ± 159 ml in the control group; p < 0.05). Also, overall fluid balance from induction of anesthesia until 48 h after EOS was more positive in patients of the GDT group (6509 ± 240 vs. 6403 ± 184 ml in the control group; p < 0.05).
Laboratory data
Hematocrit did not differ between the groups (all > 27%), except at 48 h after EOS (GDT 27 ± 0.4%, control 28 ± 0.5%; p = 0.03). Blood lactate did not differ between the groups before surgery. During the first 8 h after EOS blood lactate in the GDT group was significantly lower. At 16 h after EOS levels of blood lactate were similar in the two groups (Fig. 4).
Mechanical ventilation and ICU discharge criteria
Duration of mechanical ventilation was 12.6 ± 3.6 h in the GDT group vs. 15.4 ± 4.3 h in the control group (p = 0.002). Between the groups there were no differences in pulmonary function, defined as PaO2/FiO2 ratio (data not shown). Length of ICU stay was 57 ± 26 h in the GDT group vs. 56 ± 19 h in the control group (p = 0.29). However, patients of the GDT group reached the “fit for ICU discharge” criteria significantly earlier (25 ± 13 vs. 33 ± 17 h; p = 0.03). All patients tolerated the study regime well, and no patient died during ICU therapy.
Discussion
This study demonstrates that an algorithm-based goal-directed perioperative hemodynamic management based on early optimization of cardiac preload leads to modified fluid management during and after cardiac surgery. This led to a significant reduction in inotropic and vasopressor support. Duration of mechanical ventilation was shortened, and criteria “fit for ICU-discharge” were reached earlier.
Inadequate tissue perfusion is associated with elevated postoperative morbidity and mortality [24, 25, 26, 27, 28]. Shoemaker et al. [29] reported that perioperative optimization of the circulatory status in high-risk surgical patients led to reduced mortality and morbidity. In cardiac surgery patients, to our knowledge, only two studies have been published using GDT to optimize organ perfusion. McKendry et al. [30] evaluated a concept of postoperative nurse-directed optimization of the circulatory status, primarily defined as a stroke volume index higher than 35 ml/m2, in patients after cardiac surgery, which shortened hospital stay. Polonen et al. [19] reported a mixed venous oxygen-saturation higher than 70% and blood lactate lower than 2.0 mmol/l following cardiac surgery which resulted in faster discharge from hospital and decreased morbidity. In contrast, our concept of goal-directed hemodynamic management, based on early preload optimization, is not limited to the ICU treatment but includes the complete perioperative process starting immediately after anesthesia induction.
Organ perfusion depends essentially on blood flow and therefore on cardiac function. Cardiac function is related to the three main physiological determinants: preload, contractility, and afterload, resulting in stroke volume and finally cardiac output. All of these three determinants were assessed and if necessary, optimized according to the treatment algorithm. Initially GEDVI was assessed. This parameter has been shown to be more useful to evaluate cardiac preload and to guide volume therapy than cardiac filling pressures [2, 3, 7, 11]. We defined lower and upper threshold values of relative normovolemia (GEDVI between 640 and 800 ml/m2) based on earlier findings in cardiac surgical and septic patients [11, 20]. As a first step of preload optimization this range of GEDVI had to be reached. Assessment of cardiac contractile function was evaluated by measuring CI. Only if preload was in the upper predefined therapeutic range, was cardiac function supported by the administration of catecholamines. Finally, cardiac afterload was assessed by monitoring MAP and, if necessary, optimized by the use of a vasopressor. This treatment algorithm was based primarily on preload optimization by fluid loading. To avoid potential fluid overloading with pulmonary edema EVLWI was assessed, and an upper threshold of 10 ml/kg was set [13, 15].
In addition to measurements of blood pressure and heart rate, GEDVI, CI, and EVLWI were assessed routinely according to the treatment algorithm at specific time points. This first hemodynamic evaluation was performed immediately after induction of anesthesia to assess a baseline status (preload, cardiac function) of the patient. This was repeated immediately after termination of CPB and after EOS to assess and to correct hemodynamic alterations caused by the surgical interventions. In the ICU obligatory hemodynamic evaluation and optimization was performed routinely every 8 h. In any instance of hemodynamic instability additional measurements were made of GEDVI, CI, and EVLWI, and the appropriate algorithm-based therapeutic steps were taken.
Because of the clear advantages of diagnosing any valvular dysfunctions, acute myocardial ischemia, or pericardial hematoma transesophageal echocardiography was used if these situations were suspected [31]. The tracing of the CVP was observed during fluid loading. If CVP increased rapidly during fluid loading, right ventricular function had to be evaluated immediately by transesophageal echocardiography. However, this was not the case in any of the studied patients.
In the present study volumetric preload monitoring and subsequent optimization by volume loading in the GDT population resulted in a shortened length and reduced amount of vasopressor and catecholamine support. For fluid loading mainly colloids were used. However, no negative effect on pulmonary function, defined as PaO2/FiO2 ratio was observed. Furthermore patients of the GDT group needed a shorter period of mechanical ventilation. The need for blood products was not increased. Hematocrit values did not differ significantly between the groups until 32 h after EOS. Only 48 h after EOS did patients of the GDT group present a slightly lower but still tolerable hematocrit value. This was most possibly caused by the higher amount of fluid loading in the GDT group; however, differences in capillary leakage may also have played a role [32]. Further, patients in the GDT group showed a significantly reduced level of blood lactate in the early phase after surgery. This was probably a direct result of optimized organ perfusion caused by hemodynamic optimization. However, also the reduced need of catecholamines in the GDT group, which was also an immediate consequence of this GDT, may have been responsible for this difference.
Some specific limitations of our study need to be pointed out. This was a single-center study with a historical control group. Although the same team of surgeons and anesthesiologists treated the patients of the control group, a bias on the presented results, based on the interval between treatment of the control group and GDT group cannot be excluded. Further, a multicenter evaluation is needed to strengthen the evidence found here. This study does not definitively confirm whether the use of this perioperative goal-directed algorithm leads to an improved outcome, i.e., reduction in mortality or length of hospital stay. We used retrospective criteria to judge the “fit for ICU discharge criteria” as outcome parameter, which is a theoretical classification based on the screened parameters. Nevertheless, the results of this study provide evidence that such a goal-directed and algorithm-guided hemodynamic management leads to improvements in outcome. Further, only patients with normal cardiac function prior to surgery were included. Further studies are therefore necessary before these findings can be translated to a broader spectrum of patients. Also, integrating functional parameters of preload and fluid responsiveness, such as either left ventricular stroke volume variation or pulse pressure variation, in a treatment algorithm may further help to optimize fluid therapy [12, 33]. Finally, the question of whether epinephrine is the inotropic agent of first choice in cardiac surgery has not been determined. Although Wilson et al. [34] showed that using dopexamine instead of epinephrine may reduce morbidity in major elective surgery, this has not been verified in cardiac surgery patients.
In conclusion, the results of this study suggest that the presented algorithm-driven, perioperative hemodynamic management based on early optimization of preload and cardiac output using transcardiopulmonary thermodilution leads to an improved treatment of patients undergoing cardiac surgery.
References
Michard F, Alaya S, Zarka V, Bahloul M, Richard C, Teboul JL (2003) Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest 124:1900–1908
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Christakis GT, Fremes SE, Naylor CD, Chen E, Rao V, Goldman BS (1996) Impact of preoperative risk and perioperative morbidity on ICU stay following coronary bypass surgery. Cardiovasc Surg 4:29–35
Ryan TA, Rady MY, Bashour CA, Leventhal M, Lytle B, Starr NJ (1997) Predictors of outcome in cardiac surgical patients with prolonged intensive care stay. Chest 112:1035–1042
Weil J, Eschenhagen T, Hirt S, Magnussen O, Mittmann C, Remmers U, Scholz H (1998) Preserved Frank-Starling mechanism in human end stage heart failure. Cardiovasc Res 37:541–548
Lichtwarck-Aschoff M, Beale R, Pfeiffer UJ (1996) Central venous pressure, pulmonary artery occlusion pressure, intrathoracic blood volume, and right ventricular end-diastolic volume as indicators of cardiac preload. J Crit Care 11:180–188
Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE (2004) Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 32:691–699
Bouchard MJ, Denault A, Couture P, Guertin MC, Babin D, Ouellet P, Carrier M, Tardif JC (2004) Poor correlation between hemodynamic and echocardiographic indexes of left ventricular performance in the operating room and intensive care unit. Crit Care Med 32:644–648
Brock H, Gabriel C, Bibl D, Necek S (2002) Monitoring intravascular volumes for postoperative volume therapy. Eur J Anaesthesiol 19:288–294
Reuter DA, Felbinger TW, Moerstedt K, Weis F, Schmidt C, Kilger E, Goetz AE (2002) Intrathoracic blood volume index measured by thermodilution for preload monitoring after cardiac surgery. J Cardiothorac Vasc Anesth 16:191–195
Reuter DA, Kirchner A, Felbinger TW, Weis FC, Kilger E, Lamm P, Goetz AE (2003) Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med 31:1399–1404
Katzenelson R, Perel A, Berkenstadt H, Preisman S, Kogan S, Sternik L, Segal E (2004) Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med 32:1550–1554
Fernandez-Mondejar E, Castano-Perez J, Rivera-Fernandez R, Colmenero-Ruiz M, Manzano F, Perez-Villares J, de la Chica R (2003) Quantification of lung water by transpulmonary thermodilution in normal and edematous lung. J Crit Care 18:253–258
Boussat S, Jacques T, Levy B, Laurent E, Gache A, Capellier G, Neidhardt A (2002) Intravascular volume monitoring and extravascular lung water in septic patients with pulmonary edema. Intensive Care Med 28:712–718
Sakka SG, Ruhl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A (2000) Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 26:180–187
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J (2000) A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 90:1052–1059
Goepfert MSG, Reuter DA, Akyol D, Kilger E, Goetz AE (2004) Volume management by goal directed therapy reduces catecholamine-need in cardiosurgery patients (abstract) Crit Care Med 30[Suppl 1]:588130
Combes A, Berneau JB, Luyt CE, Trouillet JL (2004) Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med 30:1377–1383
Sakka SG, Bredle DL, Reinhart K, Meier-Hellmann A (1999) Comparison between intrathoracic blood volume and cardiac filling pressures in the early phase of hemodynamic instability of patients with sepsis or septic shock. J Crit Care 14:78–83
Sakka SG, Klein M, Reinhart K, Meier-Hellmann A (2002) Prognostic value of extravascular lung water in critically ill patients. Chest 122:2080–2086
Cheng DC, Newman MF, Duke P, Wong DT, Finegan B, Howie M, Fitch J, Bowdle TA, Hogue C, Hillel Z, Pierce E, Bukenya D (2001) The efficacy and resource utilization of remifentanil and fentanyl in fast-track coronary artery bypass graft surgery: a prospective randomized, double-blinded controlled, multi-center trial. Anesth Analg 92:1094–1102
Mythen MG, Webb AR (1994) Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med 20:99–104
Shoemaker WC, Thangathurai D, Wo CC, Kuchta K, Canas M, Sullivan MJ, Farlo J, Roffey P, Zellman V, Katz RL (1999) Intraoperative evaluation of tissue perfusion in high-risk patients by invasive and noninvasive hemodynamic monitoring. Crit Care Med 27:2147–2152
Maillet JM, Le Besnerais P, Cantoni M, Nataf P, Ruffenach A, Lessana A, Brodaty D (2003) Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest 123:1361–1366
Shoemaker WC, Patil R, Appel PL, Kram HB (1992) Hemodynamic and oxygen transport patterns for outcome prediction, therapeutic goals, and clinical algorithms to improve outcome. Feasibility of artificial intelligence to customize algorithms. Chest 102:617S–625S
Kern JW, Shoemaker WC (2002) Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med 30:1686–1692
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94:1176–1186
McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M (2004) Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ 329:258–262
Schmidlin D, Schuepbach R, Bernard E, Ecknauer E, Jenni R, Schmid ER (2001) Indications and impact of postoperative transesophageal echocardiography in cardiac surgical patients. Crit Care Med 29:2143–2148
Boldt J, Ducke M, Kumle B, Papsdorf M, Zurmeyer EL (2004) Influence of different volume replacement strategies on inflammation and endothelial activation in the elderly undergoing major abdominal surgery. Intensive Care Med 30:416–422
Pinsky MR (2002) Functional hemodynamic monitoring. Intensive Care Med 28:386–388
Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E (1999) Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 318:1099–1103
Acknowledgements
The authors thank the Ludwig Maximilian University Institute for Medical Data Processing, Biometry and Epidemiology for statistical support and calculation. D.A. R. a member of the Pulsion Medical Systems Medical Advisory Board and of a Medical Advisory Board of Fresenius-Kabi; he received a research grant from Pulsion Medical Systems which was not associated with the study submitted. A.E.G. is a member of the Pulsion Medical Systems Medical Advisory Board; he received no grants for this study but for experimental studies on functional hemodynamic monitoring in 1999–2000 (DM 40,000 from Pulsion Medical Systems, Germany) and no patents have been received with in relation to this study except for measurement of cerebral blood flow, together with Dr. Pfeiffer and Prof. Kübler. He personally received no payment with respect to this study; he received no additional honoraria except for travel reimbursements (from Abbott, Baxter, Fresenius Kabi, PMS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Goepfert, M.S.G., Reuter, D.A., Akyol, D. et al. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med 33, 96–103 (2007). https://doi.org/10.1007/s00134-006-0404-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0404-2