Abstract
Purpose
To compare the safety and efficacy of two different approaches of platelet-rich plasma (PRP) production methods as intra-articular injection treatment for knee cartilage degenerative lesions and osteoarthritis (OA).
Methods
The study involved 144 symptomatic patients affected by cartilage degenerative lesions and OA. Seventy-two patients were treated with 3 injections of platelet concentrate prepared with a single-spinning procedure (PRGF), the other 72 with 3 injections of PRP obtained with a double-spinning approach. The patients were evaluated prospectively at the enrollment and at 2, 6, and 12 months’ follow-up with IKDC, EQ-VAS and Tegner scores; adverse events and patient satisfaction were also recorded.
Results
Both treatment groups presented a statistically significant improvement in all the scores evaluated at all the follow-up times. Better results were achieved in both groups in younger patients with a lower degree of cartilage degeneration. The comparative analysis showed similar improvements with the two procedures: in particular, IKDC subjective evaluation increased from 45.0 ± 10.1 to 59.0 ± 16.2, 61.3 ± 16.3, and 61.6 ± 16.2 at 2, 6, and 12 months in the PRGF group, and from 42.1 ± 13.5 to 60.8 ± 16.6, 62.5 ± 19.9, and 59.9 ± 20.0 at 2, 6, and 12 months in the PRP group, respectively. Concerning adverse events, more swelling (P = 0.03) and pain reaction (P = 0.0005), were found after PRP injections.
Conclusions
Although PRP injections produced more pain and swelling reaction with respect to that produced by PRGF, similar results were found at the follow-up times, with a significant clinical improvement with respect to the basal level. Better results were achieved in younger patients with a low degree of cartilage degeneration.
Level of evidence
II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The social impact of degenerative diseases such as articular cartilage disease and osteoarthritis (OA) is increasing, due to the continued rise in the mean age of the population and greater emphasis on physical activity in all age groups [7, 36]. Unfortunately, the regeneration ability of cartilage is limited, and trauma, chronic overload, as well as metabolic and biological predisposition, may lead to the loss of tissue homeostasis thus resulting in accelerated joint surface damage and eventually end-stage arthritis [4], and we do not have evidence-based methods for the treatment of cartilage defects in the knee, yet [3].
Numerous approaches have been proposed as non-invasive treatment with variable success rates, but none has clearly shown an ability to alter the natural history of this disease, and therefore, none can be considered as an ideal procedure for the treatment of chronic severe chondral lesions or OA [15].
Recently, platelet-rich plasma (PRP) has been attracting attention as an innovative and promising procedure to stimulate repair or replace damaged cartilage, due to the pools of growth factors (GFs) stored in the α-granules of platelets, which have been found to take part in the regulation of articular cartilage [34]. Among these, TGF-β has shown an important role in phenotype expression, chondrogenic MSC differentiation, matrix deposition, and decreasing the suppressive effects of inflammatory mediator IL 1 on proteoglycan synthesis in cartilage [12, 26]. PDGF promotes the maintenance of hyaline-like phenotype, chondrocyte proliferation and proteoglycan synthesis [32]. IGF stimulates proteoglycan production [21], and many other bioactive molecules are involved in cartilage regeneration and metabolism independently or with synergistic interaction [25]. PRP is a simple and minimally invasive method to obtain a high concentrate of autologous GFs in physiological proportions, which can be easily and safely placed directly into the lesion site [6]. Moreover, the risk of allergy or infection is negligible, due to the autologous nature of the platelet extract [31].
Despite the worldwide clinical application of this appealing innovative treatment approach and interesting, promising findings [33], research into its clinical efficacy is still in its infancy, and in most cases, results are still preliminary and controversial. The difficulty in this field of research is increased by the numerous products used. PRP is generally defined as a blood derivate, generated by differential centrifugation of autologous whole blood, with a higher concentration of platelets compared with baseline blood, but more specific elements have not been uniformly defined in the literature. PRP concentrations have been reported to range widely, and the numerous preparation methods present many other different variables, such as the presence of other cells, activation and storage modalities, and many other aspects that are not of secondary importance for determining PRP properties and clinical efficacy [19]. In particular, the presence of leukocytes and their intra-articular injection is controversial, since some authors attribute better results to leukocyte depletion, because of the deleterious effects of proteases and reactive oxygen released from white cells; others consider them as a source of cytokines and enzymes that may also be important for the prevention of infections [10].
The aim of this study was to explore this novel biological treatment for degenerative lesions of articular cartilage and OA by comparing two products, already used in clinical practice, which are based on different preparation approaches: single- versus double-spinning procedures. The hypothesis was that the difference in platelet concentration, cellularity, and storage modality may lead to different clinical results.
Materials and methods
Clinical experimentation was approved by the Hospital Ethics Committee and Internal Review Board, and informed consent of all patients was obtained.
The following diagnostic criteria for patient selection were used: patients affected by chronic (at least 4 months) pain or swelling of the knee and imaging findings (radiograph or MRI) of degenerative changes of the joint. Patients were divided into three categories: degenerative chondral lesion (Kellgren-Lawrence 0), early OA (Kellgren-Lawrence I-III), and advanced OA (Kellgren-Lawrence IV). Exclusion criteria included systemic disorders such as diabetes, rheumatic diseases, hematological diseases (coagulopathies), severe cardiovascular diseases, infections, immunodepression, patients in therapy with anticoagulants-antiaggregants, use of NSAIDs in the 5 days before blood donation, patients with Hb values of <11 and platelet values of <150.000/mmc.
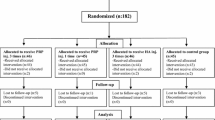
For this study, 144 patients affected by cartilage degenerative lesions and OA were enrolled and treated with intra-articular knee injections. Patients without MRI evidence of cartilage changes were excluded from the study. Symptoms were due to the degenerative knee condition and not related directly to previous trauma. For the patients who had undergone previous knee surgery, the operation was performed at least 1 year before the injective treatment. Among these patients, 72 were treated with 3 autologous PRGF injections and 72 with 3 PRP intra-articular injections. Each center performed only one treatment, and so the patient treatment allocation was due to the center the patients attended. Both centers enrolled consecutive patients following the same inclusion criteria. All the patients were prospectively evaluated at 2, 6, and 12 months’ follow-up. When lesions were bilateral, the worse knee was chosen for the clinical evaluation, being the one that determined the level achieved in the subjective scores used.
No statistically significant differences were found between the PRP and the PRGF groups regarding age, sex, number of bilateral lesions, BMI, degeneration level and previous surgery (Table 1).
Platelet concentrate preparation and injection
PRGF: The procedure consisted of a 36-ml venous blood sample for every knee treated for every injection. Four tubes of 9 ml of blood were centrifuged at 580 g for 8 min, obtaining a concentration suspended in plasma that was extracted by pipetting carefully to avoid leukocyte aspiration. All the open procedures were performed in a laminar flow chamber. Before the injection, 10% of Ca-chloride was added to the 5 ml PRGF unit to activate platelets. The procedure was repeated for every injection [38].
PRP: The procedure consisted of a 150-ml venous blood sample for every knee treated. Two centrifugations (the first at 1,800 rpm for 15 min to separate erythrocytes, and a second at 3,500 rpm for 10 min to concentrate platelets) produced 20 ml of PRP. The unit of PRP was divided into 4 small units of 5 ml each. All the open procedures were performed in an A-class sterile hood. One unit was sent to the laboratory for a quality test (platelet count and bacteriological test), 1 unit was used for the first injection within 2 h, and the other two units were stored at −30°C. Injections were administered every 21 days; for the second and third treatments, the samples were thawed in a dry thermostat at 37°C for 30′ just before application. Before the injection, 10% of Ca-chloride was added to the PRP unit to activate platelets.
In both procedures, injections were administered every 21 days. The skin was sterilely dressed, and the injection was performed through a classic lateral approach using a 22-g needle. At the end of the procedure, the patient was encouraged to bend and extend the knee a few times to allow the PRP to spread throughout the joint before becoming a gel (Fig. 1).
Platelet and cell count
To analyze the differences in concentrates obtained with the two procedures, 7 volunteers underwent blood harvesting, and both PRGF and PRP were prepared from the same blood. The mean final quantity of platelet concentrated was 315,000/μl in the PRGF group and 949,000/μl in the PRP group, with a concentration factor of 1.5× with the single-spinning procedure and 4.7× with the double-spinning procedure. The mean final number of leukocytes was 8,300/μl in the PRP group and none in the PRGF group, with a concentration factor of 0.0× with the single-spinning procedure and 1.4× with the double-spinning procedure.
Post-procedure protocol and follow-up evaluation
The patients were sent home after the injection with instructions to restrict the use of the leg and not to use non-steroidal or steroidal medication but cold therapy for pain for at least 24 h. During the cycle of injections rest or mild activities were indicated. Subsequently, a gradual resumption of normal sport or recreational activities was allowed as tolerated in both the treatment groups.
Patients were evaluated prospectively before the treatment, at 2, 6, and 12 months’ follow-up. Subjective IKDC, EQ-VAS (as recommended by ICRS evaluation package), and Tegner scores were used for clinical evaluation. Adverse events and patient satisfaction were also recorded.
Statistical analysis
All continuous data were expressed in terms of the mean and the standard deviation of the mean. One-way ANOVA was performed to assess differences between groups when the Levene test for homogeneity of variances was not significant (P < 0.05); otherwise, the Mann–Whitney test (2 groups) or the Kruskal–Wallis test (more than 2 groups) was used. The least significant difference test was performed as post hoc pair-wise analysis of the Kruskal–Wallis test. Generalized linear model for repeated measures with Bonferroni’s correction for multiple comparisons was performed to test differences of the scores at different follow-up times. The influence of grouping variables on scores at different follow-up times was investigated by the generalized linear model for repeated measures with the grouping variable as a fixed effect. Pearson’s nonparametric chi-square test evaluated by the Exact method was performed to investigate the relationships between grouping variables. Spearman’s rank correlation was used to assess the correlation between continuous variables.
A power analysis was performed for the primary endpoint of IKDC-S at the 6-month follow-up for PRP and PRGF. From a pilot study, a standard deviation of 15.8 points was found. With an alpha error of 0.05, a beta error of 0.2 and a minimal clinically significant difference of 7.4 points corresponding at 1/3 of the documented mean improvement, the minimum sample size was 72 for each group. For all tests, P < 0.05 was considered significant.
Statistical analysis was carried out by using the Statistical Package for the Social Sciences (SPSS) software version 15.0 (SPSS Inc., Chicago, USA).
Results
No severe adverse events were observed during the treatment and follow-up periods. Both groups showed a statistically significant improvement of all clinical scores from preoperative to final follow-up.
PRGF group: the IKDC subjective score showed a statistically significant improvement (P < 0.0005) at 2 months, which was maintained at 6 and 12 months (P < 0.0005) (Fig. 2). Analogously, EQ-VAS improved significantly (P < 0.0005) at 2, 6, and 12 months’ follow-up with respect to the basal level (Fig. 3). The Tegner score improved at 2 months (P < 0.0005); a further improvement was seen at 6 months, then results remained stable at 12 months (Fig. 4).
PRP group: the IKDC subjective score showed a statistically significant improvement (P < 0.0005) at 2 months, which was maintained at 6 and 12 months (P < 0.0005). Analogously, EQ-VAS improved significantly (P < 0.0005) at 2, 6, and 12 months’ follow-up with respect to the basal level (Fig. 3). The Tegner score improved at 2 months (P < 0.0005); a further improvement was seen at 6 months, then results remained stable at 12 months (Fig. 4).
When comparing the two groups, no differences were found in the subjective IKDC, EQ VAS, or Tegner scores at 2, 6, and 12 months’ follow-up. The satisfaction level was similar, too: 76.4% in the PRGF group and 80.6% in the PRP group. Moreover, there was also no difference in the level of improvement: 59 patients reported an improvement at 12 months (18 mild improvement, 36 marked improvement, 5 complete recovery) in the PRGF group and 56 in the PRP group (19 mild improvement, 32 marked improvement, 5 complete recovery) (Fig. 5).
Conversely, the two procedures showed a statistically significant difference in the number of minor adverse events observed after the injections: both pain and swelling reaction were more frequent in the PRP group (P = 0.0005 and P = 0.03, respectively) (Table 2).
Further analysis was performed to determine the parameters that influenced the clinical outcome. Inferior IKDC subjective results were observed in older patients at 12 months’ follow-up in both groups (ρ = −0.217, P = 0.009 in the PRGF group and ρ = −0.296, P = 0.012 in the PRP group) (Fig. 6). The level of joint degeneration also influenced the clinical outcome at all the follow-up times, with better results for earlier degrees of knee degeneration in both groups (Fig. 7). Other factors, such as BMI, sex, bilateral lesions, and previous surgery, did not significantly influence the final outcome in our series.
In both treatment groups, better IKDC subjective results were achieved in patients with lower degrees of knee degeneration at 2 months’ (ρ = −0.207, P = 0.029 and ρ = −0.295, P = 0.001 in the PRGF and PRP groups, respectively), 6 months’ (ρ = −0.272, P = 0.004 and ρ = −0.362, P < 0.0005 in the PRGF and PRP groups, respectively), and 12 months’ follow-up (ρ = −0.265, P = 0.005 and ρ = −0.282, P = 0.002 in the PRGF and PRP groups, respectively)
Discussion
The most important finding of the present study was that both treatment groups presented a similar statistically significant improvement in all the scores evaluated at all the follow-up times. Better results were achieved in younger patients with a lower degree of cartilage degeneration. The comparative analysis showed more swelling and pain reaction after PRP injections but similar final improvement, thus suggesting the potential of both platelet concentrates in treating joint degeneration processes.
In recent years, laboratory investigations are being focused on the possibility of preserving normal homeostasis or blocking or reversing structural damage as a therapeutic target to avoid, or at least delay, the need for more invasive surgical procedures in degenerated joints. There has been an increasing use of autologous blood products that might provide cellular and humoral mediators to favor tissue healing in tissues with low healing potential [9–11, 17, 19, 33]. The rationale is based on the GFs and bioactive molecules carried in blood.
Blood-derived products have already been studied as adjuvants for cartilage lesions or OA treatment. Frisbie [13] administrated autologous conditioned serum (ACS, a product mainly based on the presence of anti-inflammatory cytokines, including IL-1Ra, elicited by exposure of blood to glass beads) in horses with experimentally induced OA and obtained a clinical improvement in lameness, decreased synovial membrane hyperplasia, less gross chondral fibrillation and synovial membrane hemorrhage, as well as an increased synovial fluid concentration of IL 1 receptor antagonist. Anitua et al. [1] showed that autologous platelet-secreted GFs may have therapeutic effects in OA by modulating synovial cell biology and reported an increased hyaluronic acid (HA) concentration and a stabilized angiogenesis after platelet concentrate exposure. Gaissmaier et al. [14] applied human platelet supernatant to chondrocytes from articular biopsies and observed an accelerated cell expansion, whereas Mishra et al. [23] reported that PRP enhanced MSC proliferation and chondrogenic differentiation in vitro. In a rabbit model, Saito [27] reported preventive effects against OA degeneration with the administration of gelatin hydrogel microspheres containing PRP. Wu et al. [37] investigated the feasibility of PRP as an injectable scaffold for tissue engineering to support chondrogenesis: in the rabbit model, gelled PRP was successfully used to provide a 3-dimensional environment for seeded chondrocytes and deliver them to cartilage defects. Finally, Baltzer et al. [2] analyzed the effect of ACS for the treatment of patients with knee OA in a randomized double-blinded trial and showed that ACS injections considerably improved clinical signs and symptoms of OA. However, it has to be underlined that also some risks have been pointed out in the animal model [18], thus suggesting the need of studies in humans before a wide application of PRP in the clinical practice.
Clinical studies currently available in the literature support the role of PRP for the treatment of cartilage lesions. Sánchez et al. [30] treated a soccer player using PRGF for an articular cartilage avulsion and achieved an accelerated and complete healing. The same authors also reported [29] preliminary results about the effectiveness of intra-articular injections of autologous PRGF for knee OA treatment in an observational retrospective cohort study on 30 patients and suggested the safety and usefulness of this treatment approach. Wang-Saegusa et al. [35] used the same single-spinning procedure, PRGF, to treat knee OA, and the evaluation of 261 patients showed a significant increase in all the clinical scores applied, where 73.4% of patients had an improvement at 6 months’ follow-up. Sampson et al. [28] used another single-spinning procedure for the treatment of a small group of patients affected by primary and secondary knee OA and reported a favorable outcome in the majority of the patients and maintained those positive results for at least 12 months. Kon et al. [16] published a pilot study of 100 patients treated with intra-articular injections of PRP obtained with a double-spinning procedure, with evidence of safety, pain reduction and improved function. The evaluation performed at 2 years’ follow-up [8] showed an overall deterioration and a median duration of the beneficial effect of 9 months. However, the range of effect persistency was wide. In fact, a greater and longer effect was found in young men, with a low BMI and a low degree of cartilage degeneration. Finally, a comparative study recently showed better results for younger and less degenerated joints with respect to HA [20].
Due to the unique properties of platelet concentrates and the promising preliminary results reported, multiple systems have been developed to offer an easy, cost-effective strategy to obtain high concentrations of GFs for tissue healing in the clinical setting. However, different methods lead to the production of different concentrates, which may therefore present different properties and lead to different clinical results. Essentially, protocols for producing PRP can be summarized into 3 methods: selective blood filtration, single-spinning methods, and double-spinning procedures [22]. Lower costs, patient acceptance and feasibility explain the clinical application of the latter two approaches. The single-spinning approach can concentrate platelets 1 to 3 times that of baseline levels, whereas 4- to 8-fold baseline levels are achieved by double-spinning. However, double-spinning also concentrates leukocytes. Thus, whereas the single-spinning approach produces a low platelet concentration, thereby possibly inducing suboptimal effects [33], the double-spinning approach achieves a higher platelet concentration but includes white cells, which might have deleterious effects because of the proteases and reactive oxygen released, as well as a premature platelet degranulation with consequently less GFs available when the PRP is applied [24].
The aim of this study was to explore this novel biological treatment for degenerative lesions of articular cartilage and OA by comparing two products which are based on different preparation modalities: single- versus double-spinning techniques. In particular, we compared two procedures that are already used in the clinical practice and are the most documented in this field. Moreover, they also represent two opposite approaches, with marked differences that make the comparison of the clinical effect of particular interest. In fact, such differences are the focus of a scientific debate, with experts claiming better results related to different PRP properties but still no direct clinical comparison in the literature. The hypothesis was that all these differences, platelet concentration, cellularity, and storage method (the PRP group involved the use of freeze–thawed platelets), might lead to different clinical results.
Both groups showed a statistically significant improvement in all clinical scores from pre-treatment to final follow-up, with a better outcome in younger patients with lower degrees of joint degeneration. The comparative analysis failed to show any difference in any of the subjective scores used at 2, 6, and 12 months of follow-up. Satisfaction level and level of improvement were also similar. Conversely, the two procedures presented a statistically significant difference in the minor adverse events observed after the injections: both pain and swelling reaction were higher in the PRP group.
This study is simply a comparison between the experience documented by two groups using different platelet concentrates, thus study weaknesses and the absence of a biological analysis do not allow to clearly explain these findings. We could hypothesize, according to the current debate in the scientific community, that the presence of leukocytes might have caused local inflammation, thus explaining the increase in reaction. However, the increased post-injection reaction did not affect the final clinical outcome. Also this aspect is controversial, and different hypothesis can be considered: perhaps the inflammation caused was scarce and self-limiting, too low to jeopardize the overall results, or the higher number of platelets in the PRP group might have counter-balanced the negative effects of the leukocytes. However, the white cells might also play a more complex role, with an immunomodulatory capability and influence on GF concentration through their own release of GFs or by stimulating platelet release of GFs [5, 39]. Moreover, despite our attempt to minimize confounding variables using same amount and timing for the injected PRPs, variability and unanswered questions still remain concerning the role of each of the different aspects, such as number of platelets and storing procedure. With regard to this aspect, despite the well-known alteration of the morphology and decrease in platelet functional properties, which includes the degranulation of alpha-granules after storing platelets in freezing conditions [10], the good results also found in the PRP group suggest that freeze–thawing does not adversely affect platelet properties to the extent of impairing their clinical efficacy.
The limitations of this study are the lack of randomization and placebo control group as well as imaging and biological results. However, this is the first direct comparison of two platelet concentrates in the literature and the high number of homogeneous patients analyzed, together with the similarity of the injection protocol (same activation method, same number and timing of injections, same post-injective protocol), answered some questions and enabled us to draw some conclusions. The evaluation limited at 1-year follow-up could be also regarded as a limitation, but it has to be considered that, as for the other injective treatments, the procedure can be repeated cyclically making evaluations at longer follow-up difficult, and that anyway the main results are expected at short term. In fact, the main benefit is obtained at 6–12 months [8], and it is at this follow-up that it is more reasonable to determine the main difference offered by this two treatments.
Both treatments offered a significant improvement, with similar results at all follow-ups, especially in younger patients with lower degrees of joint degeneration, thus confirming findings already reported in the literature [8, 16].
The two preparation methods differ for volume of blood harvested (higher in the PRP double-spinning procedure), number of blood extractions (higher in the PRGF method, due to the use of only fresh platelets), and final concentrate, with more platelets but also leukocytes in the PRP group, and less platelets but absence of leukocytes in the PRGF group (as documented in the literature and also in this study with the direct comparative analysis of platelet concentrates obtained with the two procedures starting from the same blood of healthy volunteers). However, despite all these differences and the initial higher pain and swelling reaction in the PRP group, PRP and PRGF treatments offer same results at 12 months follow-up for the treatment of cartilage degeneration and knee OA. One last aspect to be mentioned, especially when comparing two procedures showing a similar outcome, is the cost-benefit analysis. The economic aspect in this case is not easy to be determined, since the 2-step procedure is not commercialized and is actually done for free for research purposes. However, it has to be underlined that in both cases, the material expenses are minimal, and the cost is mainly due to medical staff costs. The main practical difference can be considered the requirement of an hematology unit for the PRP, whereas PRGF can be more easily obtained and applied in the clinical setting. Therefore, the results of our clinical comparison suggest that the choice of the procedure may be done more because of practical aspects and physician preference, rather than because of differences in the outcome expected.
Further studies are needed to clarify the role of platelet concentration and white cells presence, the influence of freezing on the final platelet function, other than the changes in platelet morphology, activation and function due to the different centrifugation protocols, and if different PRP preparation and application modalities could further improve its clinical efficacy.
Conclusions
The clinical results of this study suggest that both procedures may be useful for the treatment of degenerative articular pathology of the knee. Better results were achieved in younger patients with a low degree of cartilage degeneration. The comparative analysis documented a higher pain and swelling reaction after the injective treatment in the double-spinning PRP group, but failed to show any statistically significant difference between single- and double-spinning procedures in the clinical improvement obtained up to 12 months of follow-up.
References
Anitua E, Sánchez M, Nurden AT et al (2007) Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology 46(12):1769–1772
Baltzer AW, Moser C, Jansen SA, Krauspe R (2009) Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil 17(2):152–160
Benthien JP, Schwaninger M, Behrens P (2011) We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 19(4):543–552
Buckwalter JA, Brown TD (2004) Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res 423:7–16
Castillo TN, Pouliot MA, Kim HJ, Dragoo JL (2011) Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 39(2):266–271
Creaney L, Hamilton B (2008) Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med 42(5):314–320
Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG (1997) Cartilage injuries: a review of 31, 516 knee arthroscopies. Arthroscopy 13:456–460
Filardo G, Kon E, Buda R et al (2011) Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 19(4):528–535
Filardo G, Kon E, Della Villa S et al (2010) Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop 34(6):909–915
Filardo G, Kon E, Marcacci M (2011) Reply to the letter by Dhillon and colleagues. Knee Surg Sports Traumatol Arthrosc 19(5):865–866
Filardo G, Presti ML, Kon E, Marcacci M (2010) Nonoperative biological treatment approach for partial Achilles tendon lesion. Orthopedics 33(2):120–123
Frazer A, Bunning RA, Thavarajah M, Seid JM, Russell RG (1994) Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-beta and interleukin-1beta. Osteoarthr Cartil 2(4):235–245
Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW (2007) Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res 68:290–296
Gaissmaier C, Fritz J, Krackhardt T et al (2005) Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 26:1953–1960
Hochberg MC, Altman RD, Brandt KD, et al. (1995) Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee: American College of Rheumatology. Arthritis Rheum 38(11):1541–1546
Kon E, Buda R, Filardo G et al (2010) Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 18(4):472–479
Kon E, Filardo G, Delcogliano M et al (2009) Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury 40(6):598–603
Kon E, Filardo G, Delcogliano M, et al. (2010) Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord 27(11):220
Kon E, Filardo G, Di Martino A, Marcacci M (2011) Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc 19(4):516–527
Kon E, Mandelbaum B, Buda R et al (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27(11):1490–1501
Martin JA, Buckwalter JA (2000) The role of chondrocyte-matrix interactions in maintaining and repairing articular cartilage. Biorheology 37(1–2):129–140
Mei-Dan O, Lippi G, Sánchez M, Andia I, Maffulli N (2010) Autologous platelet-rich plasma: a revolution in soft tissue sports injury management? Phys Sportsmed 38(4):127–135
Mishra A, Tummala P, King A et al (2009) Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 15(3):431–435
Nagata MJ, Messora MR, Furlaneto FA et al (2010) Effectiveness of two methods for preparation of autologous platelet-rich plasma: an experimental study in rabbits. Eur J Dent 4(4):395–402
O’Keefe RJ, Crabb ID, Puzas JE, Rosier RN (1994) Effects of transforming growth factor-beta 1 and fibroblast growth factor on DNA synthesis in growth plate chondrocytes are enhanced by insulin-like growth factor-I. J Orthop Res 12(3):299–310
Pujol JP, Chadjichristos C, Legendre F et al (2008) Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res 49(3):293–297
Saito M, Takahashi KA, Arai Y et al (2009) Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol 27:201–207
Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B (2010) Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil 89(12):961–969
Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I (2008) Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol 26(5):910–913
Sánchez M, Azofra J, Anitua E et al (2003) Plasma rich in growth factors to treat an articular cartilage avulsion: a case report. Med Sci Sports Exerc 35(10):1648–1652
Sanchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 18:93–103
Schmidt MB, Chen EH, Lynch SE (2006) A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr Cartil 14(5):403–412
Tschon M, Fini M, Giardino R et al (2011) Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci (Elite Ed) 3:96–107
Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ (2003) Articular cartilage biology. J Am Acad Orthop Surg 11:421–430
Wang-Saegusa A, Cugat R, Ares O, Seijas R, Cuscó X, Garcia-Balletbó M (2011) Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg 131(3):311–317
Widuchowski W, Widuchowski J, Trzaska T (2007) Articular cartilage defects: study of 25, 124 knee arthroscopies. Knee 14:177–182
Wu W, Chen F, Liu Y, Ma Q, Mao T (2007) Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg 65:1951–1957
Zimmermann R, Arnold D, Strasser E et al (2003) Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang 85(4):283–289
Acknowledgments
G. Altadonna, F. Balboni, S. Bassini, A. Montaperto: III Clinic—Biomechanics Lab, Rizzoli Orthopaedic Institute, Bologna, Italy. A. Gabriele, F. Pieretti, M. Vaccari, A.M. Del Vento, M. Zagarella, V. Roverini, I. Brognara, L. D’Amato, S. Ardone: Immunohematology and Transfusion Medicine Service, Rizzoli Orthopaedic Institute, Bologna, Italy. E. Pignotti, K. Smith: Task Force, Rizzoli Orthopaedic Institute, Bologna, Italy. This work was partially supported by the project Regione Emilia Romagna Programma di Ricerca Regione-Universita’ 2007–2009 (Regenerative Medicine in Osteoarticular Disease).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filardo, G., Kon, E., Pereira Ruiz, M.T. et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc 20, 2082–2091 (2012). https://doi.org/10.1007/s00167-011-1837-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1837-x