Abstract
Introduction
Neuromuscular abnormalities are found frequently in sepsis and multiorgan failure (MOF). Surprisingly, however, there are no data on maximum skeletal muscle force and fatigue in these patients.
Objectives
To test the research hypotheses that adductor pollicis (AP) force would be lower in patients with sepsis, whereas fatigue would not differ between patients and immobilized but not infected volunteers.
Design and setting
Prospective study; university intensive care unit and laboratory.
Patients
Patients with sepsis and MOF (sequential organ failure assessment (SOFA) score >10) and healthy volunteers.
Interventions
Fatigue was evoked during 20 min of intermittent tetanic ulnar nerve stimulation achieving 50% of maximum AP muscle force.
Measurements and results
We measured evoked AP muscle force and fatigue, and compound muscle action potential (CMAP), and performed standard electrophysiological tests in 13 patients, and in 7 volunteers before and after immobilization. Maximum force (20 ± 16 vs 65 ± 19 N; p < 0.01) and CMAP (3.6 ± 2.5 vs 10 ± 2.5 mV; p < 0.05) were markedly decreased in patients; however, fatigue and ulnar nerve conduction velocity did not differ from volunteers, and a decrement of CMAP was not observed with nerve stimulation frequencies up to 40 Hz. All patients with critical illness polyneuropathy, and an additional 50% of those without, had significant muscle weakness.
Conclusion
Peripheral muscle force is markedly decreased in sepsis, without evidence for an increased fatigability. Muscle weakness was most likely due to a sepsis-induced myopathy and/or axonal neuropathy, and was not the result of an immobilization atrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular abnormalities that develop as a consequence of critical illness can be found in many patients hospitalized in the intensive care unit (ICU) for 1 week or more [1]. Electromyography and histological studies in critically ill patients revealed that myopathy may coexist with neuropathies [1, 2], and both have been frequently observed in patients with sepsis [1, 2], which is the predominant cause of multiorgan failure (MOF) [3]. Immobilization of skeletal muscle alone may also cause myopathic changes [4], and there is evidence that the mechanism of critical illness myopathy and immobilization atrophy may have some similarities. In fact, local immune activation and cytokine expression in the muscle tissue were shown in both patients with critical illness myopathy [5] and in rat after limb immobilization [4]. Since skeletal muscles of patients with sepsis may be considered immobilized during ICU therapy, it would be interesting to know if sepsis adds something more to muscle dysfunction than immobilization alone.
Surprisingly, however, no quantitative in vivo data on maximum skeletal muscle force and fatigue in patients with sepsis have been reported. These compound variables of peripheral motor nerve and muscle function are of interest since muscle weakness (i.e., loss of power resulting in decreased motor function) [6, 7], and fatigue (i.e., an exercise-induced decrease in the capacity to generate force or power output) [7, 8] may compromise the quality of life of survivors of critical illness [9, 10]. Based on the results of a study on muscle force and fatigue during experimental sepsis (LPS inoculation) in rat showing muscles weakness that was not associated with increased peripheral fatigability [11], we tested the research hypotheses that adductor pollicis (AP) force (primary criterion) would be lower in patients with sepsis, whereas fatigue (secondary criterion) would not differ between patients and immobilized but not infected volunteers. The results of our study have previously been presented at an international meeting and published as an abstract [12].
Materials and methods
Subjects
After approval by the Faculty of Medicine's ethics committee we studied 13 patients and 7 healthy volunteers. We obtained patients' presumptive consent from the patients' next of kin. Volunteers provided written informed consent.
Thirteen consecutive patients with recent-onset sepsis [13] and multiorgan failure [13] (sequential organ failure assessment [SOFA] score ≥ 10) without a history of peripheral or central nervous system disease were studied within 24 h after meeting inclusion criteria. Sepsis was assumed with two or more of the following criteria as a result of infection: (a) temperature > 38 or < 36°; (b) heart rate > 90 min-1; (c) respiratory rate > 20 min-1 or PaCO2 < 32 mm Hg, or ventilator dependency; and (d) a white blood cell count > 12000 mm-3, or < 4000 mm-3, or > 10% immature (band) forms [13].
We also studied seven healthy volunteers not differing from the patients in age (patients: 58 ± 14 years; volunteers: 48 ± 22 years), weight (82 ± 16 vs 67 ± 8 kg, respectively), and height (177 ± 6 vs 174 ± 9 cm, respectively), before and after 14 days of immobilization of their arm by a lower arm cast extending from below the elbow to the metacarpophalangeal joints of the fingers and to the interphalangeal joint of the thumb, as described previously [15, 16].
Measurements
Evoked force of the AP muscle after ulnar nerve stimulation was measured with a force transducer (LB 8000, Maywood Instruments Ltd., Basingstoke, UK), using a dedicated force measurement system [17], at a thumb abduction angle of approximately 50° [18]. In parallel, we measured compound action potential amplitude (CMAP, Viking IV P, Nicolet Technologies, Madison, Wisconsin) of the ipsilateral AP muscle using surface electrodes (Nicolet Technologies, Madison, Wisconsin). A temperature transducer fixed to the skin measured thumb surface temperature continuously.
Contraction time (CT10 / 90) was calculated at 10 Hz as the time elapsed for force to increase from 10 to 90% during the first of 20 consecutive contractions. Half relaxation time (RT0.5) was calculated at 10 Hz as the time elapsed for force to decrease by 50% from peak force after the last stimulus pulse [19]. We defined the twitch/tetanus ratio as the ratio of the peak force of the first (twitch) contraction at 10 Hz, and the peak force of (tetanic) contraction at 80-Hz stimulation.
Neurophysiological testing in patients included median, ulnar, and sural nerve sensory action potentials, CMAP (including a 2-Hz ulnar nerve stimulation test) from the abductor pollicis brevis, AP, and abductor hallucis muscle (values < 3 mV were regarded as abnormally decreased) [20], as well as median, ulnar, and tibial motor nerve conduction velocities (lower threshold: 50, 50, and 40 m/s, respectively) [20]. In healthy volunteers, evoked force and CMAP of AP muscle, conduction velocity, and sensory action potential of the ulnar nerve were measured.
Protocol
Neurophysiological testing was performed before assessment of muscle force (Viking IV P, Nicolet Technologies, Madison, Wisconsin). Patients received then propofol and fentanyl for maintenance of analgesia during the painful tetanic ulnar nerve stimulation. In volunteers, analgesia was achieved by axillary brachial plexus analgesia using prilocaine 1% (40 ml). All patients had either their right or left hand and forearm in splints immobilized, and then stimulation electrodes (PNS Electrode, NDM, Dayton, Ohio) were applied randomly over the right or left ulnar nerve close to the wrist. Surface electrodes (Nicolet Technologies, Madison, Wisconsin) were then applied to the muscle motor point of the AP muscle and at the metacarpophalangeal joint of the thumb, respectively. Temperature transducers were fixed to the skin close to the EMG recording electrodes and the extremities were covered with surgical cotton to keep skin temperature above 32°C.
Following determination of supramaximal stimulation current and signal stabilization, force frequency curves were generated at baseline, during, and after fatiguing contractions as outlined in Fig. 1 and 2. In volunteers, this protocol was performed twice, i.e., before and after a 2-week immobilization period of the thumb by a lower arm cast. Values of variables obtained during the last force-frequency curve (i.e., immediately after 20 min of fatiguing contractions) were considered as post-fatigue muscle function [21].

Study protocol. After 15 min of signal stabilization (single twitch mode, 0.1 Hz), a low-frequency fatigue protocol [21] was performed. This protocol consisted of four periods (circles) of, firstly, a force frequency curve for calculation of the nerve stimulation frequency that would evoke 50% of maximum force, and secondly, a fatigue period, i.e., a subsequent 5-min period of intermittent (6 s of stimulation following 4 s of rest), non-ischemic, fatiguing contractions with the predefined (previous force – frequency curve) nerve stimulation frequency that evokes 50% of maximum force.

Original trace of stimulation current, evoked compound action potential (CMAP), and evoked adductor pollicis muscle force during ulnar nerve stimulation from a volunteer after immobilization. A Slow chart speed: adductor pollicis muscle force with increasing stimulation frequency (ulnar nerve stimulation over 2 s at each stimulation frequency) demonstrates the protocol for assessment of force – frequency curves. B Fast chart speed: adductor pollicis muscle force and CMAP during 20 stimuli at 10 Hz. Plateau values were used for acquisition of peak force.
Statistical analysis
Data (means ± SD) were analyzed using SPSS software (V 10.0., SPSS, Chicago, Ill.). Based on previous findings in animals [11], we stated the research hypotheses that AP force (primary criterion) would be lower in patients with sepsis, whereas fatigue (secondary criterion) would not differ between patients and immobilized but not infected volunteers. In any case, classical tests for differences were performed (independent and paired sample t-tests) since equivalence tests generally require much larger sample sizes [22].
Using a hierarchical sequence, repetitive measurements at different nerve stimulation frequencies could be tested with an alpha error of 5% without adjustment for multiple testing [23]. The following sequence was defined: firstly, we tested whether force differs between patients and volunteers. Possible differences in fatigue were tested subsequently only if the hypothesis of lower muscle force in patients was rejected. With statistical comparison of both main criteria (force, fatigue) we tested first if variables differed at 10-Hz nerve stimulation frequencies; thereafter, subsequent tests were performed if, and only if, all previous tests were significant to compare variables at 20, 30, 40, 50, 60, 70, and 80 Hz, respectively.
A relevant weakness in patients was defined as a maximum generable force below the lower 95th percentile of the volunteers' mean maximum generable force [24].
The sample size estimation was based on in vitro data on muscle force in patients with sepsis and controls [25]. In particular, we took into account a difference in means of muscle force of 50% and an expected standard deviation of 30%.
Results
Evoked muscle force
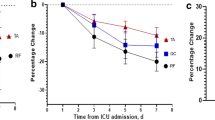
Muscle force was significantly (p < 0.02) lower at all stimulation frequencies in patients with sepsis than in volunteers after immobilization (Fig. 3). Maximum force before evoked fatigue averaged 8 ± 7 and 20 ± 16 N in patients with 10- and 80-Hz stimulation, respectively, vs 24 ± 11 and 65 ± 22 N in volunteers after immobilization (Fig. 3). Maximum force was abnormal (less than the lower 95th percentile of the volunteers' mean force, i.e., >24.2 N) in 10 of 13 patients.%

Force – frequency curves of adductor pollicis muscle during supramaximal ulnar nerve stimulation before performing the fatigue protocol (means ± SD). Force is significantly lower in patients with sepsis and multiorgan failure (full circles; n = 13) compared with healthy volunteers (triangles; n = 7) both before (open triangles) and after (solid triangles) 2 weeks of immobilization of their lower arm and thumb. ∗ p < 0.01 vs volunteers before and after immobilization. \# p < 0.05 vs volunteers before immobilization
In both patients and volunteers AP muscle force increased with stimulation frequency (p < 0.01), and maximum force generation was achieved at 30 and 50 Hz in patients and volunteers, respectively.
Maximum force was sustained even with the highest tetanic stimulation frequencies applied (Fig. 2, 3). CT10 / 90 (39 ± 9 vs. 39 ± 5 s) and RT0.5 (69 ± 17 s vs. 74 ± 15) did not differ between patients and volunteers after immobilization (Table 1). There was no apparent relationship between muscle force in patients and results of blood chemistry, requirements for vasoactive drugs, choice of antibiotic regimen, duration of mechanical ventilation, or SOFA score (Tables 2, 3).
Fatigue
The AP muscle force decreased significantly (p < 0.01) with all stimulation frequencies during the fatigue protocol, but the decrease in maximum force did not differ between patients with sepsis and MOF and healthy controls (Fig. 4). At 10-Hz nerve stimulation, however, the decrease in force after fatiguing contractions was slightly greater in patients compared with controls and averaged 28 ± 21% (patients) and 32 ± 13% (volunteers after immobilization; p < 0.05).

Effects of fatigue protocol on adductor pollicis muscle force in patients with sepsis and multiorgan failure, and in healthy volunteers after 2 weeks of immobilization of adductor pollicis muscle. Muscle force decreased significantly after 20 min of intermittent peripheral nerve stimulation (low-frequency fatigue protocol), without differences between groups. Effects on generated force of fatigue were significantly greater at low-frequency nerve stimulation. ∗ p < 0.01 vs muscle force at baseline before fatigue at the same stimulation frequency. \# p < 0.05 vs 10-Hz stimulation frequency in volunteers (immobilized)
In both patients and volunteers force after fatiguing contractions increased with increasing stimulation frequency (p < 0.0001), and amounted to 67 ± 15 and 62 ± 15% with 80-Hz stimulation, respectively (Fig. 4).
Electrophysiological measurements and muscle force
Twitch force and corresponding CMAP values of AP muscle were lower in patients than in volunteers after immobilization (4.0 ± 3.3 vs 12.6 ± 5.6 N, and 4.3 ± 5.2 vs 11.3 ± 1.7 mV, respectively, both p < 0.05). The CMAP was sustained with increasing stimulation frequency in volunteers. In contrast, in patients with sepsis, CMAP was sustained over the 10- to 40-Hz stimulation frequency range, but decreased significantly by 38 ± 20% (p < 0.01) with high-frequency stimulation (50–80 Hz). The CMAP did not decrease in patients or volunteers during fatiguing contractions. Nerve conduction velocity was unaffected in all patients and sensory nerve function was impaired only in a single patient.
Electrophysiological signs of CIP [20] were detected in 5 patients (38%), and analysis of this subgroup revealed lower CMAP values of AP muscle (1.6 ± 0.8 mV) compared with patients without CIP (4.6 ± 2.4 mV; p < 0.05). In all patients with CIP, and in 5 of 8 patients without CIP, muscle force was abnormal (i.e., lower than the 95th percentile of the controls' mean force). Muscle force of patients with CIP was also significantly lower (8.3 ± 6 vs 27 ± 16 N, at 80 Hz; p < 0.001) compared with the other patients without CIP.
Effects of lower arm immobilization
After 2 weeks of immobilization, muscle force was slightly increased (20 ± 12 vs 29 ± 13 N; p = 0.018; Fig. 3) with subtetanic stimulation (10 Hz), and this was paralleled by an increase of the twitch/tetanus ratio (32 ± 14 vs 43 ± 11%; p = 0.018), and an increase of CT10 / 90 (32 ± 2 vs 39 ± 5 ms; p = 0.018). Relaxation time (RT0.5) did not change during immobilization (63 ± 10 vs 69 ± 17 ms before vs after immobilization). Maximum force generation and endurance during fatiguing contractions were not significantly influenced by immobilization.
Patients' characteristics are given in Table 2. From the day of ICU admission onward, nutritional support was achieved in all patients with a standardized feeding schedule, intended to deliver 20–30 non-protein calories/kg per day, 1.0–1.5 g amino acids/kg day-1, vitamins, and trace elements. Insulin was administered to achieve normoglycemia [26], and 3 patients received low-dose hydrocortisol ( < 300 mg/d) during septic shock. Neuromuscular blocking agents were not given except once for percutaneous tracheotomy several days before measurements.
Discussion
In patients with sepsis AP muscle force is markedly impaired, but an increased fatigability was not observed, even in patients with CIP.
Methodological considerations
We compared skeletal muscle force and fatigue of patients with sepsis to those of healthy controls. Several variables, such as age, gender [27], as well as the history of muscular contractions before measurements [28], may influence force and fatigue. Moreover, immobilization can affect contractile properties of skeletal muscles and may decrease their maximum force [16, 29]. To compare the effects of immobilization plus sepsis on AP muscle function in our patients to that of immobilization alone, we compared the respective values of variables of patients to that of healthy controls after immobilization matched for age, weight, and gender. We observed immobilization-induced changes in contractile behavior as previously reported [16, 29, 30], i.e., an increase of force at 10-Hz stimulation frequency (Fig. 3) [30], as well as an increase of twitch/tetanus ratio axonal neuropathy [30], and contraction time [16, 29]. We did not observe a decrease in muscle force after 2 weeks of immobilization. While immobilization causes a reduction in maximal contraction force [16], our data are in accordance with the observation that a period of >3 weeks is required for these changes to occur [10].
In volunteers, we applied prilocaine for axillary plexus analgesia. This technique provides excellent analgesia during the painful tetanic nerve stimulation, and is not expected to influence muscle force after peripheral ulnar nerve stimulation [31, 32].
Impairment of muscle force
We found in patients with sepsis a marked decrease of skeletal muscle force to 30% of the controls. To our knowledge, there are only three studies which address quantitatively contractility of AP muscle in critically ill patients [25, 33, 34], but none of them provide in vivo data on maximum force and/or fatigue. Harris et al. [33] found a lower AP twitch tension in patients with "critical illness" compared with normal subjects, but the authors did not report data on maximum generable force and/or fatigue. Finn et al. [34] compared AP muscle function in patients with severe sepsis to control subjects, and found that the ratios of mean force generated at 10 Hz to that at 50 Hz was lower in patients with severe sepsis; however, the implications of that study are limited because the authors did not report data on maximum muscle force and fatigue [34].
There is only one study which reports on patients with sepsis maximum muscle force, i.e., force in isolated rectus abdominis muscle samples evoked by direct muscle stimulation [25], averaging 30% of the controls' value [25]. This compares very well with our in vivo data obtained after peripheral nerve stimulation, suggesting that skeletal muscle cell dysfunction during sepsis [25] may be a major cause of muscle weakness. This suggestion is also, at least in part, supported by our findings. Ulnar nerve conduction velocity was unaffected, and maximum force and compound motor action potential amplitude were sustained even with tetanic nerve stimulation up to 40 Hz. These results exclude a demyelinating neuropathy [20, 35] and neuromuscular transmission failure [36]; thus, muscle weakness and CMAP decrease may be explained by a myopathy and/or an axonal neuropathy [2]. We suggest that a sepsis-evoked myopathy may have been the predominant cause of muscle weakness in some (five) of our patients who did not have a CIP (i.e., CMAP and nerve conduction velocity were normal) but an abnormal muscle force.
In fact, it has been shown that muscle weakness in patients with sepsis can be induced by the effects of circulating TNF-α, which is elevated in patients with sepsis [37]. TNF-α decreases directly tetanic force of skeletal muscle [38], and also induces iNOS4, which mediates muscle weakness via local generation of peroxynitrite, a reactive oxidant formed by reaction of NO with superoxide [4, 11]. Furthermore, NO generation in skeletal muscle may also induce long-term effects that contribute to muscle weakness, i.e., increased muscle proteolysis by the ubiquitin-proteasom pathway [4, 39].
Bioenergetic failure has been suggested to be also an important mechanism underlying sepsis-associated multiorgan failure [40]; however, it is unlikely that this is relevant for impaired muscle force in our patients, as we did not observe an increase in contraction time that would be expected with a decrease of skeletal muscle energy supply [41].
Fatigue
Although muscle force was markedly impaired, we did not observe an increased fatigability in our patients. In accordance, it has been shown that atrophic muscles even may develop resistance to fatigue [42]. Fatigue resistance after immobilization can be explained, at least in part, by an immobilization-induced selective atrophy of type-2 (fast) twitch muscle fibers [43], which are susceptible to muscle fatigue [44]. Furthermore, immobilization may have protected against glycogen depletion. In fact, depletion of muscle glycogen and low blood glucose appear to be important contributing factors [45] of low-frequency fatigue, and muscle glycogen concentration increases during 2 weeks of immobilization [46].
Limitations
Our clinical study has some limitations. We evaluated in a population with severe multiple organ failure only one muscle, and it remains unclear whether the AP muscle represents the force and fatigue characteristics of other skeletal muscles. We fixed the time period of immobilization to 14 days in all volunteers. This matched the mean duration of sedation and mechanical ventilation of our patients, but did not match the full range of duration of patients' immobilization.
Potential clinical implications
Diagnosis of muscle weakness and fatigue in patients with sepsis and MOF is difficult, because clinical evaluation underestimates the true incidence of nerve and muscle dysfunction in ICU patients [1], and standard functional muscle tests such as assessment of maximal voluntary isometric contraction or assessment of muscle function by the Medical Research Council scale are not applicable when consciousness is impaired. Fortunately, skeletal muscle can be maximally activated with equal effectiveness by voluntary effort and by electrical stimulation [47]. Electrical stimulation of the AP muscle allows independent of patients' co-operation quantitative assessment of muscle force and fatigue.
Electrophysiological tests are recommended for the diagnosis of critical illness polyneuropathy [2, 20]; however, their impact has been questioned because the discriminatory value of these tests in identifying patients with clinically significant neuromuscular disorders may be low [1, 48]. In accordance, our data show that muscle weakness even occurs in half of the patients without electrophysiological signs of CIP; thus, evoked AP muscle force measurement may be more sensitive to detect relevant neuromuscular abnormalities in critically ill patients.
Our data demonstrate that peripheral muscle force is markedly decreased in patients with sepsis and MOF. In contrast, there is no evidence for an increased fatigability in these patients. Muscle weakness was most likely to due to sepsis/MOF-induced myopathy and/or axonal neuropathy, and not the result of an immobilization atrophy. Studies which compare longitudinally AP muscle force to standard electrophysiological tests are required to assess if evoked AP muscle force measurement is a viable method for the diagnosis of, or screening for, neuromuscular abnormalities in patients with sepsis and MOF.
References
Deem S, Lee CM, Curtis JR (2003). Acquired neuromuscular disorders in the intensive care unit. Am J Resp Crit Care Med 168:735–739
Hund EF (1996) Critical illness polyneuropathy clinical findings and outcomes of a frequent cause of difficult neuromuscular weaning. Crit Care Med 24:1328–1333
Deitch EA (1992) Multiorgan failure: pathophysiology and potential future therapy. Ann Surg 216:117–134
Zarzhevsky N, Menashe O, Carmeli E, Stein H, Reznick AZ (2001) Capacity for recovery and possible mechanism in immobilisation atrophy of young and old animals. Ann N Y Acad Sci 928:212–225
De Letter MA, van Doorn PA, Savekoul HF, Laman JD, Schmitz PI, Op de Coul AA, Visser LH, Kreos JM, van der Meche FG (2000) Critical illness polyneuropathy and myopathy (CIPNM): evidence for local immune activation by cytokine-expression in the muscle tissue. J Neuroimmunol 106:206–213
Riggs JE (1985) Adult-onset muscle weakness. How to identify the underlying cause. Postgrad Med 78:217–226
Edwards RHT (1981) Human muscle function and fatigue. Ciba Found Symp 1–18
Vollestad NK (1997) Measurement of human muscle fatigue. J Neurosci Methods 74:219–227
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–689
Polkey MI, Moxham J (2001) Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest 119:926–939
Boczkowski J, Lanone S, Ungureanu-Longrois D, Danialou G, Fournier T, Aubier M (1996) Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats: role on diaphragmatic contractile dysfunction. J Clin Invest 98:1550–1559
Eikermann M, Gerwig M, Beiderlinden M, Peters J (2004) Muscle force but not endurance is impaired in patients with sepsis and multiple organ failure. Anesthesiology 101:A-446
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Saeden B, Törnkwist H, Ponzer S, Höglund M (2001) Fracture of the carpal scaphoid. J Bone Joint Surg Br 83:230–234
Duchateau J, Hainaut K (1987) Electrical and mechanical changes in immobilised human muscle. J Appl Physiol 62:2168–2173
Eikermann M, Groeben H, Hüsing, J, Peters J (2004) Predictive value of mechanomyography and accelerometry for pulmonary function in partially paralyzed volunteers. Acta Anaesthesiol Scand 48:365–370
De Ruiter CJ, De Haan A (2000) Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflugers Arch 440:163–170
Vollestad NK, Sejersted I, Saugen E (1997) Mechanical behaviour of skeletal muscle during intermittent voluntary isometric contractions in humans. J Appl Physiol 83:1557–1565
Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ (1998) Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med 24:801–807
Moussavi RS, Carson PJ, Boska MD, Weiner MW, Miller RG (1989) Nonmetabolic fatigue in exercising human muscle. Neurology 39:1222–1226
Chinn S (2001) Statistics for the European Respiratory Journal. Eur Respir J 18:393–401
Bauer P (1991) Multiple testing in clinical trials. Stat Med 10:871–890
Lebowitz MD, Holberg CJ (1990) Comparisons of spirometric reference values and the proportions of abnormal subjects among male smokers and those symptomatic in a community population. Am Rev Respir Dis 141:1491–1496
Lanone S, Mebazaa A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J (2000) Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med 162:2308–2315
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in the critically ill patient. N Engl J Med 345:1359–1367
Ditor DS, Hicks AL (2000) The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol 78:781–790
Desmedt J, Hainaut K (1968) Kinetics of myofilament activation in potentiated contraction: staircase phenomenon in human skeletal muscle. Nature 217:529–532
Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL, Fitts RH (1998) Force velocity power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol 85:1949–1956
Seki K, Taniguchi Y, Narusawa M (2001) Alterations in contractile properties of human skeletal muscle induced by joint immobilisation. J Physiol 530:521–532
MacClean D, Chambers WA, Tucker GT, Wildsmith JAW (1988) Plasma prilocaine concentrations after three techniques of brachial plexus blockade. Br J Anaesth 60:136–139
Wali FA, Suer AH, Greenidge E, Tugwell AC, Hayter A (1987) Local anaesthetics inhibit influx of calcium, sodium and potassium into rat ileum, diaphragm and human isolated saphenous vein. Gen Pharmacol 18:351–355
Harris ML, Luo YM, Watson AC, Rafferty GF, Polkey MI, Green M, Moxham J (2000) Adductor pollicis twitch tension assessed by magnetic stimulation of the ulnar nerve. Am J Respir Crit Care Med 162:240–245
Finn PJ, Plank LD, Clark MA, Connolly AB, Hill G (1996) Asssessment of involuntary muscle function in patients after critical injury or severe sepsis. J Parenter Enteral Nutr 20:332–337
Rostami AM (1993) Pathogenesis of immune-mediated neuropathies. Pediatr Res 33:S90–S94
AAEM Quality Assurance Committee (2001) American Association of Electrodiagnostic Medicine. Literature review of the usefulness of repetitive nerve stimulation and single fiber EMG in the electrodiagnostic evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome. Muscle Nerve 24:1239–1247
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 9328:219–223
Hogan MC, Gladden LB, Grassi B, Stary CM, Samaja M (1998) Bioenergetics of contracting skeletal muscle after partial reduction of blood flow. J Appl Physiol 84:1882–1888
Spooner CE, Markowitz NP, Saravolaz LD (1992) The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol 62:11–17
Reid MB, Lannergren J, Westerblad H (2002) Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 166:479–484
Mitch WE, Goldberg AL (1996) Mechanism of muscle wasting. The role of the ubiquitin–proteasome pathway. N Engl J Med 335:1897–1905
Macaluso A, Vito G de (2004) Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91:450–472
Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E (2001) Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve 24:893–839
Karlsson J, Sjodin B, Jacobs I, Kaiser P (1981) Relevance of muscle fibre type to fatigue in short intense and prolonged exercise in man. Ciba Found Symp 82:59–74
Fitts RH (1994) Cellular mechanism of muscle fatigue. Physiol Rev 74:49–94
Grichko VP, Heywood-Cooksey A, Kidd KR, Fitts RH (2000) Substrate profile in rat soleus muscle fibers after hindlimb unloading and fatigue. J Appl Physiol 88:473–478
Bigland-Ritchie B, Jones DA, Woods JJ (1979) Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp Neurol 64:414–427
Craig M (2002) Electrophysiology adds little to clinical signs in critical illness polyneuropathy and myopathy. Crit Care Med 30
Acknowledgement
This work was supported by a grant (IFORES EIK-107-05350) from the Faculty of Medicine, Universität Duisburg-Essen, Germany
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eikermann, M., Koch, G., Gerwig, M. et al. Muscle force and fatigue in patients with sepsis and multiorgan failure . Intensive Care Med 32, 251–259 (2006). https://doi.org/10.1007/s00134-005-0029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-0029-x