Abstract
Objective
To investigate the effects of fenofibrate, an activator of peroxysome proliferator activated receptor (PPAR) α, on vascular endothelium and on hemostasis in a rabbit endotoxic shock model.
Design and setting
Prospective laboratory study in a university laboratory.
Subjects
36 male New Zealand rabbits weighing 2.5–3 kg.
Interventions
We determined in vitro vascular reactivity, endothelium CD31–platelet/endothelial cell adhesion molecule (PECAM) 1 immunohistochemistry, plasma coagulation factors, and monocyte tissue factor expression 5 days after onset of endotoxic shock (0.5 mg/kg intravenous bolus, Escherichia coli lipopolysaccharide) with or without treatment by fenofibrate (mixed in the chow at a concentration of 0.5%) for 15 days before lipopolysaccharide injection and 5 days afterward.
Measurements and results
Metabolic acidosis and coagulation activation confirmed presence of shock. Fenofibrate decreased monocyte tissue factor expression. It improved endothelial-dependent relaxation at 5 days (Emax=68.2±3.3%, vs. 44.2±2.5% in the non-treated group). Endotoxin-induced deendothelialization was significantly decreased by fenofibrate at 5 days (8.5±1.3% vs. 19.2±3.1% in the nontreated group).
Conclusions
These data indicate for the first time that fenofibrate, an activator of PPAR-α, inhibits monocyte tissue factor expression and protects against endothelial dysfunction and histological injury in endotoxin-induced shock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septic shock is often associated with vascular damage, hemostasis activation, and development of disseminated intravascular coagulation leading to multiple organ dysfunction and death [1]. In such conditions morphological and functional endothelial abnormalities are involved in the development of circulatory failure [2, 3, 4]. Morphological injuries are characterized by endothelial detachment and denudation (reaching approx. 20–25% of the endothelial surface [5, 6, 7]), associated with coagulation activation through monocyte tissue factor (TF) expression [1, 7] and with impaired endothelium-mediated vasorelaxation [7]. Furthermore, sepsis alters the nitric oxide pathway with a reduction in endothelial constitutive nitric oxide synthase (NOS) expression and overexpression of vascular smooth muscle cell inducible NOS (iNOS). Overall these phenomenons provide the refractory hypotension and altered tissue perfusion observed during septic shock. It has been demonstrated in human volunteers that endotoxin injection is associated with prolonged coagulation activation and endothelial injury. In a rabbit endotoxin shock model we have reported that endothelial injuries and monocyte TF expression are sustained, persisting longer than 5 days after a single injection of lipopolysaccharide (LPS) [7]. Persistence of inflammatory process via the nuclear factor (NF) κB pathway could explain at least in part the prolonged endothelial and monocyte alterations. The anatomical and functional injuries were observed to be corrected approx. 21 days after LPS injection [7]. Peroxysome proliferator activated receptor (PPAR) α belongs to the nuclear receptor superfamily [8] which by heterodimerization with the retinoid receptor binds to specific peroxysome proliferator response elements in the promoter of target genes and regulates their transcription. Particularly PPAR-α controls genes relevant to the regulation of lipid metabolism and inflammatory process [9, 10]. Recently this ligand-dependent transcription factor was localized in endothelial cells [11], as previously identified in smooth muscle cells [12] and in monocytes-macrophages [13]. Overall PPAR-α appears an interesting target to control the inflammatory process at the level of vascular wall [14]. It has recently been demonstrated that activation of PPAR-α by fenofibrate has neuroprotective effects associated with the prevention of ischemia-induced expression of vascular cell adhesion molecule (VCAM) 1 and intercellular adhesion molecule (ICAM) 1, a decrease in cerebral oxidative stress, and an improvement in middle cerebral artery sensitivity to endothelium-dependent relaxation [15] and also has a cardioprotective effect with reduced myocardial infarct size and improved postischemic contractile dysfunction [16].
Fibrates are synthetic ligands for PPAR-α, which are known mainly for their lipid-lowering activity [17, 18]. However, fibrates have recently been shown to exert vascular anti-inflammatory activity via PPAR-α by antagonizing at least in part the NF-κB pathway with a decrease in plasma concentrations of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor (TNF) α [12, 19, 20]. Furthermore, in the human monocytic leukemia THP-1 cell line PPAR-α agonists inhibit the upregulation of TF expression occurring after stimulation of these cells with LPS or IL-1β [21]. In this model it is thought that fibrates-induced modulation of TF expression occurs via cross-talk of PPAR-α with other transcription factor such as Jun, Fos, and NF-κB.
Therefore both the anti-inflammatory action and the prevention of coagulation activation could be interesting for PPAR-α agonists to prevent endothelium damage in septic shock. This study was conducted to investigate for the first time in an animal model the long-term effect of fenofibrate on endothelium in a well-characterized rabbit endotoxin-induced shock model [7, 22, 23, 24].
Methods
Study protocol
The animal experiments were approved by the French Agricultural Office for the care of animal subjects, and the care and handling of the animals were in agreement with European Union laws on animal research. We used 36 male New Zealand white rabbits weighing 2.5–3 kg (Charles River Laboratory, France). Animals were maintained throughout on a standard rabbit chow diet with food (100 g/day) and water ad libitum. Rabbits were randomly assigned to one of four groups. In two endotoxin groups conscious animals were rapidly injected intravenously with 0.5 mg/kg body weight purified LPS endotoxin (Escherichia coli serotype O55:B5 from a single batch, Sigma Chemical, St. Louis, Mo., USA) without (LPS group, n=12) or with fenofibrate supplementation (LPS+FENO group, n=8). In two sham groups animals were injected intravenously with saline without (control group, n=10) or with fenofibrate supplementation (FENO group, n=6). All animals were killed 5 days after LPS or saline injection under general anesthesia. The number of rabbits per group was chosen on the basis of our previous studies which demonstrated that at least six animals/group are necessary to show statistical differences [7, 22, 23, 24].
Fenofibrate mixed with standard chow at 0.5% (500 mg/day=200–250 mg/kg per day) was initiated 15 days before LPS or saline injection and maintained for 5 days afterward. Since it has been demonstrated that rabbits do not develop hepatomegaly at doses that do induce hepatomegaly in rats and on the basis of a previous study showing a hypolipidemic effect of fenofibrate at 0.5% [25], we chose a higher dose than that used in studies performed in rodents [26].
Arterial blood gas was analyzed 4 h after LPS or saline injection. Hematological and coagulation parameters, liver variables, and plasma creatine kinase (CPK) were measured on day 5. Bilirubin concentration was determined by the Jendrassik-Grof method. In the presence of caffeine accelerator total bilirubin couples with diazotized sulfanilic acid to form a red azobilirubin dye (color intensity proportional to the bilirubin concentration). Bilirubin is determined directly without caffeine additive. Alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT) were measured by a UV test to a standardized method (Roche Diagnostics, Mannheim, Germany). Alkaline phosphatase analysis used a colorometric assay in accordance with a standardized method (Roche Diagnostic). γ-Glutamyltranspeptidase was analyzed in accordance with an enzymatic colorimetric assay in accordance with the Szasz method (Roche Diagnostics). Analysis of all these liver parameters were performed on a Hitachi-747 automated chemistry analyzer (Roche Diagnostics). Plasma CPK concentration was determined with an enzymatic and cinetic method in presence of N-acetyl-l-cysteine used as an activator of CPK and performed on a Beckman Synchron LX20-Pro automated chemistry analyzer (Beckman Coulter). On day 5 we assessed the liver weight as a proportion of body weight assessing therapeutic observance, in vitro vascular reactivity, and endothelium CD31-PECAM1 immunoreactivity.
In vitro vascular reactivity
The descending abdominal aorta was removed rapidly by laparotomy under general anesthesia (pentobarbital, 30 mg/kg; Specia, Paris, France) and immersed in iced oxygenated Krebs-Henseleit solution (in mmol/l: NaCl 118, KCl 4.6, NaHCO3 27.2, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.75, Na2 EDTA 0.03, and d-glucose 11.1, pH=7.35–7.45). Intravenous heparin (500 IU/kg; Panpharma, France) was given before removal of the aorta to prevent coagulation. Vessels were cleaned of surrounding fat, connective tissue, cut into rings 3–4 mm long (four rings/aorta). Two rings were functionally denuded of endothelium by slightly rubbing the luminal wall with a wooden applicator. All rings were mounted progressively under 8 g resting tension (previously determined as the optimal point of their length-tension relationship) on stainless hooks in organ chambers (Radnoti Glass Technology, Monrovia, Calif., USA) filled with 40 ml warmed (37°C) and oxygenated (95% oxygen/5% CO2) Krebs-Henseleit solution [27]. Rings were connected to force transducers, and changes in isometric force were recorded continuously. The output from the transducers was amplified by signal conditioners and sent to an Intel 486-based computer for analog-to-digital conversion. After a 1-h equilibration period the presence or absence of functional endothelium was verified by addition of acetylcholine (ACh; 3.10−5 mmol/l; Sigma Chemical) to rings precontracted with phenylephrine (PE; 3.10−7 mmol/l; Sigma Chemical). After a new 30 min stabilization period at the resting tension cumulative concentration response curves were determined for PE (10−9–3.10−5 mmol/l). The presence of a vascular smooth muscle cell iNOS was determined by performing the same protocol in presence of NG-nitro-l-arginine methyl ester (L-NAME; 3.10−6 mmol/l; Sigma Chemical) in vessels without endothelium. Endothelium-derived vascular reactivity was assessed by application of the following: (a) the receptor-dependent endothelium-dependent vasodilator agonist ACh (10−9–3.10−5 mmol/l), (b) the receptor-independent endothelium-dependent vasodilator agonist calcium ionophore A23187 (10−9–3.10−6 mmol/l; Sigma Chemical), and (c) the endothelium-independent vasodilator sodium nitroprusside (SNP; 10−9–3.10−5 mmol/l, Sigma Chemical). PE, ACh, SNP, and L-NAME were dissolved in deionized water.
Immunohistochemical staining of vascular endothelium
Aortic segments were fixed with paraformaldehyde 4% and cryoprotected by immersion in sucrose 30%. Tissues were embedded in optimal cutting temperature, frozen in isopentane and stored at −80°C. Tissue sections were cut 6 µm thick. The endothelial cell layer was stained by using a CD31-PECAM1 antibody. Air-dried frozen sections were incubated with peroxidase blocking reagent, rinsed in phosphate-buffered saline (PBS) for 10 min and blocked with 10% horse serum in PBS for 10 min. The sections were incubated at 37°C overnight with a mouse-prepared monoclonal primary antibody to CD31 (Dako, Carpinteria, Calif., USA) diluted 1:20 in PBS. After PBS washings an antimouse biotinylated secondary antibody was applied for 1 h. The sections were washed with PBS and incubated with avidin-biotin-peroxidase preformed complex (Vectastain Elite ABC Peroxydase kit, Vector Laboratories, Burlingame, Calif., USA) for 1 h. The peroxidase activity was revealed using hydrogen peroxide and diaminobenzidine as a chromogen. Sections were counterstained with hematoxylin and mounted with Permount (Fisher Scientific, Elancourt, France). In each experiment negative controls without the primary antibody were included to check for nonspecific staining.
Quantification of endothelial injury used three nonconsecutive cross-sections per aortic segment photomicrographed microscopically (Axioskop20; Zeiss, France). After photographic reconstruction of each tissue section, each picture was digitalized for computerized analysis (Color Image 1.32 software). The surface area of endothelial cell injury (including three types: subendothelial vacuolization, detachment of endothelial cells, and endothelial denudation) was measured and expressed as proportion of total circumference of each section.
Hematological and coagulation studies
Hematological and coagulation variables
On day 5 arterial blood samples were collected on EDTA and used for blood cells counts (Coulter MAXM; Beckman Instruments). Total white blood cell counts were verified manually. Peripheral blood smears for differential white cell counts were stained with May-Grünwald-Giemsa. Factor II, V, and VII levels were determined by an automated clotting assay (STA; Stago, Asnières, France) using calcified rabbit brain thromboplastin and human factor deficient plasma (Stago). Prothrombin index was measured by an automated clotting assay by using calcified rabbit brain thromboplastin (Stago). Fibrinogen levels were measured by the Clauss technique (Biomérieux, Lyon, France).
Isolation of mononuclear cells and TF activity assay
Mononuclear cells were isolated by gradient centrifugation (MSL, density=1.077±0.001, Laboratories Eurobio, Les Ulis, France), washed twice, and resuspended in RPMI 1640 (Gibco Life Technologies, Eragny, France) (3×106 cells/ml). Cell viability was higher than 98% as assessed by the trypan blue test. All reagents, test tubes, and culture supplies used were free of endotoxin, as determined by the chromogenic limulus amebocyte lysate assay. The sensitivity of this assay was 0.025 U endotoxin/ml. Aliquots of cell preparations (3×106 cells/ml) suspended in RPMI 1640 without fetal calf serum were cultured for 16 h at 37°C in a humidified 5% CO2 atmosphere, without or with stimulation by endotoxin at 1 µg/ml, which corresponded to 5000 U endotoxin/ml (E. coli O55:B5, Sigma Chemical). These are referred to as unstimulated and stimulated cells, respectively. By the end of the incubation period mononuclear cells were resuspended and frozen at −80°C. TF activity was determined with a modified amidolytic assay [28, 29]. Lysed cell suspensions (50 µl) were incubated at 37°C in a microtiter plate (2 min) and were mixed with 0.25 mol/l CaCl2 (50 µl; 3 minincubation) and prothrombin concentrate complex (Laboratoire de Fractionnement et des Biotechnologies, Les Ulis, France) as a source of factor VII (50 µl, 3 UI/ml) and factor X (6 UI/ml). After addition of 50 µl chromogenic substrate S2765 (Biogenic, Maurin, France), the change in optical density at 410 nm was quantified with a microplate reader and converted to units of TF activity from log-log plots of serial dilutions of rabbit brain thromboplastin (Néoplastine CI Plus, Stago). Arbitrarily 1 ml thromboplastin was assigned a value of 1000 U/ml TF. Results were expressed as mU/1.5×105 mononuclear cells.
Statistical analysis
Results are presented as mean ±SEM, n=number of rabbits. Hematological and coagulation values were compared using Student’s unpaired t test. Liver variables and CPK plasma levels were compared using the Mann-Whitney test. The concentrations of agonist causing half-maximal contraction or relaxation (EC50) were calculated by nonlinear semilogistic regression analysis. EC50 values were compared using the Mann-Whitney test. Relaxation to the vasodilator agents is expressed as proportional reduction in the maximal contraction to PE. Mean intergroup differences were tested by repeated measures analysis of variance, followed by Scheffé’s least-significant-difference test. Significance was set at p≤0.05.
Results
Clinical parameters
Because all animals were killed at 5 days, 5-day survivors were considered permanent survivors. No death was observed in control or FENO groups. Mortality was 33.3% in the LPS group (4 deaths/12) with rabbits dying within the first 4 h following LPS injection. With fenofibrate supplementation there was a trend to decreased mortality in LPS+FENO animals to 12.5% (1 death/8; NS vs. LPS group). Compared with the baseline values there was a significant body weight loss on day 5 of 6.7±1.1% in FENO (p≤0.05), 11.7±1.5% in LPS (p≤0.05), and 17.5±1.3% in LPS+FENO groups (p≤0.05).
Ex vivo measurements
Arterial blood-gas analyses
Compensated metabolic acidosis confirmed endotoxic shock at H4 [pH 7.52±0.03, bicarbonate 15.50±0.67 mmol/l, PaCO2 19.35±0.53 (LPS) vs. pH 7.41±0.02, bicarbonate 30.00±1.53 mmol/l, PaCO2 39.33±1.45 (control), p≤0.05 for all parameters). Fenofibrate treatment was associated with a trend toward improvement in acidosis (LPS+FENO: pH 7.51±0.04, bicarbonate 21.28±2.85 mmol/l, PaCO2 23.07±2.73, p≤0.05 for bicarbonate vs. LPS).
Liver parameters
Rabbits supplemented with fenofibrate did not develop hepatomegaly. On day 5 liver weight remained stable between groups with 2.7±0.2% of body weight (control), 3.1±0.3% (FENO), 2.8±0.2% in (LPS+FENO), and 2.7±0.1% (LPS). In the FENO group alkaline phosphatase was less than control (105.5±16.7 vs. 347.6±32.9 IU/l, p=≤0.05). No significant difference in liver variables was observed between LPS and LPS+FENO groups (data not shown). Finally, treatment with fenofibrate induced a significant increase in plasma CPK [590±100 (FENO) vs. 315±17 IU/l (control), p≤0.05; 1318±314 (LPS+FENO) vs. 558±74 IU/l (LPS), p≤0.05).
Hematological and coagulation parameters
Among hematological variables LPS administration was associated with a significant increase in leukocytes and thrombocytemia (Table 1). Fernofibrate treatment also induced a significant decrease in platelet count in both endotoxinic and nonendotoxinic animals (Table 1). Among coagulation variables the value of prothrombin index was significantly higher in the LPS+FENO group than in other groups (Table 2). LPS increased fibrinogen and factors II, V, and VII plasma concentration compared to controls (Table 2). On day 5 supplementation with fenofibrate in endotoxinic animals had significantly increased these previous parameters compared to the LPS group.
Monocyte TF expression on day 5
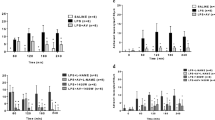
Fenofibrate alone did not induce monocyte TF expression on day 5. LPS administration increased monocyte TF expression in both unstimulated and stimulated cells compared to controls (Fig. 1). However, in unstimulated cells pretreatment with fenofibrate in septic animals blunted TF expression to 18±6 mU/1.5×105 mononuclear cells vs. 180±79 mU/1.5×105 mononuclear cells in the LPS group. In stimulated cells a higher level in TF expression was observed in the LPS+FENO group than in the LPS group, suggesting the higher ability of supplemented animals to respond to further endotoxin stimulation.
Expression of monocyte tissue factor (TF) on day 5 with or without stimulation in vitro (stimulation obtained in culture in the presence of 1 µg/ml endotoxin). CTRL Control group; LPS: animals that received LPS alone; LPS+FENO animals that received LPS and FENO; FENO animals that received fenofibrate alone; n number of rabbits. *p≤0.05 vs. CTRL; §p≤0.05 vs. LPS
In vitro vascular reactivity: vascular contraction
LPS significantly modified sensitivity to PE on day 5. The PE EC50 of rings with endothelium (E+) and rings without endothelium (E−) were similar but differed significantly from controls (Fig. 2a). Fenofibrate supplementation in LPS animals failed to restore the difference in sensitivity between E+ and E− aortic rings (LPS+FENO). In the FENO group there was no longer endothelium-dependent contraction modulation because PE EC50 was similar in E+ and E− aortic rings, as was observed in LPS group (Fig. 2a). In E− rings LPS decreased the sensitivity to PE vs. controls. This difference was abolished after in vitro incubation of L-NAME and was not observed in E− rings from fenofibrate-treated rabbits (NS, LPS+FENO vs. LPS; Fig. 2b). In the FENO group fenofibrate decreased the sensitivity to PE of E− rings vs. controls. This difference was not abolished after in vitro incubation of L-NAME (Fig. 2b). The maximal vasoconstrictor response to PE did not differ significantly between groups (data not shown).
Phenylephrine concentration eliciting 50% of maximal constraction response (EC50) in different groups. CTRL Control group; LPS animals that received LPS alone; LPS+FENO animals that received LPS and FENO; FENO animals that received fenofibrate alone; n number of rabbits. a Aortic rings in the presence of endothelium (E+) and absence of endothelium (E−). *p≤0.05 vs. CTRL E+; §p≤0.05 vs. CTRL E−. b Aortic rings (E−) incubated with or without L-NAME.* p≤0.05 vs. control E−; §p≤0.05 vs. LPS E−
Endothelium-dependent and endothelium-independent relaxation
Maximal endothelium-dependent receptor-dependent relaxation in response to ACh (Emax) was 77.2±2.7% in controls (Fig. 3a). This response was altered by LPS administration: Emax=44.2±2.5% (p≤0.05). In the same way the ACh concentration required to elicit 50% of maximal relaxation response (EC50) or sensitivity was significantly higher in the LPS group (0.41±0.09 µmol/l) than in controls (0.15±0.02 µmol/l, p≤0.05). Fenofibrate pretreatment restored ACh-induced vascular relaxation in septic rabbits (Emax=68.2±3.3%; p≤0.05 vs. LPS) but did not improve ACh sensitivity (EC50=0.38±0.07 µmol/l, NS). On the other hand, endothelium-dependent receptor-independent relaxation and sensitivity in response to calcium ionophore A23187 was not modified between groups (Fig. 3b). Similar results were observed for endothelium-dependent relaxation and sensitivity in response to SNP (data not shown).
Dose response relationships of acetylcholine vasodilator effects in isolated aortic rings. CTRL Control group; LPS animals that received LPS alone; LPS+FENO animals that received LPS and FENO; FENO animals that received fenofibrate alone; n the number of rabbits. *p≤0.05 LPS vs. CTRL, and LPS+FENO group vs. LPS group. b Dose-response relationships of calcium ionophore A23187 vasodilator effects in isolated aortic rings. CTRL Control group; LPS animals that received LPS alone; LPS+FENO animals that received LPS and FENO; FENO animals that received fenofibrate alone; n number of rings; NS no significant difference between groups
Immunohistochemical staining of vascular endothelium
In the sham groups (control and FENO) endothelial cells stained by immunohistochemical label (PECAM1/CD31) appeared intact. LPS induced three types of endothelial cell injury: subendothelial vacuolization, detachment of endothelial cells, and endothelial denudation (Fig. 4). In the LPS group injured endothelium accounted for 19.2±3.1% of the total endothelial surface area in the abdominal aorta on day 5 (p≤0.05 vs. controls). Fenofibrate pretreatment in LPS animals reduced these lesions resulting in a surface area of endothelial injury of 8.5±1.3% (p≤0.05 vs. LPS).
Discussion
The present study reports a preventive role of fenofibrate on endothelial morphological and functional injuries, and on monocyte TF expression in a documented rabbit endotoxic shock model [7, 22, 23, 24]. These beneficial effects suggest a possible role for PPAR-α in the modulation of sepsis-induced tissue injury. Our model of endotoxinic shock is characterized by circulatory failure 4 h after 0.5 mg/kg LPS injection, prolonged endothelial dysfunction, and sustained monocyte TF expression on day 5 [7, 22, 23, 24]. These characteristics are known to promote coagulation and thrombosis [30]. We previously reported that the observed LPS-induced endothelial dysfunction and histological injury can be prevented by l-arginine; this was associated with decreased mortality but not with any effect on coagulation activation and monocyte TF expression [22]. Another approach consisted of inhibiting monocyte-platelet interaction by glycoprotein IIb/IIIa inhibitor (AZ-1). AZ-1 decreased monocyte TF expression associated with reduction in endothelial injury and improved survival [23]. This suggest that decreased monocyte TF expression is of paramount importance in sepsis-induced injuries. This is consistent with results reported in the present work.
The direct role of TF in shock and mortality has been reported in E. coli induced shock [31]. In the same way disseminated intravascular coagulation is a well known cause of pejorative prognosis with high mortality rate in patients with septic shock [32]. Mortality 5 days after LPS injection was 33.3% in untreated rabbits, the majority of deaths occurring within the first 24 h. Pretreatment with fenofibrate tended to reduce mortality on day 5 to 12.5%, but not significantly. We postulate that modulation of TF expression induced by fenofibrate explains at least in part the trend decrease in mortality in this rabbit endotoxic shock model. Fenofibrate has been shown to inhibit upregulation of TF expression in monocytes and macrophages [21]. Induction of TF mRNA occurs via Jun phosphorylation and NF-κB translocation in a rapid but transient way [33]. It has been demonstrated that PPAR-α inhibits the proinflammatory activator protein 1 and NF-κB signaling pathway [12, 19]. It is likely that PPAR-α modulates TF expression by negatively interfering with the activator protein 1 and/or NF-κB pathway [21]. Our results suggest that a decrease in TF expression, by limiting coagulation activation, may limit endothelial cell injuries and thus improve endothelial function. Other mechanisms may participate in the beneficial vascular effects induced by PPAR-α agonist. First, LPS and associated inflammatory response provide activation of ICAM-1 and VCAM-1, whose expression condition leukocyte migration, a good index of vascular injury in septic rats [34]. PPAR-α activation has been shown to inhibit transcription of both ICAM-1 [35] and VCAM-1 [36] genes, preventing leukocyte adhesion and associated endothelial injury. In an ischemic brain injury model Deplanque et al. [15] demonstrated that fenofibrate significantly decreased ICAM-1 and VCAM-1 expression was associated with improved sensitivity of middle cerebral artery to endothelium-dependent relaxation. In addition, PPAR-α agonists prevent TNF-α induced VCAM-1 expression in human endothelial cells, at least in part via inhibition of NF-κB pathway [37]. Downregulation of such signaling pathway also results in inhibition of inflammatory cytokines, which are known to alter vascular endothelial relaxation [38, 39]. In addition to its effect on adhesion molecules, fenofibrate has been demonstrated to abolish the induction of chemokines, such as monocyte chemoattractant protein 1, expressed by LPS. Secondly, normal results observed with the endothelium-dependent receptor-independent relaxing agent calcium ionophore A23187 associated with impaired endothelium-dependent receptor-dependent relaxation to ACh show, as previously reported [7, 22, 23, 24], that LPS is responsible for abnormal coupling between ACh endothelial receptor and endothelial NOS. Oxidative stress has been incriminated in this uncoupling [40, 41, 42]. This preventive effect could be the result of antioxidant action as PPAR-α activation has been shown to release antioxidant enzyme as catalase and superoxide dismutase [43, 44]. As previously reported [7, 22, 23, 24], LPS induces a decreased sensitivity to PE that is improved by L-NAME. This suggests the presence of iNOS in smooth muscle cells. Induction of iNOS in response to inflammatory stimuli, such as LPS, is a well known phenomenon [45, 46]. Removal or damage of endothelial cells can also trigger the induction of iNOS in vascular smooth muscle cells [47]. In the present study the LPS-induced decrease in sensitivity to PE in smooth muscle cell was restored by fenofibrate treatment, suggesting reduction in iNOS expression. The mechanism underlying the decreased in iNOS expression in smooth muscle cell after fenofibrate treatment is unknown but may be due at least in part to restoration of endothelial structure and function induced by PPAR-α activation or direct effect on PPAR-α activation. This may also involve NF-kB pathway inhibition. Another possible explanation is a counterbalancing effect of fenofibrate which has been demonstrated to increase endothelial NOS expression [48]. This effect has been suggested to explain the cardioprotective effect of fenofibrate.
The dose of fenofibrate (0.5%) was chosen in regard to a previous work using fibrates in rabbit for its hypolipidemic action [25]. This concentration is approximately threefold higher than that used in humans. We observed a weight loss in our animals. This finding is consistent with a previous publication demonstrating that fenofibrate decreases adiposity [49]. Consistent with a previous work [25], fenofibrate did not induce hepatomegaly. Nevertheless, it was associated with significant decrease in alkaline phosphatase activity without affecting serum transaminases and γ-glutamyltranspeptidase activity. Such adverse effects have been reported previously [50, 51, 52, 53]. It seems that decrease in serum alkaline phosphastase activity is a consequence of PPAR-α activation since gemfibrozil, the sole fibrate drug without PPAR-α effect, does not modify alkaline phosphatase activity [50, 54]. Hence serum alkaline phosphatase has been recently proposed as a reliable marker for fibrate compliance [52, 53]. In our study we observed another side effect of fenofibrate not frequently reported in the literature and consisting of a decrease in platelet count. Thrombocytopenia has seldom been reported during fenofibrate treatment. Only a single case of thrombopenia has been reported in the literature in the context of pancytopenia [55]. The underlying mechanism remains unexplained. Finally, we observed a well known adverse effect of fenofibrate consisting of elevated CPK [56]. A significant increase in CPK plasma levels was observed in treated animals, with the highest concentration in the LPS fenofibrate-supplemented animals (an increase of more than twofold vs. the LPS group). This findings provides evidence of a side effect that is amplified by sepsis, suggesting the necessity of further studies to determine relationship between doses and therapeutic effects as well as between doses and adverse events.
In conclusion, the PPAR-α agonist fenofibrate decreases LPS-induced morphological injury of the endothelium. While it did not counteract systemic inflammation, our results suggest a potential mediating role for critical inflammatory processes in the vessel wall at the level of the endothelial cells. This effect was associated with an improvement in endothelial relaxation function and a decrease in coagulation activation (monocyte TF expression). Further studies are required to elucidate mechanisms implicated in the beneficial effect of fenofibrate in our rabbit endotoxin shock model, but the present work provides evidence for the first time in a living animal model that PPAR-α activation has a protective effect in septic shock.
References
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150
Kang YH. Williams R (1991) Endotoxin-induced endothelial injury and subendothelial accumulation of fibronectin in rat aorta. Anat Rec 229:86–102
Young JS, Headrick JP, Berne RM (1991) Endothelial-dependent and -independent responses in the thoracic aorta during endotoxic shock. Circ Shock 35:25–30
Parker JL, Adams HR (1993) Selective inhibition of endothelium-dependent vasodilator capacity by Escherichia coli endotoxemia. Circ Res 72:539–551
Reidy MA, Bowyer DE (1977) Scanning electron microscopy: morphology of aortic endothelium following injury by endotoxin and during subsequent repair. Atherosclerosis 26:319–328
Lee M, Schuessler G, Chien S (1988) Time dependent effects of endotoxin on the ultrastructure of the aortic endothelium. Artery 15:71–89
Leclerc J, Pu Q, Corseaux D, Haddad E, Decoene C, Bordet R, Six I, Jude B, Vallet B (2000) A single endotoxin injection in rabbit causes prolonged blood vessel dysfunctions and procoagulant state. Crit Care Med 28:3672–3678
Mangelsdorf DJ, Thummel C, Beato M, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
Isseman I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxysome proliferators. Nature 347:645–650
Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S (1992) The mouse peroxysome proliferator-activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl coA oxidase gene. EMBO J 11:433–439
Rival Y, Beneteau N, Taillandier T, Pezet M, Dupont-Passelaigue E, Patoiseau JF, Junquero D, Colpaert FC, Delhon A (2002) PPAR alpha and PPAR delta activators inhibit cyokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol 435:143–151
Staels B, Koenig W, Habib A, Merval R, Lebret M, Pineda Torra I, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf j, Tedgui A (1998) Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature 393:790–793
Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum JL, Auwerx J, Palinski W, Glass CK (1998) Expression of peroxysome proliferator activated receptor gamma (PPAR gamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci USA 95:7614–7619
Duez H, Chao Y-S, Hernandez M, Torpier G, Poulain P, Mundt S, Mallat Z, Teissier E, Burton CA, Tedgui A, Fruchart JC, Fievet C, Wright SD, Staels B (2002) Reduction of atherosclerosis by the peroxysome proliferator-activated receptor α agonist fenofibrate in mice. J Biol Chem 50:48051–48057
Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R (2003) Peroxysome proliferator-activated receptor-alpha activation: mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci 23:6264–6271
Tabernero A, Schoonjans K, Jesel L, Carpusca I, Auwerx J, Andraintsitohaina R (2002) Activation of the peroxysome proliferator-activated receptor a protects against myocardial ischaemic injury and improves endothelial vasodilation. BMC Pharmacol 2:10
Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxysome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94:4312–4317
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093
Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B (1999) PPARα negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem 274:32048–32054
Madej A, Okopien B, Kowalski J, Zielinski M, Wysocki J, Szygula B, Kalina Z, Herman ZS (1998) Effects of fenofibrate on plasma cytokine concentrations in patients with atherosclerosis, hyperlipoproteinemia IIb. Int J Clin Pharmacol Ther 36:345–349
Neve BP, Corseaux D, Chinetti G, Zawadzki C, Fruchart JC, Duriez P, Staels B, Jude B (2001) PPARα agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation 103:207–212
Wiel E, Pu Q, Corseaux D, Robin E, Bordet R, Lund N, Jude B, Vallet B (2000) Effect of L-arginine on endothelial injury and hemostasis in rabbit endotoxin shock. J Appl Physiol 89:1811–1818
Pu Q, Wiel E, Corseaux D, Bordet R, Azrin MA, Ezekowitz MD, Lund N, Jude B, Vallet B (2001) Beneficial effect of glycoprotein IIb/IIIa inhibitor (AZ-1) on endothelium in Escherichia coli endotoxin-induced shock. Crit Care Med 29:1181–1188
Wiel E, Pu Q, Leclerc J, Corseaux D, Bordet R, Lund N, Jude B, Vallet B (2004) Effects of the angiotensin-converting enzyme inhibitor perindopril on endothelial injury, hemostasis in rabbit endotoxic shock. Intensive Care Med 30:1652–1659
Hennuyer N, Poulain P, Madsen L, Berge RK, Houdebine LM, Branellec D, Fruchart JC, Fievet C, Duverger N, Staels B (1999) Beneficial effects of fibrates on apolipoprotein A-I metabolism occur independently of any peroxysome proliferative response. Circulation 99:2445–2451
Staels B, van Tol A, Andreu T, Auwerx J (1992) Fibrates influence the expression of genes involved in lipoprotein metabolism in a tissue-selective manner in the rat. Arterioscler Thromb 12:286–294
Hamon M, Vallet B, Bauters C, Wernert N, McFadden EP, Lablanche JM, Dupuis B, Bertrand ME (1994) Long-term oral administration of L-arginine reduces intimal thickening and enhances neo-endothelium-dependent acetylcholine-induced relaxation after arterial injury. Circulation 90:1357–1362
Carson S (1987) Continuous chromogenic tissue factor assay: comparison to clot-based assays and sensitivity established using pure tissue factor. Thromb Res 47:379–387
Corseaux D, Le Tourneau T, Six I, Ezekowitz MD, McFadden EP, Meurice T, Asseman P, Bauters C, Jude B (1998) Enhanced monocyte tissue factor response after experimental balloon angioplasty in hypercholesterolemic rabbit: inhibition with dietary L-arginine. Circulation 98:1776–1782
Taylor FB Jr, Wada H, Kinasewitz G (2000) Description of compensated and uncompensated disseminated intravascular coagulation (DIC) responses (non-overt and overt DIC) in baboon models of intravenous and intraperitoneal Escherichia coli sepsis and in the human model of endotoxemia: toward a better definition of DIC. Crit Care Med 28 [Suppl 9]:S12–S19
Erlich J, Fearns C, Mathison J, Ulevitch RJ, Mackman N (1999) Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun 67:2540–2546
Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, Mareyy A, Lestavel P (1992) Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 101:816–823
Hall AJ, Vos HL, Bertina RM (1999) Lipopolysaccharide induction of tissue factor in THP-1 cells involves Jun protein phosphorylation, nuclear factor κB nuclear translocation. J Biol Chem 274:376–383
Wu R, Xu Y, Song X, Meng X (2002) Gene expression of adhesion molecules in pulmonary and hepatic microvascular endothelial cells during sepsis. Chin J Traumatol 5:146–150
Delerive P, Gervois P, Fruchart JC, Staels B (2000) Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxysome proliferator-activated receptor-α activators. J Biol Chem 47:36703–36707
Van Oosten M, van de Bilt E, de Vries HE, van Berkel TJ, Kuiper J (1997) Vascular adhesion molecule-1 and intercellular molecule expression on rat liver cells after lipopolysaccharide administration in vivo. Hepatology 25:1538–1546
Marx N, Sukhova G, Collins T, Libby P, Plutzky J (1999) PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 99:3125–3131
Lefer AM, Tsao PS, Ma XL (1991) Shock- and ischemia-induced mechanisms of impairment of endothelium-mediated vasodilation. Chest 100 [Suppl 3]:S160–S163
Aoki N, Siegfried M, Lefer AM (1989) Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am J Physiol 256:H1509–H1512
Mügge A, Elwell JH, Peterson TE, Hofmeyer TG, Heistad DD, Harrison DG (1991) Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res 69:1293–1300
Keaney JF Jr, Xu A, Cunningham D, Jackson T, Frei B, Vita JA (1995) Dietary probucol preserves endothelial function in cholesterol-fed rabbits by limiting vascular oxidative stress and superoxide generation. J Clin Invest 95:2520–2529
Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20:2175–2183
Yoo HY, Chang MS, Rho HM (1999) Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene 234:87–91
Arnaiz SL, Travacio M, Llesuy S, Boveris A (1995) Hydrogen peroxide metabolism during peroxysome proliferation by fenofibrate. Biochim Biophys Acta 1272:175–180
Julou-Schaeffer G, Gray GA, Fleming I, Schott C, Parratt JR, Stoclet JC (1990) Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol 259:H1038–H1043
Szabo C, Mitchell JA, Thiemermann C, Vane JR (1993) Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol 108:786–792
Adeagbo ASO, Triggle CR (1993) Interactions of nitric oxide synthase inhibitors and dexamethasone with α-adrenoreceptor-mediated responses in rat. Br J Pharmacol 109:495–501
Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, Saito H, Kouhara H, Kasayama S, Kawase I (2004) Peroxysome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol 24:658–663
Naderali EK, Fatani S, Williams G (2004) Fenofibrate lowers adiposity and corrects metabolic abnormalities, but only partially restores endothelial function in dietary obese rats. Atherosclerosis 177:307–312
Ganotakis E, Tsimihodimos V, Bairaktari E, Rizos E, Athyros V, Seferiades C, Elisaf M (2002) Effects of various fibrates on serum alkaline phosphatase activity. Atherosclerosis 165:187–188
Steinmetz J, Morin C, Panek E, Siest G, Drouin P (1981) Biological variations in hyperlipidemic children and adolescents treated with fenofibrate. Clin Chim Acta 112:43–53
Papadakis JA, Ganotakis ES, Jagroop IA, Winder AF, Mikhailidis DP (1999) Statin+fibrate combination therapy: fluvastatin with bezafibrate or ciprofibrate in high risk patients with vascular disease. Int J Cardiol 69:237–244
Mikhailidis DP, Ganotakis ES, Spyropoulos KA, Jagroop IA, Byrne DJ, Winder AF (1998) Prothrombotic and lipoprotein variables in patients attending a cardiovascular risk management clinic: response to ciprofibrate or lifestyle advice. Int Angiol 17:225–233
Krey G, Braissant O, Ehorset F, Kalkhoven E, Perroud M, Parker MG, Wahli W (1999) Fatty acids, eicosanoids and hypolipidemic agents identified as ligands of peroxysome proliferators by coactivator-dependent receptor ligand assay. Mol Endocrinol 11:779–791
Rabasa-Lhoret R, Rasamisoa M, Avignon A, Monnier L (2001) Rare side-effects of fenofibrate. Diabetes Metab 27:66–68
Balfour JA, McTavish D, Heel RC (1990) Fenofibrate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in dyslipidemia. Drugs 40:260–290
Acknowledgements
We thank the Laboratory of Biochemistry and Molecular Biology (Profs. Degand and Porchet) from the University Hospital of Lille for the technical help in determining the concentration of the liver parameters and CPK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiel, E., Lebuffe, G., Robin, E. et al. Pretreatment with peroxysome proliferator-activated receptor α agonist fenofibrate protects endothelium in rabbit Escherichia coli endotoxin-induced shock. Intensive Care Med 31, 1269–1279 (2005). https://doi.org/10.1007/s00134-005-2730-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2730-1