Abstract
Background
Knee osteoarthritis (KOA) is a degenerative joint disease leading to pain and disability for which no curative treatment exists. Intra-articular (IA) therapies are part of this multimodal approach and are approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA). Platelet-rich plasma (PRP), hyaluronic acid (HA), and corticosteroids (CS) have been increasingly used in recent years to treat KOA.
Purpose
To determine whether IA-PRP was superior to IA-HA or IA-CS administration routes in these patients.
Material and methods
In this trial the patients were randomized to IA-HA (2 ml/week, for 3 weeks), IA-CS (1 ml) or IA-PRP (3 times, 4 ml, every 3 weeks) groups. The outcome was assessed using the Western Ontario and McMaster Universities (WOMAC) score prior to the first injection and then at 3, 6, 9 and 12 months. Pain was evaluated by a visual analogue scale (VAS) prior to treatment and after 12 months.
Results
In this study 120 patients were randomized into 3 groups. There was a significant improvement in all scores (WOMAC, VAS) in each group compared to the pretreatment values (P < 0.05). The mean WOMAC scores for the IA-HA group from pretreatment to 3, 6, 9, and 12 months were 47.23 ± 5.37, 25.02 ± 4.98, 26.38 ± 5.20, 27.86 ± 4.34, and 30.64 ± 8.36, respectively. Similar improvements were noted in the IA-CS and IA-PRP groups. There were no significant differences in the WOMAC scores between the 3 groups 3 months after treatment (P > 0.05) but IA-PRP showed significantly lower scores 6, 9 and 12 months after treatment (P < 0.05).
Conclusion
Intra-articular PRP injections into the knee for symptomatic early stages of KOA are a valid treatment option. The clinical efficacy of IA-PRP is comparable to that of the IA-HA and IA-CS forms after 3 months and the long-term efficacy of IA PRP is superior to IA-HA and IA-CS.
Zusammenfassung
Hintergrund
Kniearthrose ist eine degenerative Gelenkerkrankung, die mit Schmerzen und Einschränkungen einhergeht, für die es keine kurative Behandlung gibt. Intraartikuläre (IA) Therapien sind Teil dieses multimodalen Therapieansatzes und wurden von der Food and Drug Administration (FDA) und der Europäischen Arzneimittel-Agentur (EMA) zugelassen. Plättchenreiches Plasma (PRP), Hyaluronsäure (HA) und Kortikosteroide (CS) wurden in den letzten Jahren zunehmend zur Behandlung der Kniearthrose verwendet.
Zweck
Es sollte festgestellt werden, ob bei diesen Patienten IA-PRP einer Behandlung mit IA-HA oder IA-CS überlegen ist.
Material und Methoden
In dieser Studie wurden die Patienten in eine IA-HA-Gruppe (2 ml/Woche für 3 Wochen), eine IA-CS-Gruppe (1 ml) oder eine IA-PRP-Gruppe (3-mal 4 ml alle 3 Wochen) randomisiert. Die Ergebnisse wurden vor der ersten Injektion und dann nach 3, 6, 9 und 12 Monaten mittels des Western Ontario and McMaster Universities (WOMAC) Score ausgewertet. Die Schmerzen wurden vor der Behandlung und 12 Monate danach mittels der visuellen Analogskala (VAS) bewertet.
Ergebnisse
In dieser Studie wurden 120 Pateinten in 3 Gruppen randomisiert. In allen Scores (WOMAC, VAS) zeigte sich eine signifikante Verbesserung in jeder Gruppe im Vergleich zu den Werten vor der Behandlung (p < 0,05). Die mittleren WOMAC-Scores der IA-HA-Gruppe vom Zeitpunkt vor der Behandlung bis zu 3, 6, 9 und 12 Monaten betrugen 47,23 ± 5,37, 25,02 ± 4,98, 26,38 ± 5,20, 27,86 ± 4,34 bzw. 30,64 ± 8,36. Ähnliche Verbesserungen wurden in der IA-CS- und IA-PRP-Gruppe festgestellt. Es gab keine signifikanten Unterschiede in den WOMAC-Scores zwischen den 3 Gruppen 3 Monate nach der Behandlung (p > 0,05), aber IA-PRP zeigte signifikant niedrigere Scores 6, 9 und 12 Monate nach der Therapie (p < 0,05).
Schlussfolgerung
Intraartikuläre PRP-Injektionen ins Kniegelenk im symptomatischen Frühstadium einer Kniearthrose stellen eine valide Behandlungsoption dar. Die klinische Wirksamkeit von IA-PRP ist vergleichbar mit der Wirksamkeit der IA-HA- und IA-CS-Therapie nach 3 Monaten. Bezüglich der Langzeit-Wirksamkeit ist IA-PRP der Behandlung mit IA-HA und IA-CS überlegen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis of the knee (KOA) is a common condition associated with pain and morbidity [1]. The increasing number of patients with symptomatic KOA will continue to place an increasingly larger economic burden on global healthcare systems [1]. The third National Health and Nutrition Survey of the USA showed that the prevalence of symptomatic KOA was 12.1%, similar to that in Europe [2]. According to the latest Chinese epidemiological survey data, the prevalence of symptomatic KOA in China was 8.1%. This means that China currently has approximately 110 million KOA patients [3]. The prevalence of KOA increases gradually with age. The incidence of patients under 50 years of age is 5.2%, while it has reached 11% among those over 60 years old (Fig. 1 [4]).
Knee arthroplasty is a reliable and successful surgical treatment to address end-stage KOA. Unfortunately, the cost of and time delay to knee replacement is potentially prohibitive in some countries. In the USA potential overutilization of arthroplasty is being met with increasing scrutiny with respect to preoperative nonsurgical treatment [5]. This includes both nonpharmacological and pharmacological approaches. Intra-articular (IA) corticosteroid and viscosupplementation injections have successful, albeit short-term benefits according to several meta-analyses [6, 7], randomized controlled trials [8, 9] and large retrospective studies [10, 11]. Injections of hyaluronic acid (HA) were found to cure mild to moderate OA in patients while platelet-rich plasma (PRP) knee injections also exist in clinical trials. Clinically, the comparative efficacy and effectiveness of IA injections of PRP, HA, and CS in the treatment of KOA are unclear and controversial. Moreover, no study has directly and concurrently compared IA-HA, IA-CS and IA-PRP in early KOA. A prospective, randomized, controlled trial was therefore performed to primarily compare the efficacy of pain reduction with IA-HA, IA-CS and IA-PRP in KOA. It was hypothesized that IA-PRP would be the optimal IA administration method for the treatment of KOA.
Material and methods
This study was approved by the Ethics Committee of the Jining NO.1 People’s Hospital of Shandong.
Patients
This was a prospective, randomized study initiated in May 2016. Out of 265 patients, 120 that met the inclusion criteria received IA-HA, IA-CS and IA-PRP injections into the knee for early stages of OA (Kellgren–Lawrence grade 1–2) [12].
Inclusion criteria
Patients with symptomatic KOA (Kellgren-Lawrence grade 1–2 on radiographs) between the ages of 40 and 65 years, having a body mass index (BMI) < 30, with stable knees without malalignment or maltracking of the patella were included in the study. Additional inclusion criteria were patients having pain with no relief using anti-inflammatory agents even after 3 months, normal blood results and coagulation profile (platelets 150,000–450,000/l), patients who had not undergone any surgery on the affected knee within 2 years prior to the first injection and zero, traces or 1+ effusion on the grading scale based on the Stroke test [13].
Exclusion criteria
Patients diagnosed with tricompartmental OA, rheumatoid arthritis or concomitant hip OA were not included in the study. A previous high tibial osteotomy or cartilage transplantation procedure, grades 2+ and 3+ effusion in the knee joint (requiring aspiration) based on the Stroke test, blood diseases, systemic metabolic disorders, immunodeficiency, hepatitis B or C, HIV positive status, local or systemic infection, ingestion of anti-platelet medication within 7 days prior to the injection and treatment with IA or oral corticosteroids in the 3 months prior to the first injection were considered criteria for exclusion in addition to patients who refused to participate.
PRP preparation
Samples of 8 ml blood were obtained from the cubital vein and centrifuged for 5 min at 1500 g centrifugal force (RCF) or 3500 pm as per the recommendations of the manufacturer. This system did not use a second centrifugation process. Centrifugation of whole venous blood takes advantage of differing density gradients of the components in blood to concentrate platelets. Erythrocytes, which are most dense, remain as the packed cell layer at the bottom of the centrifuge container. The buffy coat of white blood cells is above this while the platelets are at the highest concentration in the plasma just above the buffy coat and decrease in concentration towards the top of the plasma layer. After centrifugation, platelet recovery was >80% (twofold increase) and total leucocyte concentration was below the normal level-specific granulocyte depletion >95% in 4 ml of PRP. Leucocyte poor-PRP (LP-PRP) was obtained according to Dohan Ehrenfest et al. classification [14] which was P2 Bb as per the PAW classification [15]. The PRP was aspirated into a syringe and a topical anesthetic skin refrigerant was applied locally before IA infiltration by a suprapatellar approach using sterile aseptic precautions. The PRP was activated in vivo when the platelets were exposed to collagen or von Willebrand factor, leading to aggregation. After treatment, patients were allowed weight bearing and local ice application was recommended for 20 min every 2–3 h for 24 h. Vigorous activities of the knee were not recommended for 48 h.
HA and CS preparation
The IA injection of HA (sodium hyaluronate, molecular weight 500 – 730 kDa) was provided by the biochemical industry corporation (SK chemical research co., LTD, Tokyo, Japan) and 2 ml was injected into the knee of the patients each week for 3 weeks; IA injection of 1 ml CS produced by Shanghai Schering-Plough pharmaceutical company (Shanghai, China) was similarly injected into the knee. The injection method was same as for the IA-PRP group and after treatment patients were allowed weight bearing and local ice application was recommended for 20 min every 2–3 h for 24 h. Vigorous activities of the knee were not recommended for 48 h. All operations in the same laminar flow room were administered by the same group of persons for IA injection.
Outcome measures
Outcome following treatment was assessed using the Western Ontario and McMaster Universities (WOMAC) and visual analogue scales (VAS, 0 = no pain up to 10 = worst possible pain) [15] scoring systems which were recorded through questionnaires completed by the patients prior to the first injection and then at 3, 6, 9 and 12 months follow-up. Data were recorded in SOCRATESTM (2012, Ortholink PTY Ltd., Balmain, New South Wales, Australia) orthopedic outcomes software. Any adverse events occurring within 12 months postoperatively were recorded at the time of follow-up.
Statistical analysis
Distributions of demographic data, baseline data, and outcomes were assessed using measures of central tendency (mean, standard deviation) for quantitative variables and with percentages for qualitative variables. The general linear model for repeated measurement tests was performed to investigate within time variations for the continuous variables (WOMAC, VAS) for all patients and each evaluated subgroup. Categorical variables were compared using the χ2 and Fisher’s exact tests. All data analyses were performed using SPSS for Windows, Version 19.0 (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0.05.
Results
Patient demographics
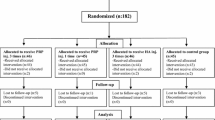
During the recruitment period from May 2016 to October 2017 a total of 265 patients were scheduled to receive IA injections into the knee and were systematically followed up from the start of treatment. Among these patients, 84 were ineligible, while 61 were excluded from participation. Hence, the trial was completed with 120 patients. No patients were lost or excluded during the follow-up (Fig. 2).
The mean patient age (and standard deviation) was 54.5 ± 1.2 years, 65 patients (54%) were men and 55 (46%) were women. The mean patient BMI was 24.75 ± 3.62 kg/m2. Baseline characteristics were comparable among the allocation groups (Table 1).
WOMAC scores
There was a significant improvement in WOMAC scores at each follow-up compared to the pretreatment value (P < 0.05). When comparing the effect of treatment between the 3 groups after 3 months they showed similar and significant improvement (P > 0.05); however, the results at 6, 9, and 12 months in the IA-PRP group showed a significant difference to the IA-HA and IA-CS groups and with a greater improvement in results in the patients (P < 0.05) (Table 2; Fig. 3).
VAS pain scores
The VAS scores decreased from 4.52 at baseline assessment to 2.14 at 12-month follow-up for the IA-HA group. For the IA-CS group they decreased from 4.64 to 2.26 and in IA PRP group the VAS scores decreased from 4.57 to 1.98. The benefit in pain reduction as measured by the VAS was significant (P < 0.05) in the IA-HA, IA-CS and IA-PRP groups compared to pretreatment (Table 3).
From pretreatment to the final follow-up, none of patients sustained low-grade fever, a deep venous thrombosis (DVT) or an infection (Table 4). Mild complications such as pain, nausea, and dizziness, which were of short duration, were observed in 2 patients (1.7%) in the IA-HA group, 3 patients (2.5%) in the IA-CS group, and 5 patients (4.2%) in the IA-PRP group (Table 4). These conditions were generally relieved after 24 or 48 h.
Discussion
It was determined that IA-PRP injections significantly improved the clinical outcomes in symptomatic KOA. The use of PRP was also shown to be significantly better than HA or CS for the treatment of symptomatic KOA at the time of follow-up. Treating OA nonoperatively has been ongoing for several decades. Multiple studies have reported the use of HA, PRP, and corticosteroids, among other agents, in the nonoperative treatment of OA. While there are a number of studies documenting the use of HA or CS in the treatment of OA, there are limited studies documenting the use of PRP for the same purpose. More importantly, there are very limited studies comparing the use of PRP with that of HA or CS in the treatment of KOA. To address these concerns, this prospective, randomized, controlled study was conducted to compare the similarities and difference between the three groups.
Current treatments focus on pain reduction, exercise therapy and in end-stage OA joint replacement. No curative treatment exists for OA. Since joint arthroplasties have a limited lifespan, there is a great need for disease-modifying drugs or therapies in the early stages. Therefore, a biological therapy for tissue injury that has emerged in recent years is treatment with PRP, which is a plasma product extracted from whole blood that contains at least 1.0 × 106 platelets per µl [16]. The platelets undergo degranulation, after which they release growth factors and cytokines such as transforming growth factor beta (TGF-beta), platelet-derived growth factor (PDGF) [16,17,18], insulin-like growth factor-1 (IGF-1), basic fibroblast growth factor and vascular endothelial growth factor (VEGF). Both PDGF and TGF-beta are two important factors in tissue healing. From preclinical research it is known that PRP promotes the proliferation of cells derived from human synovium and cartilage [19, 20] and that PRP-treated chondrocytes repair cartilage better than nontreated chondrocytes [21]. These cells in turn produce more superficial zone protein, which functions as a boundary lubricant that helps to reduce friction and wear [20, 22, 23]. The PRP itself was also shown to reduce friction in bovine articular cartilage explants [20]. The anti-inflammatory effects of PRP have been demonstrated both in a co-culture system of osteoarthritic cartilage and synovium [20] and in human osteoarthritic chondrocytes, where it reduced multiple proinflammatory effects induced by interleukin 1b [24]. Furthermore, in a canine OA model, multiple PRP injections were shown to have beneficial effects on pain and functional impairment but no effect on the severity of radiographic OA [25]. Moreover, several clinical trials in OA have concluded that IA-PRP injections are safe and have a beneficial effect on OA symptoms, such as pain and swelling for up to 12 months [26,27,28,29,30]. Patel et al. [31] compared the outcome following single and double PRP injections compared to a control group for early OA at 6 weeks, 3 and 6 months. They concluded that there was a significant improvement in WOMAC scores at all follow-ups when PRP was administered, with no difference between single and double injections. Hart et al. [32] in a prospective study of 50 patients administered 9 injections in 1 year to assess if PRP can increase tibiofemoral cartilage regeneration in the knee. They reported improvement in all scores at 12 months but with no significant cartilage regeneration. Torrero et al. [33] in a prospective study included patients aged 18–65 years and reported significant improvement in the KOOS and VAS score after a single injection up to 6 months after the treatment. Filardo et al. [34] compared a single spin and double spin method of preparation of PRP in 144 patients, demonstrating a significant clinical improvement in both groups with better results in younger patients. Similarly, in a comparative study to assess the efficacy of PRP and hyaluronic acid in 150 patients over 6 months, Kon et al. [35] showed improved IKDC and VAS scores in both groups after 2 and 6 months with better results in the PRP group.
To the best of our knowledge, this is the first study to compare the clinical improvement between IA-HA, IA-CS, and IA-PRP in KOA. There are very few randomized control trials (RCT) comparing IA injections of PRP, HA, and CS. From February 2015, 5 supplementary RCT were published: 4 RCT comparing IA-PRP to IA-HA [29, 34, 36, 37] and 1 RCT comparing IA-PRP to IA-CS [38]. The most important finding of this study was that IA-PRP was superior to IA-HA and IA-CS in reducing pain and recovering physical function in the long term for KOA.
Although this study was carefully designed, several limitations exist. First, this study only included patients between the ages of 40 and 65 years, who are nonprofessional athletes in order to eliminate bias which could occur due to extremes of age. In some previous studies the age group has been as wide as 18–81 years [33, 39]. Second, this study only recorded the WOMAC scores prior to the first injection and then at the end of 12- month follow-up. We can’t find the results of 18 months or 2 years. In a prospective study including 91 patients, a follow-up of 24 months was reported [40, 41]; patients received three IA-PRP injections at monthly intervals, and all parameters worsened at 2 years with significantly lower levels of IKDC objective, subjective and EQ-VAS scores with respect to the 12-month evaluation (IKDC objective fell from 67% to 59% of normal and nearly normal knees; IKDC subjective score was reduced from 60% to 51%, although they remained higher than the basal level). Jang et al. [42] showed deterioration in scores within the 1st year. Finally, in this study the outcomes between the three groups with WOMAC and VAS scores were compared but did not have post-treatment MRI results for every patient; therefore, the changes in the knee cartilage at the end of 12 months cannot be seen.
The optimal IA administration for KOA has remained unclear and controversial. Corticosteroids IA injections are a part of the pharmacological treatment of the acute phase or flare of KOA and IA-HA injections are part of the pharmacological treatment of the chronic phase of KOA. Although multiple studies have reported the safety of using of PRP in the early stages of osteoarthritis of the knee [26,27,28,29,30,31,32,33,34,35, 38], high-quality randomized control trials are needed to determine the safety and improvements of IA-PRP.
Conclusion
The use of IA-PRP injections into the knee for symptomatic early stages of KOA are a valid treatment option. There is a significant reduction in pain and clinical improvement after 3 months, which can be further improved at 12 months. Although the optimal method of IA treatment remains a matter of debate in the literature, the results of the experiment show an encouraging improvement in all scores compared to the pretreament values.
Abbreviations
- BMI:
-
Body mass index
- CS:
-
Corticosteroids
- DVT:
-
Deep venous thrombosis
- EQ-VAS:
-
EuroQol-VAS
- HA:
-
Hyaluronic acid
- HIV:
-
Human immunodeficiency virus
- IA:
-
Intra-articular
- IGF-1:
-
Insulin-like growth factor 1
- IKDC:
-
International Knee Documentation Committee
- KOA:
-
Knee osteoarthritis
- KOOS:
-
Knee injury and osteoarthritis outcome score
- MRI:
-
Magnetic resonance imaging
- PRP:
-
Platelet-rich plasma
- RCF:
-
Relative centrifugal force
- TGF-beta:
-
Transforming growth factor beta
- VAS:
-
Visual analog scale
- VEGF:
-
Vascular endothelial growth factor
- WOMAC:
-
Western Ontario and McMasters Universities
References
Curl WW, Krome J, Gordon ES, Rushing J (1997) Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy 13(4):456–460
Litwic A, Edwards MH, Dennison EM et al (2013) Epidemiology and burden of osteoarthritis. Br Med Bull 105(1):185–199
Tang X, Wang S, Zhan S et al (2016) The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol 68(3):648–653
Ling X, Michael CN (2003) High prevalence of knee, but not hip or hand osteoarthritis in Beijing elders: comparison with data of Caucasian in United States. Chung Hua I Hsueh Tsa Chih 83(14):1206–1209
Riddle DL, Jiranek WA, Hayes CW (2014) Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol 66(8):2134–2143
Altman RD, Devji T, Bhandari M, Fierlinger A (2016) Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum 46(2):151–159
He WW, Kuang MJ, Zhao J, Sun L (2017) Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg 39:95
Nguyen C, Boutron I, Baron G (2017) Evolution of pain at 3 months by oral resveratrol in knee osteoarthritis (ARTHROL): protocol for a multicentre randomised double-blind placebo-controlled trial. BMJ Open 7(9):e17652
Tammachote N, Kanitnate S, Yakumpor T, Panichkul P (2016) Intra-articular, single-shot hylan G‑F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am 98(11):885–892
Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE (2015) Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 162(1):46–54
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32(3):495–505
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ (2009) Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther 39(12):845–849
Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 27(3):158
DeLong JM, Russell RP, Mazzocca AD (2012) Platelet-rich plasma: the PAW classification system. Arthroscopy 28(7):998–1009
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62(4):489–496
Lacci KM, Dardik A (2010) Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med 83(1):1–9
Nikolidakis D, Jansen JA (2008) The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev 14(3):249–258
Osterman C, McCarthy MB, Cote MP et al (2015) Platelet-rich plasma increases anti-inflammatory markers in a human coculture model for osteoarthritis. Am J Sports Med 43(6):1474–1484
Sakata R, McNary SM, Miyatake K et al (2015) Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med 43(6):1467–1473
Zhou Q, Xu C, Cheng X et al (2016) Platelets promote cartilage repair and chondrocyte proliferation via ADP in a rodent model of osteoarthritis. Platelets 27(3):212–222
Sakata R, Reddi AH (2016) Platelet-rich plasma modulates actions on articular cartilage lubrication and regeneration. Tissue Eng Part B Rev 22(5):408–419
Sundman EA, Cole BJ, Karas V et al (2014) The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med 42(1):35–41
van Buul GM, Koevoet WL, Kops N et al (2011) Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med 39(11):2362–2370
Cook JL, Smith PA, Bozynski CC et al (2016) Multiple injections of leukoreduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J Orthop Res 34(4):607–615
Campbell KA, Saltzman BM, Mascarenhas R et al (2015) Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping Meta-analyses. Arthroscopy 31(11):2213–2221
Gobbi A, Lad D, Karnatzikos G (2015) The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 23(8):2170–2177
Laudy AB, Bakker EW, Rekers M et al (2015) Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med 49(10):657–672
Montanez-Heredia E, Irızar S, Huertas PJ et al (2016) Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the Spanish national health care system. Int J Mol Sci 17(7):E1064
Smith PA (2016) Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med 44(4):884–891
Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A (2013) Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med 41(2):356–364
Hart R, Safi A, Komzák M, Jajtner P, Puskeiler M, Hartová P (2013) Platelet-rich plasma in patients with tibiofemoral cartilage degeneration. Arch Orthop Trauma Surg 133(9):1295–1301
Torrero JI, Aroles F, Ferrer D (2012) Treatment of knee chondropathy with platelet rich plasma. Preliminary results at 6 months of follow-up with only one injection. J Biol Regul Homeost Agents 26(2 Suppl 1):71–78
Filardo G, Kon E, Pereira Ruiz MT et al (2012) Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc 20(10):2082–2091
Kon E, Mandelbaum B, Buda R, Filardo G et al (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27(11):1490–1501
Gormeli G, Gormeli CA, Ataoglu B et al (2017) Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 25(03):958–965
Paterson KL, Nicholls M, Bennell KL et al (2016) Intra-articular injection of photo-activated platelet rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 17(1):1–9
Forogh B, Mianehsaz E, Shoaee S et al (2015) Effect of single injection of Platelet-Rich Plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness 56(7–8):901–908
Napolitano M, Matera S, Bossio M et al (2012) Autologous platelet gel for tissue regeneration in degenerative disorders of the knee. Blood Transfus 10(1):72–77
Filardo G, Kon E, Buda R et al (2011) Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 19(4):528–535
Kon E, Buda R, Filardo G et al (2010) Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 18(4):472–479
Jang SJ, Kim JD, Cha SS (2013) Platelet-rich plasma (PRP) injections as an effective treatment for early osteoarthritis. Eur J Orthop Surg Traumatol 23(5):573–580
Acknowledgements
The authors would like to thank the research assistants from Jining No. 1 People’s Hospital of Shandong for their support and the patients enrolled in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Huang, X. Liu, X. Xu and J. Liu declare that they have no competing interests.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the Jining No. 1 People’s Hospital of Shandong. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Huang, Y., Liu, X., Xu, X. et al. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis. Orthopäde 48, 239–247 (2019). https://doi.org/10.1007/s00132-018-03659-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00132-018-03659-5
Keywords
- Degenerative joint disease
- Visual analog scale
- Multimodal treatment
- Assessment, outcomes
- Viscosupplementation