Abstract
Objective
To investigate whether the temperature recorded by an iThermonitor has better concordance with the core temperature than the bladder temperature recorded by a Foley catheter sensor in laparoscopic rectal surgery.
Methods
Eighty-two adults undergoing laparoscopic rectal surgery were enrolled. Temperatures were continuously measured by a distal oesophageal probe (the reference core temperature), axillary iThermonitor and Foley catheter sensor (bladder temperature) in each patient during surgery. Pairs of axillary and core temperatures or pairs of bladder temperature and core temperatures were compared and summarized using linear regression and the repeated-measured Bland–Altman method during the whole surgical period and pneumoperitoneum period.
Results
There were 3303 pairs of temperature measurements during the whole surgical period. The mean difference between iThermonitor and oesophageal was 0.05 °C ; the limits of agreement were − 0.48 to 0.56 °C. The mean difference between the oesophagus and bladder was 0.28 °C; the limits of agreement were − 0.39 to 0.94 °C (P < 0.001, F-test vs. iThermonitor). Ninety -five% of all iThermonitor values were within 0.5 °C of oesophageal temperature, whereas the proportion for oesophageal and bladder differences within 0.5 °C was only 84% (95% confidence interval 80–88%). Lin’s CCC for the iThermonitor and bladder measurements were 0.842 (95%CI: 0.831–0.851) and 0.688 (95%CI: 0.673–0.703) respectively. Similar results were found during the pneumoperitoneum period.
Conclusions
The temperature recorded by iThermonitor has better concordance with the core temperature than the bladder temperature recorded by Foley catheter sensor in laparoscopic rectal surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Maintaining perioperative normothermia is critical for surgical patients since both hypothermia and hyperthermia are closely related to several perioperative complications [1,2,3,4,5,6]. Inadvertent perioperative hypothermia occurs more often in clinical practice and is known to be associated with surgical site infection, coagulopathy, cardiovascular side events and delayed postanaesthetic recovery [1,2,3, 7]. Therefore, core temperature monitoring is essential for detecting thermal disturbances during the perioperative period. As a result, the monitoring of body temperature is included as one of the items in the surgical safety checklist of the World Health Organization guidelines (WHO 2015) [8].
During the perioperative period, especially during the intraoperative hours, monitoring the core temperature is relatively difficult [9]. The appropriate sites for core temperature monitoring include the pulmonary artery, distal oesophagus, nasopharynx, or tympanic membrane, of which the distal oesophagus is most often chosen during general endotracheal anaesthesia [10]. However, placing a probe in the distal oesophagus is an invasive method, thus precluding those patients with oesophageal disease or a coagulation dysfunction. Moreover, this method cannot be used in awake patients or when there is a blocked supraglottic airway.
If the sites mentioned above are not feasible in some circumstances, there are various sites thought to estimate the core temperature, such as oral, axillary and bladder sites. The oral method is not suitable as an intraoperative measurement but is good as a postoperative measurement. On the other hand, kidneys receive about 25% of the cardiac output; therefore, if the urine flow rate is within a normal range, the urinary bladder temperature would closely match the core temperature. To facilitate bladder temperature measurement, special Foley catheters have been invented with temperature sensors. Once the catheter has been inserted, temperature data can be obtained. Many anaesthesiologists prefer bladder temperature measurement during perioperative period, because the insertion of an indwelling urinary catheter is required in most major surgeries. However, the bladder temperature might have poor concordance with the core temperature during laparoscopic surgery due to decreased renal perfusion [10, 11]. Yet few studies have investigated the concordance between bladder temperature and core temperature during laparoscopic surgeries.
The new wireless axillary thermometer, iThermonitor WT701 (Raiing Medical, Beijing, CN), is an innovative continuous temperature monitoring device that uses a high-precision thermistor probe attached to the axillary root (lateral body surface of the axillary artery), a printed circuit board with Bluetooth chip, an embedded algorithm based on amount of experimental data accumulation historically to predict and correct core body temperature. Its accuracy has been recognized as “near core” due to its compensation algorithm in many clinical scenarios [12]. The compensation algorithm contains two parts, one is the basic noise-reduction algorithm (filtered and anti-interfered), and the other is an innovative algorithm based on large samples of different types of data in anesthesia environment, such as room temperature, human bladder, esophageal, axillary temperature experimental data from different parts of human body structure. The current study investigated whether the temperature recorded by an iThermonitor has better concordance with the core temperature than the bladder temperature recorded by a Foley catheter sensor in laparoscopic rectal surgery.
2 Methods
2.1 Study design and participants
The study was a prospective observational study. Eighty-two adults undergoing laparoscopic rectal surgery were enrolled from September 2020 to May 2021 in a Chinese tertiary, referral teaching hospital. We excluded patients in whom oesophageal temperature monitoring was impractical. This study was approved by KY20202116-C-1 from Approval Form I E C of our hospital. Participating patients provided written consent.
2.2 Intraoperative management
The attending anaesthesiologist was free to autonomously choose the type of general anaesthesia (balanced, total intravenous, volatile). The respiratory, neuromuscular and pain management, as well as the fluid therapy, were performed according to the standard ERAS (Enhanced Recovery After Surgery) protocol. Intravenous infusions were given through an arm vein, and the fluids were warmed or not per anaesthesiologist preference. An arterial catheter was inserted into either wrist. All patients were supine with both arms adducted during surgery. The ambient temperature was kept between 22 and 25 °C.
2.3 Measurements
The patient characteristics such as age, height, weight, sex, and the American Society of Anaesthesiologists physical status were recorded, along with the details of the surgery, including the duration of procedure, whether there was pneumoperitoneum, and the fluid or transfusion volume. The pressure of pneumoperitoneum was set to be lower than 15 mmHg.
The standard monitoring of vital signs during general anaesthesia was integrated with the core temperature monitoring. The axillary temperature was recorded from an iThermonitor wireless module. A hypoallergenic adhesive patch provided by Raiing Medical was used to securely position the iThermonitor in the axilla before anaesthesia induction (Fig. 1). After the induction of general anaesthesia, an oesophageal probe (Model 21,075 A; Philips Medical Systems, Andover, MA) was inserted through a nostril under laryngoscopic vision with approximately 30–35 cm. A 14 F Foley catheter with a temperature sensor (Model 12,511,407; Jinhu Industrial Estate Hualong, Pan Yu, Guangdong, China) was placed into the urinary bladder. The oesophageal thermometers were removed at the end of surgery. The Foley catheter and axillary thermometers were removed or retained per the clinical need of the patient. Temperatures were recorded at 5-minute intervals throughout surgery.
2.4 Data analysis
We considered the oesophageal temperature to be the reference core temperature. We defined ± 0.5 °C to be a clinically important temperature deviation. This value has been used in past studies and corresponds to the normal circadian variation of ± 0.5 °C [13,14,15]. As the primary outcome measure, the proportion of temperatures within 0.5 °C of the reference was calculated for each patient and is summarized as the mean of the proportion of the patients with the 95% confidence interval (CI), and this was estimated by bootstrapping, accounting for within-in patient correlation. From a clinical point of view, a proportion greater than 90% was considered sufficiently accurate. The secondary outcome measures were the bias (iThermonitor or bladder temperature minus oesophageal temperature) and 95% CIs determined by the repeated-measures Bland–Altman analysis. To compare the accuracy of the iThermonitor bias with the bladder, an F-test was performed. The concordance correlation coefficient, which summarizes the agreement between 2 methods by measuring the variation of their linear relationship from the 45° line through the origin,[16] was estimated via variance components of a linear mixed model using restricted maximum likelihood approach [17, 18]. Assuming that the bias of iThermonitor is 0.15 with an SD of 0.3, the 95% CI for proportion of measurements within 0.5 °C would be from 0.81 to 0.85 and the 95% CI for concordance correlation coefficients would be from 0.78 to 0.86 with 80 subjects and an average of 30 measurements each.
3 Results
We enrolled and analysed 82 qualified patients. All of them completed the study and were included in the final analysis. The demographic and perioperative characteristics are summarized in Table 1.
3.1 Patient characteristics and surgical information
The mean age of the patients was 60 ± 11 (SD) years, and the median intraoperative recording duration was 187.2 ± 67.7 (SD) min. Among the 82 patients, 37 patients (45%) were female. The mean volumes of peritoneal lavage and intraoperative fluid therapy were 736.7 ± 448.1 ml and 2286.9 ± 632.1 ml, respectively.
3.2 Analysis of the difference between the iThermonitor or the bladder temperature and the reference measurements
3.2.1 During the whole surgical period
A total of 3303 sets of iThermonitor and reference measurements were obtained from 82 patients (40 sets per patient on average). As shown in Fig. 2, the iThermonitor temperature and the reference oesophageal temperature agreed well overall. The bias was 0.05 °C with an SD of 0.26, and the Pearson correlation Coefficient was 0.849 (Table 2). We also obtained 3303 pairs of bladder and reference measurements. The bias between them was 0.28 °C with an SD of 0.34 (Table 2), which was more than 5 times that between the iThermonitor and reference values (P < 0.001, F-test).
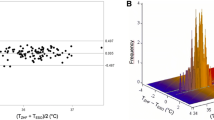
The 95% limits of agreement (LOA) for the iThermonitor were relatively narrow, with an estimated upper limit of 0.56 °C and the lower limit of − 0.48 °C, indicating reasonably good ± 0.51 °C agreement across the range of mean temperatures from 35.1 to 38.3 °C (Fig. 3). The proportion of patients with an average difference within ± 0.5 °C was 95.21% (95% CI, 91.88–98.55%) (Table 2). For the bladder temperature, the proportion of values that had an average difference within 0.5 °C was only 83.90% (95% CI, 80.27-87.52%) (Table 2).
(A) Bland-Altman chart for iTh VS esophageal temperature and bladder VS esophageal temperature during whole surgical period; (B) Bland-Altman chart for iTh VS esophageal temperature and bladder VS esophageal temperature during pneumoperitoneum period. The x-axis shows the average of the experimental and reference temperatures; the y-axis is the difference between the 2 measurements. The solid line shows mean bias; the dashed lines show 95% limits of agreement; and shaded area shows the 95% for limits of agreement
The iThermonitor detected fever with a sensitivity of 0.92 (95% CIs 0.74–0.99) and a specificity of 1 (0.999–1.00). Sensitivity and specificity for hypothermia were 0.724 (0.683–0.763) and 0.922 (0.911–0.932), respectively. The bladder sensor detected fever with a sensitivity of 0.72 (95% CIs 0.506–0.879) and a specificity of 1 (0.999–1.00). Sensitivity and specificity for hypothermia were 0.946 (0.922–0.964) and 0.765 (0.749–0.78), respectively. Lin’s CCC was excellent for correlation of the iThermonitor measurements with oesophageal temperature (CCC = 0.842 (95%CI: 0.831–0.851)); in contrast, CCC for the bladder measurements was only 0.688 (95%CI: 0.673–0.703).
3.2.2 During pneumoperitoneum period
During the pneumoperitoneum period, 2024 sets of iThermonitor, bladder and reference measurements were obtained. The bias between the iThermonitor and reference was 0.04 °C with an SD of 0.27 °C; the limits of agreement were − 0.48 to 0.56 °C (Table 2; Fig. 3). Whereas the bias between the bladder and reference measurements was as high as 0.32 °C with an SD of 0.38 °C (P < 0.001, F-test vs. iThermonitor), the limits of agreement were − 0.42 to 1.05 °C (Table 2; Fig. 3). A total of 93.62% of iThermonitor values were within ± 0.5 °C of the reference, and the data for the bladder were only 83.92% (Table 2). Lin’s CCC for the iThermonitor and bladder measurements were 0.785 (95%CI: 0.768–0.801) and 0.603 (95%CI: 0.581–0.624) respectively.
4 Discussion
A total of 95.21% of iThermonitor measurements were within ± 0.5 °C of the reference core temperature during the whole surgical period. During the pneumoperitoneum period, the proportion was approximately 94%. From a clinical point of view, a proportion greater than 90% was considered sufficiently accurate. Therefore, we consider the axillary iThermonitor to be sufficiently accurate for clinical use during laparoscopic rectal surgeries. For the bladder temperature, both of the proportions during the whole surgical and pneumoperitoneum periods were approximately 83.9%, which were lower than 90%. Therefore, the Foley catheter sensor is not sufficiently accurate for temperature monitoring during laparoscopic rectal surgery.
Temperature monitoring via the urinary bladder has become common in the operating room. Especially in surgery when the insertion of an indwelling urinary catheter is required, the bladder temperature measurement is considered an appropriate choice for temperature monitoring [19, 20]. Several previous studies have demonstrated that the bladder temperature measured by a Foley catheter sensor was a clinically acceptable method to monitor the core temperature during abdominal surgery [19, 21,22,23]. However, this method had a poor performance in determining the core temperature in the situations where there was a low urine flow rate or a rapid thermal change. In laparoscopic surgeries where a pneumoperitoneum is required prior to the procedure, the bladder temperature might respond more significantly than the core temperature. From our results, we consider that the urinary bladder temperature is inappropriate for patients undergoing laparoscopic rectal surgery.
In general, the axilla is not considered the ideal site for core temperature measurement. However, the wireless axillary iThermonitor in our study uses a proprietary system to adjust for the ambient temperature, thermal condition of the skin interface and changes in arm position, and it tries to estimate the core temperature. Our results showed that the axillary iThermonitor measurements accurately estimated the core temperature during the whole surgery and during the pneumoperitoneum periods.
Besides the monitoring tool we assessed in this study, we have searched clinical and technical articles in which other novel non-invasive core body temperature thermometry solutions were used and assessed. We realize that more studies should be conducted to explore a certain non-invasive measurement tool’s applicability and accuracy in a wider range of clinical settings but not just different types of surgery, i.e. Intensive Care Unit (with longer durations and wider temperature ranges) or more heat stress exposure (more rapid temperature changes and external influences). For example, Zero-Heat Flux Thermometer applied on the forehead (SpotOn™, 3 M, Minnesota, USA) had a bias of 0.06 ± 0.45 °C and 95% limits of agreement of -0.83 to 0.95 °C compared to the esophageal probe in post-cardiac arrest care scenario (Targeted Temperature Management (TTM)) in intensive care unit [24].The “Dräger” Double Sensor thermal measurement device has a better agreement with rectal temperature during the heat exposure phases than during resting under comfort conditions [25].
This study had several limitations. First, we postulated that bladder temperature showing poor agreement with core temperature during laparoscopic surgery was due to decreased kidney perfusion and influence of CO2 pneumoperitoneum temperature. However, we didn’t give evidence to support it. Moreover, we did not use error grid analysis to compare these two methods, which is thought to provide much information on the clinical relevance of the respective statistical findings. We will construct the error grid for core temperature monitoring basing on expert’s opinions in the future study.
In summary, we demonstrated the hypothesis that the concordance between the axillary iThermonitor measurements and the core temperature is better than that between the urinary bladder temperature and the core temperature in laparoscopic rectal surgery. The axillary iThermonitor measurements but not the bladder temperature measurements represent the core temperature well in that circumstance.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to statutory provisions regarding data and privacy protection.
References
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–15.
Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–92.
Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–23.
Spiotta AM, Stiefel MF, Heuer GG, et al. Brain hyperthermia after traumatic brain injury does not reduce brain oxygen.2008; 62: 864–72.
Kotani N, Kushikata T, Matsukawa T, et al. A rapid increase in foot tissue temperature predicts cardiovascular collapse during anaphylactic and anaphylactoid reactions. Anesthesiology. 1997;87:559–68.
Davis M, Brown R, Dickson A, et al. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br J Anaesth. 2002;88:508–15.
Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277(14):1127–34.
World Health Organization. WHO guidelines for safe surgery. apps.who.int/iris/bitstream/10665/44185/1/9789241598552_eng.pdf 2008.
Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655–64.
Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21(2):152–60.
Horrow R. Does urinary catheter tempeature reflect core temperature during cardiac surgery? Anesthesiology. 1988;69(6):986–9.
Hong SH, Lee J, Jung J-Y, Shim JW, Jung HS. Simple calculation of the optimal insertion depth of esophageal temperature probes in children. J Clin Monit Comput. 2020;34:353–9.
Sessler DI. Perioperative Temp Monit Anaesthesiol. 2021;134(1):111–8.
Eshraghi Y, Nasr V, Parra-Sanchez I, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014;119:543–9.
Langham GE, Maheshwari A, Contrera K, You J, Mascha E, Sessler DI. Noninvasive temperature monitoring in post-anesthesia care units. Anesthesiology. 2009;111:90–6.
Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68.
Carrasco JL, King TS, Chinchilli VM. The concordance correlation coefficient for repeated measures estimated by variance components. J Biopharm Stat. 2009;19:90–105.
Carrasco JL, Phillips BR, Puig-Martinez J, King TS, Chinchilli VM. Estimation of the concordance correlation coefficient for repeated measures using SAS and R. Comput Methods Programs Biomed. 2013;109:293–304.
Tayefeh F, Plattner O, Sessler DI, Ikeda T, Marder D. Circadian changes in the sweating-to-vasoconstriction interthreshold range. Pflugers Arch. 1998;435:402–6.
Lilly B, Zekan. Urinary bladder temperature monitoring: A new index of body core temperature. Crit Care Med 198;8(12): 742–744.
Earp F. Urinary bladder/pulmonary artery temperature ratio of less than 1 and shivering in cardiac surgical patients. Am J Crit Care. 1992;1(2):43–52.
Stone JG, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed. Anesthesiology. 1995;82(2):344–51.
Russell F. Comparison of bladder, oesophageal and pulmonary artery temperatures in major abdominal surgery. Anaesthesia. 1996;51(4):338–40.
Fiorini K, Tamasi T, Dorie J, Hegazy AF, Lee T-Y, Slessarev M. Non-Invasive Monitoring of Core Body Temperature for Targeted Temperature Management in Post-Cardiac Arrest Care. Front Med. 2022;9:810825.
Mazgaoker S, Ketko I, Yanovich R, Heled Y, Epstein Y. Measuring core body temperature with a non-invasive sensor. J Therm Biol. 2017;66:17–20.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Dr. Huang Nie and Yanran Dai conceptualized and designed the study. Huang Nie and Mengjia Luo drafted the manuscript. Xiangying Feng and Feifei Liu performed data collection. Yanran Dai and Mengjia Luo performed data analysis. All authors interpreted the data.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Y., Luo, M., Liu, F. et al. Temperature measurements of a wearable and wireless axillary sensor iThermonitor but not a bladder probe represents the core temperature during laparoscopic rectal surgery. J Clin Monit Comput 37, 303–309 (2023). https://doi.org/10.1007/s10877-022-00892-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00892-4