Abstract
Objective.
Comparisons of urinary bladder, oesophageal, rectal, axillary, and inguinal temperatures versus pulmonary artery temperature.
Design.
Prospective cohort study.
Setting.
Intensive Care Unit of a University-Hospital.
Patients.
Forty-two intensive care patients requiring a pulmonary artery catheter (PAC).
Intervention.
Patients requiring PAC and without oesophageal, urinary bladder, and/or rectal disease or recent surgery were included in the study. Temperature was simultaneously monitored with PAC, urinary, oesophageal, and rectal electronic thermometers and with axillary and inguinal gallium-in-glass thermometers. Comparisons used a Bland and Altman method.
Measurements and main results.
The pulmonary arterial temperature ranged from 33.7 °C to 40.2 °C. Urinary bladder temperature was assessed in the last 22 patients. A total of 529 temperature measurement comparisons were carried out (252 comparisons of esophageal, rectal, inguinal, axillary, and pulmonary artery temperature measurements in the first 20 patients, and 277 comparisons with overall methods in the last patients). Nine to 18 temperature measurement comparisons were carried out per patient (median = 13). The mean differences between pulmonary artery temperatures and those of the different methods studied were: oesophageal (0.11±0.30 °C), rectal (−0.07±0.40 °C), axillary (0.27±0.45 °C), inguinal (0.17±0.48 °C), urinary bladder (−0.21±0.20 °C).
Conclusion.
In critically ill patients, urinary bladder and oesophageal electronic thermometers are more reliable than the electronic rectal thermometer which is better than inguinal and axillary gallium-in-glass thermometers to measure core temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In critically ill patients, temperature measurement is a routine care task and can lead to important decisions regarding investigations and treatments. Because the temperature of blood perfusing the hypothalamus is not currently measurable, many methods have been developed to measure temperature [1]. Historically, the rectal route was preferred to measure a patient's core temperature. However, rectal temperature significantly lags behind other core sites such as the oesophageal site, especially during acute temperature alterations [2, 3]. This method also poses the potential risk of rectal perforation and could be uncomfortable for the patient. Axillary and inguinal temperature measurements using classical mercury or gallium-in-glass thermometers have replaced rectal temperature measurement because they are easy to use. However, these methods do not correlate well with core temperature and do not allow continuous monitoring in intensive care units [3, 4]. Therefore, electronic thermometers have been developed to continuously monitor temperature in esophageal, rectal, urinary bladder, and oral sites [3, 5, 6, 7]. Furthermore, these methods allow a quick and easy measurement of core temperature. Core temperature can also be assessed using tympanic thermometers. However, this method is not available in every intensive care unit as it is time-consuming and requires trained operators [5, 8, 9]. Moreover, it has been shown that tympanic thermometry is not reliable in critically ill patients [10]. As many methods are available to measure core temperature in critically ill patients, we decided to compare temperature measurement using an electronic probe in rectal, esophageal, and urinary bladder sites and temperature measurement in inguinal and axillary sites versus pulmonary artery core temperature. The latter is considered as the "gold standard" for the measurement of core body temperature [3, 5, 6, 7, 10].
Materials and methods
This prospective study was approved by the local Ethics Committee and oral informed consent was obtained by the patient or his/her closest relative.
Patients
From October 1998 to May 2001, patients >18 years requiring a pulmonary artery catheter (PAC) monitoring were included in the study. Non-inclusion criteria were: patients <18 years, patients who did not give their informed consent, patients with a recent history of rectal, oesophageal or urinary bladder surgery, and patients with oesophageal and/or rectal varicose with potential risks of haemorrhage.
Temperature measurements
In each patient, PAC (OptiQ SvO2/CCO, Abbott critical care systems, Abbott Laboratories, North Chicago, Ill., USA) was inserted using a central venous catheterization. The correct position of the catheter tip was checked by chest radiography and the catheter was connected to the cardiac monitor (Qvue Continuous cardiac output computer, Abbott critical care systems) that allows the measurement of the temperature of blood in the pulmonary artery. Oesophageal and rectal temperatures were measured with electronic probes (YSI, Siemens, Sweden) that were connected to the monitor. The tip of the probe was inserted up to 20 cm into the oesophagus and into the rectum as normal procedure. However, the correct position was not checked by radiography to avoid excessive X-ray. The urinary bladder temperature was measured with a thermistor Foley catheter (Mon.a.therm Foley Temp, Mallinckrodt, Saint Louis, Mo., USA) that was connected to the monitor. Axillary and inguinal temperatures were measured with manual Gallium thermometers (Magnien SA, Saint Brice sous Forêt, France). Every 2 h they were placed in the inguinal and axillary sites by an aid-nurse and a nurse. After 2–3 min, they were removed and the temperatures were collected separately by a nurse and an aid-nurse to allow a blinded data collection. Urinary bladder temperature was not measured in the first 20 patients because the thermistor Foley catheter was not yet available.
Time of measurements
In each patient, the temperature measurements were performed within the first 48 h of pulmonary artery catheter insertion. Measurements were collected by nurses and aid-nurses every 2 h for 24 h. Nurses and aid nurses first collected the values of inguinal and axillary temperatures before collecting the values of oesophageal, rectal, urinary bladder, and pulmonary artery temperatures recorded on the screens of the monitor and cardiac output monitor.
Studied parameters
In each patient, age, gender, height, weight, and APACHE II score were recorded [12]. The requirements of mechanical ventilation, sedation, and inotropic and/or vasopressive drugs were also recorded. Finally, the patient's outcome (death or discharge from the unit) was recorded.
Statistical analysis
Results are expressed as the median value with 5th and 95th percentiles. To compare axillary, inguinal, oesophageal, rectal, and urinary bladder temperature versus pulmonary artery core temperature, a Bland and Altman method was used [13].
Results
During the study period, 909 patients were admitted in the unit. Among these, 81 required a pulmonary artery catheter. Twenty-eight patients were not included because of esophageal, rectal or urinary bladder disease or recent surgery. Six patients were excluded because of a moribund state and there was a protocol violation in five other patients (lack of temperature measurements with one of the studied methods). Therefore, 42 patients were included in the study. Urinary bladder temperature was assessed in the last 22 patients. Patients' characteristics, underlying diseases, and the use of sedation or vaso-active drugs are shown in Table 1.
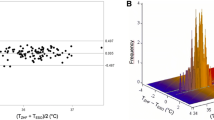
Five hundred and twenty-nine temperature measurement comparisons were carried out (252 comparisons of oesophageal, rectal, inguinal, axillary, and pulmonary artery temperature measurements in the first 20 patients and 277 comparisons of oesophageal, rectal, inguinal, axillary, urinary bladder, and pulmonary artery temperature measurements in the last 22 patients). The pulmonary arterial temperature ranged from 33.7 °C to 40.2 °C. Nine to 18 temperature measurement comparisons were carried out per patient (median = 13). The mean differences with pulmonary artery temperature were: esophageal (0.11±0.30 °C), rectal (−0.07±0.40 °C), axillary (0.27±0.45 °C), inguinal (0.17±0.48 °C), and urinary bladder (−0.21±0.20 °C). Bland and Altman diagrams are shown in Fig. 1.
Discussion
The present study shows that, using a Bland and Altman diagram, the mean differences with blood temperature of pulmonary artery were 0.11±0.30 °C, −0.07±0.40 °C, 0.27±0.45 °C, 0.17±0.48 °C, and −0.21±0.20 °C for oesophageal, rectal, axillary, inguinal, and urinary bladder temperatures, respectively.
The core temperature is routinely monitored in intensive care units. The value of the core temperature influences decision-making regarding potential investigation (for instance, blood culture) and treatment (for instance, anti-infective therapy). Historically, rectal temperature was first measured. However, it could be uncomfortable and painful for the patient [14]. Inguinal and axillary gallium glass thermometers, that are noninvasive, are also used. However, they do not allow a continuous monitoring of temperature. Therefore, electronic thermometers allowing a continuous measurement have been developed to permit a reliable measurement. The present study included patients in whom a pulmonary artery catheter was required. The severe state of these patients was confirmed by a high APACHE II score and the frequent association with respiratory and haemodynamic failures. Moreover, measured temperatures ranged from 33.7 °C to 40.2 °C as in other studies [3, 5, 7].
Although the temperature of blood perfusing the hypothalamus is the reference of core body temperature, the pulmonary artery temperature is considered as the gold standard in anesthesia and in the critical care unit [1, 3, 4, 5, 6, 7] because it has been shown to be closest to the temperature in the high internal jugular vein and core body temperature [11]. Moreover, the statistical analysis used a Bland and Altman method that has been recommended since 1986 to compare methods measuring the same clinical parameter [13].
The tympanic thermometer was not used in the present study. First, tympanic measurement does not allow a continuous monitoring of temperature and is less cost-effective than inguinal and axillary methods with gallium-in-glass thermometers. Second, several studies have demonstrated that tympanic measurements were not reliable compared to oesophageal and urinary bladder methods [3, 5, 7]. Third, Giuliano et al. [5] argued that tympanic measurement requires highly experienced nurses to accurately measure the temperature of the tympanic membrane. Electronic thermometers with disposable probe-covers for oral temperature measurement allow a rapid, easy, and accurate temperature measurement [5, 7]. However, as in the Unit, this method remains rarely used in critically ill patients [5,16].
Previous studies were performed to assess a new method and/or did not compare methods (tympanic and oral methods) that are not routinely used in critically ill patients [3, 5, 7]. Moreover, these studies involved small numbers of investigators who were trained before the study begun. In contrast, the present study assessed several temperature measurement methods that are routinely used in critically ill patients. Moreover, all nurse and aid-nurse staff were involved. Temperature measurements were carried out in the study as they were performed daily in the Unit. Nevertheless, results obtained with oesophageal, rectal, urinary bladder, and axillary methods were similar to those reported in studies assessing one of these methods. Biases ranged from 0.0±0.2 °C to 0.0±0.3 °C, from −0.8±0.3 °C to 0.1±1.4 °C, from 0.1±0.7 °C to 0.7±0.6 °C, and from 0.1±0.5 °C to 0.1±0.5 °C for urinary bladder, rectal, axillary, and oesophageal methods, respectively [3, 7, 17, 18, 19]. The discordance between axillary and inguinal methods could be explained by the initial (misplacement by nurse) or secondary (patient's movement) misplacement of the gallium-in-glass thermometer. When the thermometer was not placed directly over the femoral and/or axillary artery, deviations in temperature measurement occurred [3]. Therefore, we decided to not assess the repeatability of gallium-in-glass thermometers. Moreover, the assessment of repeatability would need numerous, nearly simultaneous, measurements that would not reflect actual daily clinical practice.
The reliability of the temperature measurement technique was not defined before the study. However, this definition has never been established. In clinical practice, Giuliano [5] and Robinson [3] considered that a method of temperature measurement was reliable when the standard deviation ranged from 0.3 °C to 0.5 °C. According to these assumptions, oesophageal and, especially, urinary bladder methods were the most accurate. However, other methods showed a standard deviation inferior to 0.5 °C. Whatever the standard deviation considered, this means that for a measured temperature, the actual temperature has a 95% chance to be in the limits of agreement (i.e., measured temperature ±2 SD, i.e., 0.6 °C or 1°C). For instance, for a measured temperature of 38 °C, the actual temperature has 95% chance to be between 37.4 °C and 38.6 °C or between 37 °C and 39 °C. Therefore, it could be concluded that in the most critically ill patients, the urinary bladder technique is preferable because its accuracy is ±0.4 °C. Oesophageal temperature could be an alternative when the urinary bladder temperature cannot be used because the oesophageal probe is easily inserted in intubated and sedated patients..
Nevertheless, the present study has some limitations. First, it involved the most critically ill patients requiring a pulmonary artery catheter and frequent mechanical ventilation and sedation. The use of a urinary catheter was required in this kind of patient. Electronic oesophageal and rectal thermometers could be easily inserted. However, rectal measurements could be affected by stools, and the proper positioning of the oesophageal probe could not be checked (ideally in the lowest quarter of the oesophagus) [20]. In the same way, the use of patched probes could perhaps decrease the bias and the standard deviation observed in axillary and inguinal sites. Second, the results cannot be extrapolated in patients breathing spontaneously. Although oxygen administration does not affect oral temperature measurements, we observed a great discordance between PAC and oesophageal temperature measurements in one patient breathing spontaneously. This finding could be explained by the cooling of a misplaced oesophageal probe by nasal oxygen administration. Therefore, in conscious and spontaneously breathing patients, methods such as tympanic, oral, axillary, and inguinal remain available even if their accuracy and reliability are inferior to urinary bladder and oesophageal thermometers.
References
Cork RC, Vaughan RW, Humphrey LS (1983) Precision and accuracy of intraoperative temperature monitoring. Anesth Analg 62:211–214
Molnar GW, Read RC (1974) Studies during open-heart surgery on special characteristics of rectal temperature. J Appl Physiol 36:333–336
Robinson J, Charlton J, Seal R, Spady D, Joffres MR (1998) Esophageal, rectal, axillary, tympanic, and pulmonary artery temperatures during cardiac surgery. Can J Anaesth 45:317–323
Schmitz T, Bair N, Falk M, Levine C (1995) A comparison of five methods of temperature measurement in febrile intensive care unit patients. Am J Crit Care 4:186–192
Giuliano KK, Scott SS, Elliot S, Giuliano AJ (1999) Temperature measurement in critically ill orally intubated adults: a comparison of pulmonary artery core, tympanic, and oral methods. Crit Care Med 27:2188–2193
Lilly JK, Boland JP, Zekan S (1980) Urinary bladder temperature monitoring: a new index of core body temperature. Crit Care Med 8:742–744
Erickson KS, Kirklin SK (1993) Comparison of ear-based, bladder, oral, and axillary methods for core temperature measurement. Crit Care Med 21:1528–1534
Terndrup TE, Rajk J (1992) Impact of operator technique and device on infrared emission detection tympanic thermometry. J Emerg Med 10:683–687
Peterson MH, Hauge HN (1997) Can training improve the results with infrared tympanic thermometers? Acta Anaesthesiol Scand 41:1066–1070
Staven K, Saxholm H, Smith-Erichsen N (1997) Accuracy of infrared ear thermometry in adult patients. Intensive Care Med 23:100–105
Eichna LW, Berger AR, Rader B, Buckaroo WH (1951) Comparison of intracardiac and intravascular temperatures with rectal temperatures in man. J Clin Invest 30:353–359
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307–310
Hasler ME, Cohen JA (1982) The effect of oxygen administration on oral temperature assessment. Nurs Res 31:265–268
Shinozaki T, Deane R, Perkins FM (1988) Infrared tympanic thermometer:,evaluation of a new clinical thermometer. Crit Care Med 16:148–150
Franchesi VT (1991) Accuracy and feasability of measuring oral temperature in critically ill adults. Focus Crit Care 18:221–228
Nierman DM (1991) Core temperature measurement in the intensive care unit. Crit Care Med 19:818–823
Erickson RS, Meyer LT (1994) Accuracy or infrared ear thermometry and other temperature methods in adults. Am J Crit Care 3:40–54
Romano MJ, Fortenberry JD, Autrey E, (1993) Infrared tympanic thermometry in the pediatric intensive care unit. Crit Care Med 21:1181–1185
Whitby JD, Dunkin LJ (1968) Temperature differences in the esophagus. Br J Anaesth 40:991–995
Acknowledgments.
The authors very sincerely thank nurses and aid-nurses for their kind and skilled assistance in temperature measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lefrant, JY., Muller, L., de La Coussaye, J.E. et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 29, 414–418 (2003). https://doi.org/10.1007/s00134-002-1619-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-002-1619-5