Abstract
Haemodynamic goal-directed therapies (GDT) may improve outcome following elective major surgery. So far, few data exist regarding haemodynamic optimization during emergency surgery. In this randomized, controlled trial, 50 surgical patients with hypovolemic or septic conditions were enrolled and we compared two algorithms of GDTs based either on conventional parameters and pressure pulse variation (control group) or on cardiac index, global end-diastolic volume index and stroke volume variation as derived from the PiCCO monitoring system (optimized group). Postoperative outcome was estimated by a composite index including major complications and by the Sequential Organ Failure Assessment (SOFA) Score within the first 3 days after surgery (POD1, POD2 and POD3). Data from 43 patients were analyzed (control group, N = 23; optimized group, N = 20). Similar amounts of fluid were given in the two groups. Intraoperatively, dobutamine was given in 45 % optimized patients but in no control patients. Major complications occurred more frequently in the optimized group [19 (95 %) versus 10 (40 %) in the control group, P < 0.001]. Likewise, SOFA scores were higher in the optimized group on POD1 (10.2 ± 2.5 versus 6.6 ± 2.2 in the control group, P = 0.001), POD2 (8.4 ± 2.6 vs 5.0 ± 2.4 in the control group, P = 0.002) and POD 3 (5.2 ± 3.6 and 2.2 ± 1.3 in the control group, P = 0.01). There was no significant difference in hospital mortality (13 % in the control group and 25 % in the optimized group). Haemodynamic optimization based on volumetric and flow PiCCO-derived parameters was associated with a less favorable postoperative outcome compared with a conventional GDT protocol during emergency surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiple trauma and sepsis share common pathophysiological pathways (e.g., the release of cytokines, oxygen-free radicals, and prostanoids) that cause major alterations in tissue oxygen delivery leading to multiple organ dysfunction and even death [1, 2]. Conventionally, resuscitative management with fluids and drugs is targeted to restore mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP) and urine output (UO) [3–5]. However, these vital signs lag behind physiological markers of tissue hypoxia and fail to identify ongoing hypovolemia, myocardial depression and vasoplegic syndrome [6, 7].
Stressing the importance of matching oxygen delivery to oxygen consumption in critically ill patients, Shoemaker et al. [8, 9] hypothesized that therapies driving systemic oxygen transport to supra-physiological values might improve clinical outcome. Tuchschmidt et al. [10] and Rivers et al. [11] demonstrated the benefits of early resuscitation with fluids and cardiovascular drugs in septic patients. The concept of “goal-directed therapies” (GDT) to maximize tissue oxygen delivery while avoiding fluid overload has been extended to elective major surgical procedures where pulmonary artery catheters have been replaced by minimally invasive monitors) [12–16]. Compared with the liberal approach of fluid management, implementation of GDTs has been associated with reductions in postoperative complications, shorter duration of hospital stay and valuable cost savings, regardless of the type of monitoring [17–19]. Importantly, the greatest benefits of GDTs were found amongst the highest surgical risk groups and when a combination of inotropes and fluids was administered [20, 21]. Contrasting with these favorable inotrope-related effects, caution has been raised regarding the increased morbidity and mortality associated with supra-physiologic oxygen delivery in septic or other critically-ill patients [22, 23].
Although the efficacy and safety of GDTs have been investigated in patients with severe sepsis and trauma admitted to the intensive care unit (ICU) or the emergency department, few studies have been focused on the intraoperative phase. Given these unsettled controversies [24], we compared two GDT strategies in high risk patients requiring emergency surgery and tested the hypothesis that intraoperative haemodynamic optimization based on cardiac index (CI), stroke volume variation (SVV), and global end-diastolic volume index (GEDVI) provides better clinical outcome compared with a conventional algorithm based on pressure pulse variation (PPV) and standard “static” haemodynamic parameters.
2 Materials and methods
2.1 Patient selection and study design
After approval by the Ethics Committee of the University Hospital of Geneva (NAC 09-044B), this open prospective randomized trial was conducted in a single academic centre and complied with the Helsinki declaration (ClinicalTrial.gov NCT0165-3977).
Adults (older than 18 years) were screened for eligibility by an emergency physician or an anesthesiologist after their admission in the emergency department, surgical ward or ICU. Inclusion criteria consisted in severe sepsis or hypovolemic conditions (see “Appendix”) [25, 26] and requirement of an emergency surgical procedure under general anaesthesia with an expected duration exceeding 120 min. The “emergency” criterion was defined by the need to proceed to surgery within 6 h after medical consultation.
Exclusion criteria were neurological injuries, pregnancy, severe cardiac arrhythmia, severe valvular disease, intracardiac shunt, burn injuries, liver failure (Child-Pugh class C), any contraindication to femoral artery catheterization, emergency thoracotomy, cardiac arrest with resuscitation, do-not-resuscitate order and expected death within 48 h of admission.
Information regarding the study protocol was given to family members or next of kin and written consent was obtained from each participant after postoperative recovery of cognitive function. Randomization was performed using a “block of 6″ and stratified according to sepsis and hypovolemia by computer-generated random numbers using opaque sealed envelopes. Patients were allocated to the optimized or control groups. In the control group, the administration of fluids and cardiovascular supportive drugs was guided by PPV and conventional physiological targets (MAP, HR, UO, Hb, lactate); this protocol had been implemented in the anesthesia department since 2009, and was therefore routinely applied by the emergency anaesthesia team. In the optimized group, PiCCO-derived parameters were used [CI, GEDVI and extravascular lung water index (EVLWI)] in addition to conventional haemodynamic parameters.
2.2 Protocol
Pre- and postoperative medical care was left at the discretion of the ICU physicians who were blinded to the group allocation.
In the operating theater, patients were equipped with two intravenous lines, a central venous catheter and standard monitoring devices including an ECG, pulsed oximetry, capnometry, central body temperature probe, bispectral index of the EEG and neuromuscular testing. In the control group, a cannula (Seldicath 3 French, Plastimed, Saint Lieu la Forêt, France) was inserted in the radial artery and connected to a Philips monitor (IntelliVue MP70) with real-time display of PPV that was determined over four consecutive 8 s windows (averaged PPV calculated as [PPmax − PPmin)]/PPmean). In the optimized group, a 5-French thermistor-tipped catheter (PV2025 L20, Pulsiocath; Pulsion Medical Systems, Munich, Germany) was inserted in the femoral artery and connected to a monitor for transpulmonary thermodilution and arterial pulse contour analysis (PiCCOplus; Pulsion Medical Systems). All pressure transducers were positioned at the mid-axillary level and zeroed at atmospheric pressure.
After anesthesia induction (etomidate or ketamine) and tracheal intubation, the lungs were ventilated with an air-oxygen mixture at an inspiratory oxygen fraction between 0.4 and 0.8. A multimodal standardized program was applied in all patients and entailed protective lung ventilation (tidal volume of 7–8 ml/kg ideal body weight, recruitment maneuvers, 4–8 mmHg positive end-expiratory pressure), BIS-guided titration of anaesthetic agents, blood sparing techniques (cell saver, thromboelastometry and transfusion algorithm) as well as warming devices (intravenous fluids, blankets and forced air) to maintain normothermia.
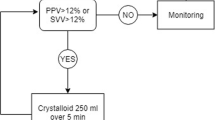
As illustrated in Fig. 1, all patients were given intravenous saline (8–10 ml/kg IBW) before surgical incision. Thereafter, an infusion of crystalloids was set at a continuous rate of 3–4 ml/kg/h. In both groups, 250 ml intravenous crystalloids (or balanced hydroxyxethyl starch 6 % 130/0.4, Voluven® %, Fresenius Kabi, Bad Homburg, Germany) were given over 5 min, as long as SVV or PPV remained above 12 % intraoperatively. Utilization of cardiovascular drugs was left at the discretion of the care-giving anaesthesiologist in the control group whereas the administration of inotropes (dobutamine, epinephrine) and vasopressors (norepinephrine, phenylephrine) was guided by PiCCO-derived parameters in the optimized group to treat circulatory disturbances (e.g., vasoplegia, cardiac depression). In the ICU, the lung protective ventilation strategy was continued and physicians were mindful to restrict fluid infusion in order to minimize positive fluid balance. Measurements
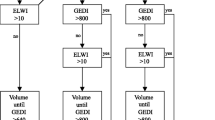
Algorithms of goal-directed therapy in the control and optimized groups. PPV pulse pressure variation, MAP mean arterial pressure, HR heart rate, CVP central, venous pressure, SVV stroke volume variation, SV stroke volume, CI cardiac index, GEDVI global end-diastolic volume index, EVLWI extravascular lung water index, ScvO 2 central venous oxygen saturation
Besides haemodynamics and body temperature monitoring, arterial blood samples were obtained intraoperatively for gas analysis and determination of the concentrations of lactate, hemoglobin, glucose and electrolytes. Achievement of appropriate physiological targets was also recorded according to group allocation. The rate and dose of inotropes (dobutamine, epinephrine) and vasopressors (norepinephrine, phenylephrine), the volume of crystalloids, colloids and blood products administered during surgery and the first three postoperative days were all recorded. The Physiological and Operative Severity Score for the enumeration of Mortality and morbidity (POSSUM) was calculated using 12 physiological and 6 surgical variables, each assigned on a 4-grade scale, to provide the physiological score (ranging from 12 to 88) and the operative severity score (ranging from 6 to 44) [27].
Clinical outcomes and their surrogate biomarkers (e.g. lactate, troponin-I, pro-BNP) were traced daily and recorded by extracting specific data from the electronic medical file until hospital discharge. In addition to hospital mortality, major adverse events were categorized as related to surgery (surgical site infection, re-operation) or to specific organ systems, namely, cardiac (myocardial infarction, congestive heart failure), cerebral (stroke), pulmonary (acute lung injury, bronchopneumonia), surgical, and renal complications [28].
The simplified acute physiology score (SAPS) was calculated from the worst values obtained at ICU admission. The severity of postoperative organ dysfunction syndrome (MODS) was estimated using the Sequential Organ Failure Assessment (SOFA) score at the first, second and third postoperative day (POD1, POD2 and POD3) [29, 30].
2.3 Study endpoints
The primary study endpoints were the intraoperative change in arterial blood lactate (Δ lactate = lactatepreop − lactateend of surgery) and a short-term organ dysfunction index expressed by the SOFA score. Hospital and ICU duration of stay as well as a composite morbidity index including specific complications were considered as secondary endpoints.
2.4 Statistical analysis
A study population of 250 patients was required to demonstrate a difference greater than 15 % in lactate levels between the two groups (with alpha level set at 0.05 and power of 90 %). Interim analyses were planned after recruiting the first 50 patients with a more stringent level of significance being set for the trial to be stopped (P < 0.01). Descriptive statistics were used to characterize demographic, clinical and surgical variables of each study group. Values were reported as means (standard deviation), medians (interquartiles) or numbers (percentages). To compare the two groups (control vs. optimized), Mann–Whitney or Student t tests were used for continuous data and Fisher’s exact tests for categorical data. Statistical significance was assumed for P < 0.05.
3 Results
An interim analysis on efficacy and safety was run after enrolling 50 patients from October 2010 to September 2013. Twenty seven patients were randomly assigned to the control group and 23 to the optimized group (Fig. 2). Seven patients were secondarily excluded due to missing data (N = 1) and surgical procedure lasting less than 120 min (N = 4 in the control group and N = 2 in the optimized group). Hence, completed data of 43 patients were analyzed, 23 in the control group and 20 in the optimized group. There were no complications related to the cannulation of the femoral artery.
All patients required laparotomies and the majority of patients were males (67 %), presenting with sepsis (72 %) and POSSUM ranging from 16 to 60 for physiological scores and from 16 to 31 for operative scores. The two groups were well matched for patient demographic and clinical characteristics, surgical procedures and risk scores (Table 1).
Intraoperatively, there was no difference in the amount of crystalloids, colloids and blood products administered in the two groups (Table 2). However, dobutamine treatment was initiated in 9 out 20 patients in the optimized group whereas no patients received dobutamine in the control group. The administration of norepinephrine and epinephrine did not differ between the two groups, whereas the total dose of phenylephrine was higher in the control group.
As shown in Table 3, blood lactate levels changed little throughout surgery and behaved similarly in the two groups (−0.2 ± 1.2 mm/l in the control group and −1.2 ± 1.4 mm/l in the optimized group, P = 0.078). Likewise, MAP, HR, ScvO2, PaO2/FIO2 and haemoglobin levels remained stable throughout surgery and did not differ between the two groups. Of note, haemodynamic targets (HR, MAP, CVP, SVV or PPV, lactate, haemoglobin, diuresis and body temperature) were achieved similarly in the two groups and PiCCO-derived flow and volumetric targets were achieved in more than 70 % of “optimized” patients towards the end of surgery (Table 4).
Over the first 3 days after surgery, the amount of fluids and cardiovascular drugs administered was similar in the two groups (Table 5). As reported in Table 6, the SOFA scores were higher in the optimized group at POD1 (10.2 ± 2.5 versus 6.6 ± 2.2 in the control group, P = 0.001), POD2 (8.4 ± 2.6 vs 5.0 ± 2.4 in the control group, P = 0.002) and POD 3 (5.2 ± 3.6 and 2.2 ± 1.3 in the control group, P = 0.011). Likewise, major postoperative complications occurred more frequently in the optimized group [19 (95 %) vs 10 (40 %) in the control group, P < 0.001; median of 3 vs 1 complication, P = 0.038]. Hospital mortality as well as the length of stay in ICU and in hospital did not differ between the two groups. Importantly, the observed mortality (13 % in the control group and 25 % in the optimized group) was much less than the predicted mortality based on POSSUM (60 % ± 20 in the control group and 62 % ± 22 in the optimized group).
To determine the relationship between the administration of inotropic drug and postoperative outcome, data were re-analyzed after grouping patients into 2 categories, those with and without intraoperative inotropic support. As shown in Table 7, patients receiving any inotrope (dobutamine or epinephrine) had a higher SOFA score within the first 2 days after surgery, compared with patients not receiving inotropes, although the two groups were similar regarding their operative risk score. Of note, trauma and septic patients did not differ regarding their preoperative POSSUM scores and blood lactate levels as well as postoperative SAPS and SOFA scores (data not shown).
4 Discussion
In this randomized controlled trial, two GDT protocols were applied in high risk patients during the intraoperative phase of emergency surgical procedures. Compared with a conventional approach based on standard physiological targets, haemodynamic optimization based on PiCCO-derived parameters led to greater intraoperative utilization of inotropes that was associated with a less favorable outcome as reflected by a higher rate of major complications and more severe organ dysfunction within the first 3 days following surgery. Based on this interim analysis, the trial was interrupted.
Although fluid management and cardiovascular drug support is part of routine perioperative care, the optimal haemodynamic strategy remains largely unclear [24, 31]. A growing body of scientific knowledge supports that both fluid restriction and oxygen maximization strategies in haemodynamically stable patients undergoing elective surgery improve postoperative clinical outcome, whereas fluid overload and tissue oxygen deficit have been incriminated in the pathogenesis of complications and organ failure [18, 32, 33]. So far, the myriad of GDT protocols and haemodynamic endpoints has generated confusion in selecting appropriate and individualized therapies in surgical patients with different risk profiles [34].
In the current study, the perioperative changes in blood lactate and in the SOFA score following emergency surgery were considered as the primary outcome measures. Blood lactate levels have been shown to predict outcome both in multiple trauma and in septic patients [35, 36]. Likewise, the SOFA score—a proxy of postoperative physiological disturbances—has been validated as a useful tool in predicting outcome and in guiding physician’ decisions for further diagnostic and therapeutic interventions in surgical patients [37, 38]. We only observed minor changes in blood lactate levels throughout surgery in both groups of patients, which could be explained by the relatively low preoperative basal lactate levels owing to appropriate early resuscitative interventions that preceded the intraoperative phase.
Importantly, we observed greater postoperative impairments in organ function as expressed by the SOFA score and a two-fold higher complication rate in the optimized group than in the control group. These results contrast with clinical studies showing that GDT using a combination of inotropes and fluids reduces postoperative complications and the length of hospital stay among high risk surgical patients, particularly those with an expected mortality exceeding 20 % [20, 39]. The current study also differs from previous trials because of: (1) the “very high risk” profile of the surgical population, (2) the routine application of a multimodal standardized program in all patients and (3) the comparison of two standardized GDT protocols (instead of GDT versus “usual care”).
In most recent studies focusing on the intraoperative phase, emergency procedures have been excluded or represent a very small proportion of the total population (<5 %) [18, 39]. A similar trial was conducted by Harten et al. [40] in 29 patients undergoing emergency abdominal laparotomy. Although these patients were at lower risk (e.g., shorter duration of surgery and lower POSSUM), the GDT approach based on PPV-fluid loading failed to improve functional parameters and to reduce postoperative complications, compared with a non-standardized “usual care” approach.
In our study, the patient population was considered at “very high risk” as we anticipated perioperative mortality rates exceeding 35 and 50 % based on SAPS and POSSUM [27]. The lower than expected mortality (25 and 12 % in the control and optimized groups, respectively) could be attributed to the beneficial impact of the GDT haemodynamic protocols and the multimodal standardized program (protective ventilation, haemostatic therapy and blood savings) that have been shown to minimize the risk of perioperative complications [41]. As shown by Takala et al. [42], simple utilization of haemodynamic monitoring without clearly defined treatment goals does not improve clinical outcome in critically-ill patients. In the present study, the so-called dynamic indices of fluid responsiveness, SVV and PPV allowed individual titration of patient cardiac preload and resulted in the administration of similar volumes of crystalloids and colloids in the two groups. Whereas conventional static haemodynamic parameters were used in control patients, PICCO-derived parameters of cardiac preload and pulmonary compartment were added in the optimized group to guide pharmacological drug support and resulted in the administration of dobutamine in 9 out 20 patients. Further data analysis by grouping patients receiving beta-adrenergic agents suggested possible detrimental effects associated with inotropic drug support as indicated by worst SOFA scores within the first days after surgery.
Although protocol driven administration of inotropes has been shown to improve systemic haemodynamics, improvement in microcirculatory and inflammatory parameters as well in organ function indices could not be demonstrated in the early phase of sepsis and in some surgical patients [43–45]. Consistent with our results, poorer clinical outcomes have been reported in large observational studies including cardiac surgical patients exposed to beta-adrenergic drugs [46, 47]. Likewise, placebo-controlled trials in nonsurgical acute or chronic heart failure also indicated either the absence of beneficial effects or even a higher incidence of adverse events (e.g., arrhythmias, hypotension, myocardial infarct) in patients treated with inotropic agents [48]. In the setting of ischemia–reperfusion injuries, potential mechanisms of catecholamine-induced toxicity include worsening of calcium overload within the cardiomyocytes and reduced cardiac metabolic efficiency owing to enhancement of free fatty acid oxidation [49, 50]. Increased bacterial growth and virulence, immunosuppression, thrombogenicity as well as insulin resistance and hyperglycemia associated with excessive adrenergic stimulation could further contribute to deleterious outcomes [51]. Conversely, mitigation of the high sympathetic drive with titrated doses of a betablocker has recently been shown to improve cardiac work efficiency and to lower mortality in septic shock while HR and systemic oxygen delivery were reduced [52]. Together these data challenge the recommendations of maximizing systemic oxygen delivery during the intraoperative period. Although individualized titration of fluids and inotropes aimed to increase oxygen delivery may be beneficial in patients undergoing elective major surgery and those admitted in ICU, alternative protocols should be tested in critically-ill patients undergoing emergency surgery aiming to provide efficient cardiac function and sufficient tissue oxygen delivery to match organ metabolic demands.
This study has some limitations and weaknesses. We enrolled two different categories of patients—septic and hypovolemic—that were equally distributed in the two treatment arms. Given the results of the interim analysis, the trial was interrupted prematurely and the small number of enrolled cases precluded a meaningful subgroup analysis to identify different haemodynamic responses. The slow pace of recruitment was mainly attributed to the limited availability of the research team over this 4 year period and several patients were not eligible due to pre-existing arrhythmia or a contraindication to cannulate the femoral arteries. In these patients, other minimally-invasive monitoring devices would have been suitable to optimize the haemodynamic state. Technologies using arterial pressure wave form analysis and lithium dilution have been validated in perioperative settings to individualize fluid and drug administration while facilitating the fast-tracking processes [53]. Finally, the care-giving physicians could not be blinded to the treatment protocol. Hence, to minimize potential bias and practice variability among physicians, we compared two standardized haemodynamic strategies and found similar adhesion to GDT protocols as reflected by the rate of achieved haemodynamic goals in each group. Moreover, care-giving nurses and physicians in ICU as well the research assistants collecting clinical and physiological outcome data were all blinded to group allocation.
Finally, maximal SOFA score at 24 h after surgery differed by 3.5 (1.1) between the two groups. Data from Ferreira et al. [54] indicate that a two point increase in SOFA score correlates with an average 10 % increase in mortality. Thus to target a relevant 30 % reduction in SOFA score and assuming usual error rates (α = 0.05; β = 0.20), 64 patients per group would be required. Given the non-Gaussian data distribution and the use of non-parametric statistics, the number of patients to be randomized has to be increased by 10 %. Thus, in future trials, the total sample size would set to at least 140 patients.
In conclusion, in high-risk patients undergoing emergency major non-thoracic surgery, haemodynamic optimization guided by an algorithm using volumetric and flow PiCCO-derived parameters was not superior compared to an algorithm using conventional haemodynamic parameters. Intraoperative optimization with a combination of inotropes and fluids was associated with a less favorable outcome. These preliminary results should be challenged in a larger multicenter trial testing different GDTs during the vulnerable intraoperative phase in high risk surgical patients.
References
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensiv Care Med. 2013;39:165–228.
Wenzel V, Russo S, Arntz HR, Bahr J, Baubin MA, Bottiger BW, Dirks B, Dorges V, Eich C, Fischer M et al. Anaesthesist. 2006, 55:958–966, 968–972.
Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14:218–25.
Ekbal NJ, Dyson A, Black C, Singer M. Monitoring tissue perfusion, oxygenation, and metabolism in critically ill patients. Chest. 2013;143:1799–808.
Fouche Y, Sikorski R, Dutton RP. Changing paradigms in surgical resuscitation. Crit Care Med. 2010;38(Suppl):S411–20.
Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg. 1973;106:630–6.
Shoemaker WC, Mohr PA, Printen KJ, Brown RS, Amato JJ, Carey JS, Youssef S, Reinhard JM, Kim SI, Kark AE. Use of sequential physiologic measurements for evaluation and therapy of uncomplicated septic shock. Surg Gynecol Obst. 1970;131:245–54.
Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992;102:216–20.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;34:1368–77.
Preisman S, Pfeiffer U, Lieberman N, Perel A. New monitors of intravascular volume: a comparison of arterial pressure waveform analysis and the intrathoracic blood volume. Intensiv Care Med. 1997;23:651–7.
Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8.
Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100.
Lobo SM, de Oliveira NE. Clinical review: what are the best hemodynamic targets for noncardiac surgical patients? Crit Care. 2013;17:210.
Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, et al. Hemodynamic monitoring in shock and implications for management. International consensus conference, Paris, France, 27–28 April 2006. Intensiv Care Med. 2007;33:575–90.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care. 2005;9:R687–93.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90.
Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–92.
Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, Cecconi M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 2014;112:648–59.
Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, Hamilton M, Rhodes A. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209.
Dunser MW, Ruokonen E, Pettila V, Ulmer H, Torgersen C, Schmittinger CA, Jakob S, Takala J. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care. 2009;13:R181.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22.
Wilms H, Mittal A, Haydock MD, van den Heever M, Devaud M, Windsor JA. A systematic review of goal directed fluid therapy: rating of evidence for goals and monitoring methods. J Crit Care. 2014;29:204–9.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
Kobayashi L, Costantini TW, Coimbra R. Hypovolemic shock resuscitation. Surg Clin N Am. 2012;92:1403–23.
Williams DJ, Walker JD. A nomogram to calculate the physiological and operative severity score for the enUmeration of Mortality and morbidity (POSSUM). Br J Surg. 2014;101:239–45.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Fueglistaler P, Amsler F, Schuepp M, Fueglistaler-Montali I, Attenberger C, Pargger H, Jacob AL, Gross T. Prognostic value of sequential organ failure assessment and simplified acute physiology II score compared with trauma scores in the outcome of multiple-trauma patients. Am J Surg. 2010;200:204–14.
Ochiai T, Hiranuma S, Takiguchi N, Ito K, Kawaguchi A, Iwai T, Arii S. SOFA score predicts postoperative outcome of patients with colorectal perforation. Hepatogastroenterology. 2004;51:1007–10.
Lees N, Hamilton M, Rhodes A. Clinical review: goal-directed therapy in high risk surgical patients. Crit Care. 2009;13(5):231.
Boland MR, Noorani A, Varty K, Coffey JC, Agha R, Walsh SR. Perioperative fluid restriction in major abdominal surgery: systematic review and meta-analysis of randomized, clinical trials. World J Surg. 2013;37:1193–202.
Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K. Optimisation systematic review steering G: perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a cochrane systematic review. Br J Anaesth. 2013;111:535–48.
Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, Minto G. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108:53–62.
Ghneim MH, Regner JL, Jupiter DC, Kang F, Bonner GL, Bready MS, Frazee R, Ciceri D, Davis ML. Goal directed fluid resuscitation decreases time for lactate clearance and facilitates early fascial closure in damage control surgery. Am J Surg. 2013;206:995–9.
Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37(10):2827–39.
Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Crit Care Clin. 2000;16:353–66.
Meyer ZC, Schreinemakers JM, Mulder PG, de Waal RA, Ermens AA, van der Laan L. The role of C-reactive protein and the SOFA score as parameter for clinical decision making in surgical patients during the intensive care unit course. PLoS One. 2013;8:e55964.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402.
Harten J, Crozier JE, McCreath B, Hay A, McMillan DC, McArdle CS, Kinsella J. Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696). Int J Surg. 2008;6:197–204.
Licker M, Diaper J, Villiger Y, Spiliopoulos A, Licker V, Robert J, Tschopp JM. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009;13:R41.
Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM. Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial. Crit Care. 2011;15:R148.
Takala J, Meier-Hellmann A, Eddleston J, Hulstaert P, Sramek V. Effect of dopexamine on outcome after major abdominal surgery: a prospective, randomized, controlled multicenter study. European multicenter study group on dopexamine in major abdominal surgery. Crit Care Med. 2000;28:3417–23.
Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, Florez J, Castro R, Aquevedo A, Pairumani R, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensiv Care Med. 2013;39:1435–43.
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403–8.
Shahin J, DeVarennes B, Tse CW, Amarica DA, Dial S. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care. 2011;15:R162.
Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–108.
Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensiv Care Med. 2012;38:359–67.
Rhodes SS, Ropella KM, Camara AK, Chen Q, Riess ML, Pagel PS, Stowe DF. Ischemia-reperfusion injury changes the dynamics of Ca2+—contraction coupling due to inotropic drugs in isolated hearts. J Appl Physiol. 2006;100:940–50.
Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC. Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2008;294:H954–60.
Singer M. Catecholamine treatment for shock—equally good or bad? Lancet. 2007;370:636–7.
Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–91.
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–97.
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrial.gov NCT0165-3977.
Appendix
Appendix
Severe Hypovolemia corresponding to class III or IV hemorrhagic shock according to the following criteria:
-
HR > 100 mmHg with systolic arterial pressure (SAP) < 100 mmHg after fluid loading (10 ml/kg) or with a blood lactate level > 4mm/L
Severe sepsis refers to sepsis-induced tissue hypoperfusion or organ dysfunction with any of the following thought to be due to the infection:
-
Sepsis-induced hypotension (SAP < 90 mmHg or MAP < 70 mmHg or SAP decrease more than 40 mmHg or two standard deviations below normal age in the absence of other causes of hypotension
-
Lactate above upper limits of laboratory normal
-
Urine output <0.5 mL/kg/hr for more than 2 h despite adequate fluid resuscitation
-
Acute lung injury with PaO2/FIO2 < 250 in the absence of pneumonia as infection source
-
Acute lung injury with PaO2/FIO2 < 200 in the presence of pneumonia as infection source
-
Creatinine > 2 mg/dL (176.8 μmol/L)
-
Bilirubin >4 mg/dL (34.2 μmol/L)
-
Platelet count <100,000 μL–1
-
Coagulopathy (INR > 1.5)
Sepsis-induced hypotension is defined as a systolic blood pressure (SBP) <90 mmHg or MAP <70 mmHg or a SBP decrease >40 mmHg or less than two standard deviations below normal for age in the absence of other causes of hypotension.
Rights and permissions
About this article
Cite this article
Pavlovic, G., Diaper, J., Ellenberger, C. et al. Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial. J Clin Monit Comput 30, 87–99 (2016). https://doi.org/10.1007/s10877-015-9691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9691-x