Abstract
This study aims to verify the relevance of Brachidontes pharaonis to assess the ecotoxicological status of polluted sites. For this, the levels of some heavy metals (i.e. Zn, Cu, Pb, and Cd) and a battery of biomarkers including metallothionein (MT), malondialdehyde (MDA), reduced glutathione (GSH), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) were assessed in mussels collected from the harbor of Rades (North), and the harbor of Zarzis (South). Moreover, abiotic parameters including temperature, salinity, pH, and dissolved oxygen were assessed. Results from the ICP-OES showed that the southern population exhibited a higher metal pollution index with significantly higher Zn, Cu, and Pb concentrations. Moreover, the specimens from Zarzis displayed significantly higher levels of MDA, MT, GSH, GPx, SOD, and CAT reflecting higher levels of oxidative and chemical stress. These results emphasize the potential utility of B. pharaonis for the monitoring of heavily impacted sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the decades following the opening of the Suez Canal in 1869, many Indo-Pacific species have successfully reached the Mediterranean sea and have established dense populations mainly in the eastern basin (Çinar et al. 2021). Among these species, the lessepsian invader Brachidontes pharaonis (Fischer 1870) has colonized many rocky habitats in the Mediterranean littorals (Tsangaris et al. 2016). Since its first record in 1876 at the eastern Mediterranean basin (Port Said, Egypt, 1876), B. pharaonis propagated rapidly to other areas in the western parts and it is expected to invade the whole Mediterranean basin soon (Sarà et al. 2018). Recently, a new introduction of this alien species has been detected in the central Mediterranean basin, particularly in Tunisia (Antit et al. 2018).
Harbors constitute one of the major pathways for marine alien species introduction, principally via the discharge of ballast water and biofouling (Saebi et al. 2020). They constitute ‘stepping-stones’ for the establishment and spread of some invasive species in new environments (Keller et al. 2010). Besides being a hotspot for invasive species, harbors are particularly subjected to chronic chemical contamination and are at high pollution incident risk. Heavy metals (HM) are among the most hazardous chemicals affecting these environments due to their toxicity, persistence, and bioaccumulation throughout the food web (Gokkus and Berber 2019). The accumulation of HM in biota can cause several damages at cellular and tissue levels principally through the overproduction of reactive oxygen species (ROS) and oxidative injuries (Valko et al. 2016).
Besides HM bioaccumulation measurements, which provide a time-integrated estimation of the ecotoxicological status of a given site, the determination of their adverse biological effects on the biota through biological markers constitutes a powerful tool for the assessment of marine pollution (Schettino et al. 2012). Small cysteine-rich proteins such as metallothioneins (MTs) are one of the most established specific biomarkers for the biomonitoring of HM contamination due to their strong binding-metal affinity (Amirad et al. 2006). Other thiol compounds like glutathione (GSH), known for their metal-binding and ROS scavenging capacities, have also been widely used to assess metal contamination in aquatic organisms (Le Saux et al. 2020). Moreover, other oxidative stress biomarkers of exposure such as antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx), as well as lipid peroxidation metabolites [e.g. malondialdehyde (MDA)] are routinely used as reliable biochemical markers of pollution contamination, including metals (Le Saux et al. 2020; Telahigue et al. 2022).
Due to its high ability to accumulate a wide range of pollutants including metals, its wide geographic distribution, sedentary lifestyle, filter feeding behavior, and ease of collection, the invasive Red Sea mussel B. pharaounis has been proposed as an alternative sentinel species for marine pollution biomonitoring (Karayakar et al. 2007; Tsangaris et al. 2016; Hamed et al. 2020; Benaltabet et al. 2021). Yet, despite the interest that B. pharaonis may have for in situ-biomonitoring of environmental perturbations, no information is available concerning this species in Tunisian waters.
The aim of the present study was to investigate the relevance of B. pharaonis to detect the contamination level in the harbor environment and to assess the ecotoxicological status of these heavily and chronically impacted aquatic ecosystems. To accomplish this goal, levels of some HM (i.e. Cd, Pb, Zn, and Cu), known as the main sources of pollution in the harbor environment (Tang et al. 2018) and frequently detected at particularly high levels on Tunisian shores (Annabi-Trabelsi et al. 2021), were determined in B. pharaonis soft tissues collected from two harbors on the northern and southern coasts. In addition, several biochemical biomarkers, including MT, GSH, MDA, GPx, SOD, and CAT were assessed.
Materials and Methods
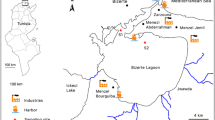
Two selected sampling sites (Fig. 1) along the Tunisian coast were chosen based on their pollution level and the presence of important B. pharaonis populations. The first sampling site (S1) is located at Rades harbor (36.801248 latitude and 10.276192 longitude) in the Northern East of Tunisia. This harbor is one of the largest in the country and plays an important role in the national transport chain (mostly container traffic and rolling units) (LCAs 2021). The second sampling site (S2) is located at the Zarzis harbor (33.495138 latitude and 11.120114° longitude) in the Southern East of Tunisia. This harbor serves as a regional commercial exchange, primarily for the export of marine salt and crude petroleum, as well as the import of white oil products (LCAs 2021).
A total of 270 immature specimens of B. pharaonis with a shell length of 7.1 ± 0.8 mm and a total weight of 64.5 ± 6.4 mg were collected by hand during July 2017 from the two sites. During the sampling campaigns, the abiotic parameters (temperature, salinity, pH, and dissolved oxygen) were assessed in situ (in triplicate) using a multiparameter portable meter (WTW). The samples were transported to the laboratory in a liquid nitrogen container. Due to the small size of individuals, the whole body soft tissues of B. pharaonis were used for biochemical and chemical analysis. Furthermore, samples were pooled as follows: 6 pools of 30 individuals each for biomarker analysis and 3 pools of 30 each for trace metal measurements. To prevent contamination risks, acid-cleaned laboratory materials were used during sample collection and analytical determination.
For the HM analysis, samples of the soft tissues of B. pharaonis were initially washed with Milli-Q water and then digested in a microwave oven (Start D, Milestone, Sorisole, Italy) using nitric acid. The samples were then diluted with ultrapure water, filtrated, and analyzed by an inductively coupled plasma—optical emission spectrometry (Optima 8 × 00 ICP-OES, Perkin Elmer, USA). All the samples were analyzed in triplicate. Shellfish tissue [certified by the International Atomic Energy Agency (IAEA 407)] was used as reference material. The recovery for most analytes was around 95%. The concentrations were expressed as µg g−1 dry weight of the sample.
To compare the total metal content in the two studied B. pharaonis populations, the Metal Pollution Index (MPI) was calculated according to the following equation (Usero et al. 1997):
where Cfn is the concentration of the nth metal in the analyzed sample.
For the biochemical analysis, soft tissues from B. pharaonis were homogenized with phosphate buffer (0.1 M, pH 7.4) on ice-cold using ultra Turrax. The mixture was then centrifuged for 20 min at 10,000×g and 4°C. Finally, the obtained supernatant was stored at -80°C until analysis.
To estimate the total protein content in the B. pharaonis soft tissues, Lowry et al. (1951) method was applied. The Draper and Hadley (1990) assay was applied to determine the extent of the lipid peroxidation measured as MDA level. The TBA–MDA complex absorbance was measured at 532 nm and results were expressed as nmol of MDA per mg of protein. The GSH content was determined based on the reaction between the 5,5-dithiobtis-2- nitro benzoic acid (DTNB) and compounds containing sulfhydryl according to Ellman (1959) technique modified by Jollow et al. (1974). The absorbance of the resulting yellow color was read at 412 nm and GSH content was given as mg per g of tissue. The colorimetric method of Viarengo et al. (1997) modified by Petrović et al. (2001) was used to estimate the MT content. The absorbance was measured at 412 nm and MT concentrations were given as micromoles GSH per gram of tissue. As for the antioxidant enzyme activities, the method of Flohé and Günzler (1984) was applied to assess the activity of GPx. The absorbance was read at 420 nm and the GPx activity was expressed as nmoles of GSH per min per mg of protein. The SOD activity was estimated by monitoring the photochemical reduction of nitro blue tetrazolium (NBT) in the presence of riboflavin at 560 nm as described in Beauchamp and Fridovich (1971). The enzyme activity was given as units (U) per mg of protein, where U corresponds to the amount of enzyme needed for the inhibition of the rate of NBT by 50%. Finally, the CAT activity was determined according to the method of Aebi (1984) which measure the H2O2 decomposition at 240 nm (ε = 40 mM−1 cm−1). The unit of expression of CAT activity was µmol H2O2 consumed per min per mg of protein.
The statistical analysis and graph generation were carried out using R version 4.0.2 (Core Team 2021). Prior to analysis, the Shapiro–Wilk test was performed to verify normality. A Mann–Whitney test was performed to check the significance when the data was not normally distributed. In the case of normally distributed data, the Barlett test was used to assess the equality of variance. Then, statistical comparisons were performed using the Student test in the case of equal variance and the Welch test in the case of different variance. A p value < 0.05 was considered to be statistically significant.
Results and discussion
The physicochemical parameters of seawater measured at the two sampling sites are given in Table 1. Results showed that compared to the harbor of Rades, the seawater in Zarzis harbor had significantly lower pH values (p < 0.05) and slightly higher temperature, salinity, and DO values (p > 0.05). Overall, the obtained results are in line with the physicochemical gradients (from the North to the South) recorded along the Tunisian coast for some parameters, mainly temperature and salinity (Quéméneur et al. 2020).
HM concentrations and MPI from B. pharaonis collected at Rades and Zarzis harbors are given in Table 2. A similar bioaccumulation pattern characterized by the dominance of Zn followed by Cu, Pb, and then Cd was reported in the two studied populations. However, significantly higher (p < 0.05) HM loads were found in the southern population, except for Cd, which did not display a significant difference between the two B. pharaonis populations (p > 0.05). In addition, the present data showed that the Zarzis population had a higher MPI (1.28) than that of Rades (0.59). These findings reflect a higher degree of pollution in the port of Zarzis. Besides the ballast water and the cargo ship sewage, which are generally known as the main sources of HM (namely Pb, Zn, and Cu) in harbor waters (Tang et al. 2018), the particularly high levels of HM found in the southern population could be originated from the phosphate industry effluents. According to Mansouri et al. (2020), the littoral zone of the gulf of Gabes, extending from Sfax to Boughrara lagoon, is highly impacted by the phosphogypsum, which represents the major source of metal contamination in the gulf of Gabes. In a recent work, Annabi-Trabelsi et al. (2021) have found that Zn, Cd, Pb, and Cu levels in the gulf of Gabes seawater exceed by far concentrations in other Mediterranean coastal waters, illustrating the region as a pollution “hotspot”. Apart from the ecological hazard caused by the industrial effluents, the southward-increasing gradients of some physicochemical parameters, supported by bibliographic evidence (Quéméneur et al. 2020), could explain the difference observed in HM concentrations between the two studied B. pharaonis populations. Indeed, it is widely accepted that abiotic parameters (such as temperature, salinity, and pH) can have a significant impact on HM uptake and bioaccumulation in bivalves (Krishnakumar et al. 2018). For example, increased temperature was found to affect both metal chemistry and bivalve physiology, leading to their enhanced bioavailability, uptake, and thereby toxicity (Rouane-Hacene et al. 2015).
Overall, the HM concentrations obtained in this study were in accordance with those of Benaltabet et al. (2021), who have reported quite similar concentrations of Zn, Cu, and Cd (but not Pb) in B. pharaonis from the gulf of Aqaba. Other authors have reported much lower concentrations in B. pharaonis from the Alexandria coast (Hamed et al. 2020), while much more elevated HM concentrations were recorded by Karayakar et al. (2007) in this species from the Mersin coasts (Turkey). In addition to extrinsic factors, intrinsic parameters such as size, gender, and reproductive condition can also affect an organism’s ability to bioaccumulate heavy metals (Krishnakumar et al. 2018).
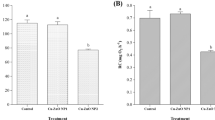
Biomarker responses in the soft tissues of Brachidontes pharaonis collected at two sites along the Tunisian coasts, the Rades harbor in the North (N) and the Zarzis harbor in the South (S). a Malondialdehyde (MDA); b metallothionein (MT); c reduced glutathione (GSH); d glutathione peroxidase (GPx); e superoxide dismutase (SOD); f Catalase (CAT). *Statistically different (p < 0.05)
When comparing the biochemical biomarker responses between the two studied B. pharaonis populations (Fig. 2), a significantly higher level (p < 0.05) of MDA was recorded in mussels from Zarzis harbor (Fig. 2a). This result could reflect that the southern population is subjected to a more prominent oxidative stress condition than the northern one. The obtained result fitted well with the particularly high HM levels above recorded in the southern B. pharaonis population. Indeed, the increase of MDA level, a byproduct of free radical-mediated oxidative damage to lipids, was found to closely correlate with the HM accumulation in marine bivalves (Géret et al. 2002; Telahigue et al. 2022). To buffer the ROS excess and neutralize HM toxicity, cells possess an efficient antioxidative defense compromising enzymatic and nonenzymatic mechanisms. Thiols such as MTs are among the major intracellular antioxidant agents involved in HM detoxification and cellular antioxidative defense owing to their metal-binding and ROS scavenging capacities (Amirad et al. 2006). In the current study, the total MT levels measured in the soft tissues of B. pharaonis (Fig. 2b) were found to be significantly higher in the samples collected from Zarzis harbor in comparison to those from Rades harbor (p < 0.05). The obtained results could be attributed to the higher HM load recorded in this population and could mirror a protective response against metal toxicity. According to the literature, MT is highly linked to the metal loads in marine organisms and can be regarded as a common defense reaction to metal exposure (Chen et al. 2014; Le Saux et al. 2020). Moreover, the induction of MT in mussels may greatly depend on several abiotic factors such as salinity, temperature, and DO (Lavradas et al. 2016). The activation of the thiol-dependent cellular defenses to cope with the metal contamination challenge was here further evidenced by the significantly (p < 0.05) higher level of GSH recorded in the Zarzis population (as given in Fig. 2c). This important detoxifying agent that serves as a scavenger of free radicals and an essential cofactor of several enzymes has been found to increase in some marine bivalves inhabiting contaminated sites (Lavradas et al. 2016; Telahigue et al. 2022). Within the cellular defense system, antioxidant enzymes including GPx, SOD, and CAT act collectively as a first defensive line to suppress or mitigate the formation of ROS in the cell. Firstly, SOD catalyzes the dismutation of superoxide anions into hydrogen peroxide, which is in turn reduced into water by both GPx and CAT activities (Freitas et al. 2019). In the current study, significantly higher (p < 0.05) GPx, SOD, and CAT activities were recorded in B. pharaonis from Zarzis harbor when compared to that from Rades, as given in Fig. 2d to f, respectively. This result could be attributed to an adaptative response adopted by B. pharaonis to prevent oxidative insult and to increase its tolerance and resistance in this highly contaminated area, as reported by Livingstone (2001) for other organisms. These differences could also be attributed to the changes in the physicochemical parameters which may impact the metabolic activity of the organism and thereby its capacity to biotransform and detoxify xenobiotic chemicals, as previously reported by de Lorenzo (2015).
From the data obtained, it is evident that the southern population exhibits significantly higher HM (i.e. Zn, Cu, and Pb) bioaccumulation and more prominent biomarker (i.e. MDA, MT, GSH, GPx, SOD, and CAT) responses reflecting a more stressful environment. These differences could be attributed to several parameters, including pollution sources and environmental conditions. This study provided preliminary results on the relevance of B. pharaonis as a bioindicator of marine pollution in heavily impacted sites. However, further investigations, taking into account other chemicals and biomarkers on a larger spatio-temporal scale, are required for a more reliable interpretation.
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202. https://doi.org/10.1016/j.aquatox.2005.08.015
Annabi-Trabelsi N, Guermazi W, Karam Q, Ali M, Uddin S, Leignel V, Ayadi H (2021) Concentrations of trace metals in phytoplankton and zooplankton in the Gulf of Gabès, Tunisia. Mar Pollut Bull 168:112392. https://doi.org/10.1016/j.marpolbul.2021.112392
Antit M, Amor N, Urra J, Alagaili AN, Farjallah S (2018) Genetic variability of the Lessepsian migrant mussel Brachidontes pharaonis (Bivalvia: Mytilidae) in Tunisia. Afr J Mar Sci 40:211–217. https://doi.org/10.2989/1814232X.2018.1476265
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Benaltabet T, Gutner-Hoch D, Torfstein A (2021) Heavy metal, rare earth element and Pb isotope dynamics in mussels during a depuration experiment in the gulf of Aqaba, Northern Red Sea. Front Mar Sci 8:669329. https://doi.org/10.3389/fmars.2021.669329
Chen L, Ma L, Bai Q et al (2014) Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J Biol Chem 289:22413–22426. https://doi.org/10.1074/jbc.M114.548677
Çinar ME, Bilecenoğlu M, Yokeş MB et al (2021) Current status (as of end of 2020) of marine alien species in Turkey. PLoS ONE 16:e0251086. https://doi.org/10.1371/journal.pone.0251086
Core Team R (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
de Lorenzo V, Sekowska A, Danchin A (2015) Chemical reactivity drives spatiotemporal organisation of bacterial metabolism. FEMS Microbiol Rev 39:96–119. https://doi.org/10.1111/1574-6976.12089
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Meth Enzymol 105:114–121. https://doi.org/10.1016/S0076-6879(84)05015-1
Freitas R, Coppola F, Costa S et al (2019) Does salinity modulates the response of Mytilus galloprovincialis exposed to triclosan and diclofenac? Environ Pollut 251:756–765. https://doi.org/10.1016/j.envpol.2019.04.115
Géret F, Serafim A, Barreira L, Bebianno MJ (2002) Response of antioxidant systems to copper in the gills of the clam Ruditapes decussatus. Mar Environ Res 54:413–417
Gokkus K, Berber S (2019) Heavy metal pollution in Inebolu and Bartin Ports, Black Sea, Turkey. Indian J Geol-Mar Sci 48:1600
Hamed ESAE, Khaled A, Ahdy H, Ahmed HO, Abdel Razek FA (2020) Health risk assessment of heavy metals in three invertebrate species collected along Alexandria Coast, Egypt, Egypt. J Aquat Res 46:389–395. https://doi.org/10.1016/j.ejar.2020.11.001
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4- bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Karayakar F, Erdem C, Cicik B (2007) Seasonal variation in copper, zinc, chromium, lead and cadmium levels in hepatopancreas, gill and muscle tissues of the mussel Brachidontes pharaonis Fischer, collected along the Mersin coast, Turkey. Bull Environ Contam Toxicol 79(3):350–355. https://doi.org/10.1007/s00128-007-9246-z
Keller RP, Drake JM, Drew MB, Lodge DM (2010) Linking environmental conditions and ship movements to estimate invasive species transport across the global shipping network. Divers Distrib 17:93–102
Krishnakumar PK, Qurban MA, Geetha Sasikumar G (2018) Biomonitoring of trace metals in the coastal waters using bivalve molluscs. In: Saleh HEM, El-Adham E (eds) Trace elements—human health and environment. IntechOpen, London. https://doi.org/10.5772/intechopen.76938
Lavradas RT, Rafael RCC, Bordon ICAC et al (2016) Differential metallothionein, reduced glutathione and metal levels in Perna perna mussels in two environmentally impacted tropical bays in southeastern Brazil. Ecotoxicol Environ Saf 129:75–84. https://doi.org/10.1016/j.ecoenv.2016.03.011
LCAs (2021) Logistics capacity assessments. Available at https://dlca.logcluster.org/display/public/DLCA/LCA+Homepage. Accessed on 06 Jan 2022
Le Saux A, David E, Betoulle S et al (2020) New insights into cellular impacts of metals in aquatic animals. Environments 7:46. https://doi.org/10.3390/environments7060046
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666. https://doi.org/10.1016/s0025-326x(01)00060-1
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mansouri B, Gzam M, Souid F, Telahigue F, Chahlaoui A, Ouarrak K, Kharroubi A (2020) Assessment of heavy metal contamination in Gulf of Gabès coastland (southeastern Tunisia): impact of chemical industries and drift currents. Arab J Geosci 13:11–05. https://doi.org/10.1007/s12517-020-06163-3
Petrović S, Ozretić B, Krajnović-Ozretić M, Bobinac D (2001) Lysosomal membrane stability and metallothioneins in digestive gland of mussels (Mytilus galloprovincialis Lam.) as biomarkers in a field study. Mar Pollut Bull 42:1373–1378. https://doi.org/10.1016/S0025-326X(01)00167-9
Quéméneur M, Bel Hassen M, Armougom F, Khammeri Y, Lajnef R, Bellaaj-Zouari A (2020) Prokaryotic diversity and distribution along physical and nutrient gradients in the Tunisian coastal waters (South Mediterranean Sea). Front Microbiol 11:593540. https://doi.org/10.3389/fmicb.2020.593540
Rouane-Hacene O, Boutiba Z, Belhaouari B, GuibboliniSabatier ME, Francour P, Risso-De Faverney C (2015) Seasonal assessment of biological indices, bioaccumulation and bioavailability of heavy metals in mussels Mytilus galloprovincialis from Algerian west coast, applied to environmental monitoring. Oceanologia 57(4):362–374. https://doi.org/10.1016/j.oceano.2015.07.004
Saebi M, Xu J, Curasi SR, Grey EK, Chawla NV, Lodge DM (2020) Network analysis of ballast-mediated species transfer reveals important introduction and dispersal patterns in the Arctic. Sci Rep 10(1):1–15
Sarà G, Porporato EM, Mangano MC, Mieszkowska N (2018) Multiple stressors facilitate the spread of a non-indigenous bivalve in the Mediterranean Sea. J Biogeogr 45:1090–1103. https://doi.org/10.1111/jbi.13184
Schettino T, Caricato R, Calisi A, Giordano ME, Lionetto MG (2012) Biomarker approach in marine monitoring and assessment: new insights and perspectives. Open Environ Sci 6:20–27. https://doi.org/10.2174/1876325101206010020
Tang H, Ke Z, Yan M et al (2018) Concentrations, distribution, and ecological risk assessment of heavy metals in Daya Bay, China. Water 10:780. https://doi.org/10.3390/w10060780
Telahigue K, Rabeh I, Chouba L et al (2022) Assessment of the heavy metal levels and biomarker responses in the smooth scallop Flexopecten glaber from a heavily urbanized Mediterranean lagoon (Bizerte lagoon). Environ Monit Assess 194(6):397. https://doi.org/10.1007/s10661-022-10071-2
Tsangaris C, Moschino V, Strogyloudi E et al (2016) Biochemical biomarker responses to pollution in selected sentinel organisms across the Eastern Mediterranean and the Black Sea. Environ Sci Pollut Res 23:1789–1804. https://doi.org/10.1007/s11356-015-5410-x
Usero J, Rega–lado EG, Gracia I (1997) Trace metals in bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of southern Spain. Environ Int 23:291–298. https://doi.org/10.1016/S0160-4120(97)00030-5
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90(1):1–37. https://doi.org/10.1007/s00204-015-1579-5
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Acknowledgements
The authors are thankful to the editor and the anonymous reviewers for their acceptance to review this work and their kind suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Telahigue, K., Antit, M., Rabeh, I. et al. Heavy Metal Bioaccumulation and Oxidative Stress Profile in Brachidontes pharaonis (Bivalvia: Mytilidae) from the Tunisian Coast: Insight into Its Relevance as Bioindicator of Marine Pollution. Bull Environ Contam Toxicol 109, 831–838 (2022). https://doi.org/10.1007/s00128-022-03593-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-022-03593-5