Abstract
The aim of this study was to investigate biomarkers of oxidative stress: сatalase (CAT), glutathione (GR), glutathione S-transferase (GST), low-molecular antioxidant—reduced glutathione (GSH) and lipid peroxide oxidation marker—malondialdehyde (MDA) and to measure heavy metal (НМ) concentrations in Dreissena polymorpha soft tissues at three different sampling sites in the Rybinsk reservoir (one of the largest in Europe) in order to assess exposure to anthropogenic pollution. The reservoir has a considerable source of pollution in its northern part due to a large industrial complex in Cherepovets. Mussels were collected at three sites which differ in the levels of anthropogenic load: more contaminated sites 1 (Cherepovets) and 2 (Koprino). Site 3 (Vesyegonsk) is relatively clean and is used as a control. In soft tissues of mussels from sites 1 and 2 significantly higher concentrations of HM (Pb, V, Cr, Ni, Cu, Mn, Zn, Cd) were detected than in mussels from site 3. Mussels from sites 1 and 2 subjected to long-term contamination from Cherepovets industry showed considerable CAT, MDA and GSH induction compared to mussels from relatively clean site 3. The correlation was found between elevated HM content and increased levels of CAT, MDA and GSH. The obtained data suggest that HM may contribute to the oxidative stress induction in mussel. MDA, CAT and GSH sensitivity in D. polymorpha demonstrated a possible use of these biomarkers in freshwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic exposure to anthropogenic contaminants can lead to injuries in aquatic inhabitants (fish, mussels, insects etc.). Mussels can tolerate some amount of contaminant-induced stress, but long-term exposure can exhaust repair and coping mechanisms resulting in pathological changes at higher levels of biological organization. Biomarkers can be characterized as functional measures of exposure to stressors, which are usually expressed at the cellular and molecular level of biological organization. Heavy metals have long been recognized as serious contaminants of the aquatic environment. They cause serious impairment in metabolic, physiological and structural systems (Binelli et al., 2015) Heavy metals accumulated in tissues of aquatic organisms may cause multiple toxic impacts and also reactions that generate reactive oxygen species (ROS) (Livingstone, 2001). Then, ROS can lead to pollution-induced oxidative stress. Oxidative stress biomarkers (catalase (CAT), glutathione reductase (GR), reduced glutathione (GSH), malondialdehyde (MDA) now successfully used to assess the effects of chronic exposure to different pollutants (Habig et al., 1974). Evaluation of pollutant bioaccumulation by various organisms is widely used in ecotoxicology as it reflects their bioavailability in the ecosystem (Pesnya et al., 2015, Phillips et al., 1993). Zebra mussel Dreissena polymorpha (Pallas, 1771) (Bivalvia: Dreissenidae) is widely used as a model organism for biomonitoring purposes since late 1970’s. This is a sedentary active filtrator, an active bioaccumulator of mineral and organic pollutants (Binelli et al., 2010; Bolognesi et al., 2004). As invasive species zebra mussel widely distributed in Russia. The use of biomarkers of zebra mussels has allowed to detect specific biological response to particular pollutant in laboratory studies or to mixed anthropogenic contamination in field studies (Faria, Huertas et al., 2010). According to Binelli et al. (2015) D. polymorpha may represent the freshwater counterpart of Mutilus in ecotoxicological studies. Present paper is devoted to the first study of oxidative stress induction in D. polymorpha by chronic anthropogenic HM pollution in aquatic environment of Rybinsk reservoir. We have tried to compare oxidative stress indices with HM accumulation in tissues. However, it was impossible to measure all of the pollutants presented in sample sites of Rybinsk reservoir. Oxidative stress biomarkers including enzymes of antioxidant defense system were studied. Catalase is involved in detoxifying reactive oxygen species (CAT E.C. 1.11.1.6, takes part in H2O2 utilization). Glutathione reductase enzyme takes part in reduction of oxidized glutathione (GR, E.C. 1.8.1.7) (Canesi et al., 1999). Glutathione S-transferase enzyme is included in phase II biotransformation xenobiotic and participating in neutralization of organic hydroperoxides (Regoli, Principato, 1995; Richardson et al., 2008). We have also studied the level of low-molecular antioxidant—reduced glutathione (GSH) and lipid peroxide oxidation marker— malondialdehyde (MDA) (Habig et al., 1974). Concentration of Pb, V, Cr, Mn, Co, Ni, Cu, Zn and Cd was measured in D. polymorpha soft tissues.
MATERIALS AND METHODS
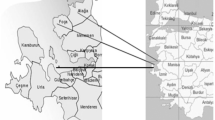
Study sites. Sampling was performed on three sites in the Rybinsk reservoir in June 2013. The Rybinsk Reservoir (58°22′30″ N, 38°25′04″ E) is located in the European part of Russia and belongs to the Volga Cascade of reservoirs. It is the largest artificial waterbody in Europe (4550 km2) (Fig. 1).
Anthropogenic contamination of the RR is of great concern in terms of public and environmental health. The reservoir has a considerable source of pollution in its northern part due to a large industrial complex encompassing over 60 plants in Cherepovets. Wastewaters from some facilities are discharged directly or through a wastewater treatment plant into the Sheksna River and then flow into the RR. A number of settlements located along the shores of the RR contribute contamination of the waterbody by surface runoff as well as by local atmospheric transfer. Locations of sampling sites were chosen to be contrasting in terms of anthropogenic load. Results of perennial monitoring of the reservoir have shown that sampling sites 1 and 2 are characterized by the highest degree of HM pollution (Table 1) (Bakanov et al., 2000; Gapeeva, 2013).
Site 1 (59°08′38.8″ N, 37°58′23.2″ E) is situated in the most polluted part of the reservoir, in the Sheksna stretch, close to industrial metallurgical complex of Cherepovets. City’s and industrial wastewaters bring various pollutants into the environment of Rybinsk Reservoir. There observed intensive HM pollution (Cr, Cu, Ni, Zn, Cd, Pb), with Cu, Zn and Pb concentrations exceeding MPC (Gapeeva, 2013). Ecosystem components (water, bottom sediments, benthos and fish) contained both organic pollutants polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAH) and heavy metals such as Cd, Zn, Cu, Pb (Bakanov et al., 2000; Flerov et al., 2000; Gapeeva, 2013). Site 2 is situated in the Volga stretch of the reservoir near settlement Koprino (58°04′09″ N, 38°17′17″ E). Bottom sediments at this site contain high concentration of Zn, Cd, Cr, Cu (Bakanov, Tomilina et al., 2000). Site 3 is situated in the Mologa stretch of the reservoir (58°41′32.2″ N, 37°10′6.3″ E) in a relatively clean part of the reservoir near settlement Vesyegonsk. Concentrations of HM and organic chemicals are lowest here (Bakanov et al., 2000; Chuiko et al., 2010).
Sample collection and preparation. Immediately after capture mussels were frozen at –195°C and transported to laboratory in liquid nitrogen. In laboratory, mussels of 20 mm length were chosen and sexed using light microscopy (×400) (Vlastov, Kachanova, 1959). Twelve tissue samples containing an equal amount of males and females were taken for biochemical indices analysis and HM content determination.
Biochemical determinations. For the biochemical determinations soft tissues (wet mass 2 g) homogenized in a 1 : 5, weight: volume ice cold phosphate buffer 0.1 M (pH 7.5). All measurements (concentration of MDA, GSH and enzyme activity) were carried out using a dual-beam spectrophotometer LAMBDA 25 (Perkin Elmer, Waltham, MA, USA). GSH was measured using DTNB (5,5′-dithiobis-2-nitrobenzoic acid) at 412 nm (Moron, Depierre et al., 1979), and malondialdehyde (MDA) assay using thiobarbituric acid at 535 nm (Stalnaya, Garishvili, 1977). For antioxidant enzymes assays the homogenate was centrifuged for 40 minutes at 22 000 g and 0°С. Supernatant was measured for CAT by the degradation of H2O2 and ammonium molibdate as a staining reagent et 25°С and λ = 410 nm (Koroluk et al., 1988). GR activity was based on followed the oxidation NADPH by GR in the presence of oxidized glutathione (GSSG) at 25°С and λ = 340 nm (Carlberg, Mannervik, 1985). GST activity was measured by 1-chloro-2,4-dinitrobenzene (CDNB) described by at 27°С and λ = 349 nm (Habig et al., 1974). Protein contents were determined using the Bradford dye-binding assay with bovine serum albumin as the standard (Bradford, 1976).
Determination of metals in samples mussels. Determination of HM (Pb, V, Cr, Mn, Co, Ni, Cu, Zn and Cd) content in mussel’s soft tissues was performed with the use of microwave digestion systems SpeedWareMWS-3+ (Berghof GmbH, Germany), for 20 minutes at 200°С according to the protocol of EPA Method 3050B. HM concentration were analyzed using an inductively coupled plasma mass spectrometer ELAN DRC-e (Perkin Elmer, USA). Results expressed as mkg of HM in 1 gramm of wet tissue.
Statistical analysis. Statistical analysis was carried out in Statistica 6 software (StatSoft Inc., USA). Comparison of antioxidant activity of D. polymorpha from different stations and of different sexes was made by two-way ANOVA. Differences were detected with Fisher’s LSD approach with 5% confidence level. Correlation between oxidative stress and soft tissues' metal content was calculated as Pearson’s R coefficient. 288 mussels were used in the study. At each site were collected 96 mussels for analysis of biochemical parameters and HM contents. In each sample were combined soft tissues of 8 mussels of the same sex. For each site were analyzed 6 samples of male and 6 samples of female (totally 12 samples of both sex).
RESULTS
Mussels from different parts of the reservoir have significant (р ≤ 0.05) differences in oxidative stress indices and metal content in tissues. Minimal levels of all observed HM concentrations and biomarkers (р ≤ 0.05) were registered in D. polymorpha from relatively clean site 3. No significant differences in biomarkers and HM concentrations between male (M) and female (F) individuals were registered, so further comparison was performed between sites 1, 2 and 3 with the use of averaged values of M and F (Table 2). It was found that mussels from the polluted sites 1 and 2 had significantly higher concentrations of many HM in tissues: Pb, Cr, Ni, Cu. However, mussels from site 1 contained maximum amounts of Pb, V, Cr, Ni, Mn and Zn in comparison to site 3 (by 6; 12.5; 6.3; 3.4; 2.6 and 2 times higher, correspondingly) while mussels from site 2 contained V, Cr, Mn and Cd by 9; 5.7; 6; 3.4 times higher than that at the site 3, correspondingly. Significantly higher values of MDA, CAT and GSH were found in the mussel`s tissue from polluted sites 1 and 2 in comparison with relatively clean site 3. At the site 2 the level of MDA and activity of CAT considerably higher than in both site 1 (by 1.2 and 1.5 times) and site 3 (by 1.9 and 2.8 times). At the site 1 CAT activity and MDA level significantly higher than that’s at the site 3 by 1.6 and 1.9 times correspondingly (Table 3). GSH level in D. polymorpha tissue on both sites 1 and 2 was found to be 7 times higher in comparison with control site 3. There are no significant differences in GST and GR activity were detected between all three sites. Correlation was registered between biomarkers levels and HM content in D. polymorpha tissue. There was a correlation between MDA and Cd, CAT and Mn, GSH and Cu (Table 4).
DISCUSSION
Zebra mussels collected on site 1, close to industrial cluster of Cherepovets and on a remote site 2 are exposed to toxic pollutants. Their tissues accumulate large concentrations of HM which strengthening oxidation processes and therefore, increase MDA concentration. Products of POL are important diagnostic indices of elevated ROS formation (Huggett et al., 1992). Many of the metals found are prooxidants and stimulate peroxide oxidation of lipids as well as development of oxidative stress (Livingstone, 2001). Transition metals (Mn, Cu, Zn) catalyze ROS production by means of Fenton reaction (Winston, Di Giulio, 1991) while many other metals (Ni, Pd, Cd) lead to increased oxidation processes through some toxic mechanisms. Cadmium, for example, disrupts cellular membrane balance and disorganizes metabolism Ca2+ (Suzuki et al., 2004). An increase of MDA in D. polymorpha sampled on sites with different levels of pollution was noted by de Lafontaine et al. (2000). Their results have also shown the sensitivity of POL to HM exposure. Other researchers Vlahogianni and Valavanidis (2007) have studied toxic effect of HM Cu(II), Cd(II), Pb(II) on another bivalve Mytilus galloprovincialis and all HM have also caused an increase in MDA concentration. According to Naimo (1995) the most toxic heavy metals for freshwater bivalve is Hg, Cd, Cu and Zn. Moreover, Chu and Chow (2002) showed that different heavy metals together can cause synergistic toxicity in biologicals model. Results of the present study showed that the increase in the intensity of oxidation processes in D. polymorpha in polluted sites 1 and 2, resulted in the activation of different AOD mechanisms: increased activity of CAT and GSH level of growth. Timely induction of antioxidant enzymes is an important protective reaction in the development of oxidative stress caused by anthropogenic pollution (Sole, 2000). CAT, as well as MDA—is an oxidative damage marker which can be both caused by POPs and HM (Binelli et al., 2015). Simultaneous increase in MDA and CAT in D. polymorpha has also been observed in laboratory experiments with exposure to Cd (34 mg/L) (Faria et al., 2009). On site 1 ROS production in D. polymorpha tissue may be due to the complex of HM (Pb, Cr, Ni, Cu, Zn) of anthropogenic origin, with the source of their income into the Rybinsk reservoir ecosystem from industrial metallurgical complex of Cherepovets (Flerov et al., 2000).
Detected positive correlation of CAT with Mn, and MDA with Cd affirms that HM caused POL amplification processes. Higher content of metals: Pb and Zn was observed in mussel`s tissues. Similar results were obtained for D. polymorpha in field trials (Faria et al., 2010). In polluted stations mussels accumulated higher concentrations of HM: Ni, Zn, Cd, Pb, As and PCBs so that the antioxidant system response was also expressed in the activation of CAT and other enzymes except GST and GR. The increase of CAT activity has been described by many authors for some other species like fish and invertebrates sampled in areas contaminated with HM (Huggett et al., 1992). Mussels from site 2 had maximum values of MDA content and CAT activity, with a high content of shellfish accumulating Mn and Cd (Table 2). This may be due to their bioavailability. Manganese—a metal with strong oxidizing properties (Leonard et al., 2004), and cadmium, is known for its multiple toxic effects (Son et al., 2001). Their combined toxic effects may doubly enhance ROS formation and leads to an increase in POL processes, which explains the significant correlation between the activity of CAT contents of the MDA, and the concentration of Cd and Mn in the tissues of mussels.
The reduced GSH detoxifies metals, POPs and catches ROS, therefore, constituting the first line of defense against their toxic effects (Bocchetti et al., 2008). GSH level is higher in D. polymorpha from polluted sites 1 and 2. This is due to the fact that mussels accumulated higher quantities of HM (Pb, Zn, Mn, V, Cd) here. Detected HM could be the cause of increased GSH level, which is confirmed by its positive correlation with Cu. A similar trend with increased GSH level was found in the Black Sea mussel Mytilus galloprovincialis in the natural environment, on polluted sites, with the accumulation of metals: Cr, Fe, Cu, and Mn (Regoli, Principato, 1995). Previously, other studies on bivalve mollusk (Mytilus galloprovincialis) and fish (Dicentrarchus labrax, Abramis brama) described reduction of GSH under the influence of HM and POPs in the laboratory and in the field (Viarengo et al., 1997; Bocchetti et al., 2008; Morozov et al., 2012). Some studies reported short-term increase in the content of GSH and its subsequent depletion in different species of animals and plants (Huggett et al., 1992). Analysis of literature data and results of their studies, may allow us to conclude that the changes in GSH level depends on the concentration of pollutants and different exposure times, and it is difficult to interpret the results obtained in the field. In our field study GSH. level in D. polymorpha from sites 1 and 2 with chronic exposure to pollutants was 7 times higher in comparison to relatively clean site 3, that`s may be due to an adaptive response to the action of HM. Endogenous enzymes glutathione synthesis is carried out by c-glutamyl-cysteine synthetase and glutationsinthetase, (Canesi et al., 1999), and GR restores its oxidized form (Carlberg, Mannervik, 1985). So it can be assumed that these enzymes at the moment of our study, together provided sufficient cell renewal resource for reduced GSH in the tissues of mussels exposed to pollution (contamination) (Faria et al., 2009).
CONCLUSIONS
Obtained data suggest that HM may contribute to the oxidative stress induction in mussel. Bivalves D. polymorpha have accumulated high HMs concentrations in different sites of the Rybinsk reservoir with a high level of anthropogenic pollution (sites 1 and 2). This stimulates the formation of ROS and POL induction. As a consequence, there have been changes in the antioxidant defense system functioning (CAT and GSH increase), that is an adaptive compensatory mechanism on HM action. Consequently, the measured biomarkers in D.polymorpha reflect the level of anthropogenic load on different parts of the Rybinsk reservoir. Due to it’s sensitivity biomarkers in D. polymorpha can be recommended to assess HM pollution in freshwater ecosystems.
REFERENCES
Bakanov, A.I., Tomilina, I.I., and Gapeeva, M.V., Sediment quality assessment of the Upper Volga reservoirs using elements of the triad approach, Inland Water Biol., 2000, no. 1, p. 102.
Binelli, A., Cogni, D., Parolini, M., and Provini, A., Multi-biomarker approach to investigate the state of contamination of the R. Lambro/R. Po confluence (Italy) by zebra mussel (Dreissena polymorpha), Chemosphere, 2010, vol. 79, p. 518. https://doi.org/10.1016/j.chemosphere.2010.02.033
Binelli, C., Della Torre, S., et al., Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review, Environ. Pollut., 2015, vol. 196, p. 386. https://doi.org/10.1016/j.envpol.2014.10.023
Bocchetti, R., Fattorini, D., Pisanelli, B., et al., Contaminant accumulation and biomarker responses in caged mussels, Mytilus galloprovincialis, to evaluate bioavailability and toxicological effects of remobilized chemicals during dredging and disposal operations in harbour areas, Aquat. Toxicol., 2008, vol. 89, p. 257. https://doi.org/10.1016/j.aquatox.2008.07.011
Bolognesi, C., Buschini, A., Branchi, E., et al., Comet and micronucleus assays in zebra mussel cells for genotoxicity assessment of surface drinking water treated with three different disinfectants, Sci. Total Environ., 2004, vol. 333, p. 127. https://doi.org/10.1016/j.scitotenv.2004.05.018
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein dye binding, Anal. Biochem., 1976, vol. 72, p. 248.
Canesi, L., Viarengo, A., Leonzio, C., et al., Heavy metals and glutathione metabolism in mussel tissues, Aquat. Toxicol., 1999, vol. 46, p. 67. https://doi.org/10.1016/S0166-445X(98)00116-7
Carlberg, I. and Mannervik, B., Glutathione reductase, Methods Enzymol., 1985, vol. 113, p. 484. https://doi.org/10.1016/S0076-6879(85)13062-4
Chu, K.W. and Chow, K.L., Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative, Aquat. Toxicol., 2002, vol. 61, p. 53. https://doi.org/10.1016/S0166-445X(02)00017-6
Chuiko, G.M., Tillitt, D.E., Zajicek, J.L., et al., Chemical contamination of the Rybinsk Reservoir, northwest Russia: Relationship between liver polychlorinated biphenyls (PCB) content and health indicators in bream (Abramis brama), Chemosphere, 2007, vol. 67, p. 527. https://doi.org/10.1016/j.chemosphere.2006.09.046
Chuiko, G.M., Zakonnov, V.V., Morozov, A.A., et al., Spatial distribution and qualitative composition of polychlorinated biphenyls and organochlorine pesticides in bottom sediments and bream (Abramis brama L.) from the Rybinsk Reservoir, Inland Water Biol., 2010, vol. 3, no. 2, p. 193. https://doi.org/10.1134/S199508291002015X
Faria, M., Huertas, D., Soto, D.X., et al., Contaminant accumulation and multi-biomarker responses in field collected zebra mussels (Dreissena polymorpha) and crayfish (Procambarus clarkii), to evaluate toxicological effects of industrial hazardous dumps in the Ebro river (NE Spain), Chemosphere, 2010, vol. 78, p. 232. https://doi.org/10.1016/j.chemosphere.2009.11.003
Faria, M., Carrasco, L., Diez, S., et al., Multibiomarker responses in the freshwater mussel dreissena polymorpha exposed to polychlorobiphenyls and metals, Comp. Biochem. Physiol., 2009, vol. 149, p. 281. https://doi.org/10.1016/j.cbpc.2008.07.012
Flerov, B.A., Tomilina, I.I., Clivlend, L., et al., A comprehensive assessment of the status of bottom sediments of the Rybinsk Reservoir, Biol. Vnutr. Vod, 2000, no. 2, p. 148.
Gapeeva, M.V., Heavy metals in the water and sediments of Rybinsk Reservoir, Water:Chem. Ecol., 2013, vol. 5, p. 3.
Habig, W.H., Pabst, M.J., and Jacoby, W.B., Glutathion-S-transferase: the first step in mercapture acid formation, J. Biol. Chem., 1974, vol. 249, p. 7130.
Halliwell, B. and Gutteridge, J.M.C., Free Radicals in Biology and Medicine, Oxford: Oxford Univ. Press, 1999.
Huggett, R.J., Kimerle, R.A., Mehrle, P.M., and Bergman, H.L., Biomarkers: Biochemical, Physiological, and Histological Markers of Anthropogenic Stress, SETAC Special Publication Series, Boca Raton: Lewis Publishers, 1992.
Koroluk, M.A., Ivanova, L.I., Mayorova, I.G., and Tokarev, V.E., Method for determination of catalase activity, Lab. Work, 1988, no. 1, p. 16.
de Lafontaine, Y., Gagneґ, F., Blaise, C., et al., Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada), Aquat. Toxicol., 2000, vol. 50, p. 51. https://doi.org/10.1016/S0166-445X(99)00094-6
Leonard, S.S., Harris, G.K., and Shi, X., Metal-induced oxidative stress and signal transduction, Free Radic. Biol. Med., 2004, vol. 37, p. 19. https://doi.org/10.1016/j.freeradbiomed.2004.09.010
Livingstone, D.R., Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms, Mar. Pollut. Bull., 2001, vol. 42, p. 656. https://doi.org/10.1016/S0025-326X(01)00060-1
Livingstone, D.R., Oxidative stress in aquatic organisms in relation to pollution and aquaculture, Revue Méd. Vét., 2003, vol. 154, no. 6, p. 427.
Manduzio, H., Rocher, B., Durand, F., Galap, C., and Leboulenger, F., The point about oxidative stress in molluscs, Invertebr. Survival J., 2005, vol. 2, p. 91.
Menshikova, E.B., Lankin, V.Z., Zenkov, N.K., et al., Oxidative Stress: Prooxidants and Antioxidants, Moscow: Slovo, 2006.
Moron, M.S., Depierre, J.W., and Mannervik, B., Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver, Biochim. Biophys. Acta, 1979, vol. 582, p. 67.
Morozov, A.A., Chuiko, G.M., and Brodskii, E.S., Functional state of the liver antioxidant system of the bream Abramis brama (L.) from Rybinsk Reservoir regions with different anthropogenic loads, Inland Water Biol., 2012, vol. 5, no. 1, p. 147. https://doi.org/10.1134/S1995082911040134
Naimo, T.J., A review of the effects of heavy metals on freshwater mussels, Ecotoxicology, 1995, vol. 4, p. 341.
Navarro, A., Faria, M., Barata, C., and Pina, B., Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults, Environ. Pollut., 2011, vol. 159, p. 100. https://doi.org/10.1016/j.envpol.2010.09.018
Pesnya, D.S., Romanovsky, A.V., Chuiko, G.M., et al., Antioxidant system of freshwater bivalve Anodonta cygnea Linn. under the short-term change of salinity, Water:Chem. Ecol., 2015, vol. 6, p. 80.
Phillips, D.J.H. and Rainbow, P.S., Biomonitoring of Trace Aquatic Contaminants, 1st ed., Environmental Management Series, London: Elsevier Applied Science, 1993.
Regoli, F., Frenzilli, G., Bocchetti, R., et al., Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment, Aquat. Toxicol., 2004, vol. 68, p. 167. https://doi.org/10.1016/j.aquatox.2004.03.011
Regoli, F. and Principato, G., Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilis galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers, Aquat. Toxicol., 1995, vol. 31, p. 143.
Richardson, B.J., Sharon De Luca-Abbott, E.M., Michae, M., McClellan, K., and Lam Paul, K.S., Antioxidant responses to polycyclic aromatic hydrocarbons and organochlorine pesticides in green-lipped mussels (Perna viridis): do mussels “integrate” biomarker responses?, Mar. Pollut. Bull., 2008, vol. 57, p. 503. https://doi.org/10.1016/j.marpolbul.2008.02.032
Sole, M., Assessment of the results of chemical analyses combined with the biological effects of organic pollution on mussels, TrAC, Trends Anal. Chem. (Pers. Ed.), 2000, vol. 19, p. 1. https://doi.org/10.1016/S0165-9936(99)00174-0.
Son, M.H., Kanga, K.W., Lee, C.H., and Kim, S.G., Potentiation of cadmium induced cytotoxicity by sulfur amino acid deprivation through activation of extracellular signal-regulated kinase1/2 (ERK1/2) in conjunction with p38 kinase or c-jun N-terminal kinase (JNK) Complete inhibition of the potentiated toxicity by U0126 an ERK1/2 and p38 kinase inhibitor, Biochem. Pharmacol., 2001, vol. 62, p. 1379. https://doi.org/10.1016/S0006-2952(01)00780-8
Stalnaya, I.D. and Garishvili, T.G., Method for determination of malondialdehyde with the use of thiobarbituric acid, in Modern Methods in Biochemistry, Moscow: Medicine, 1977, p. 66.
Suzuki, N., Yamamoto, M., Watanabe, K., et al., Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: the scale is a good model for the evaluation of heavy metals in bone metabolism, J. Bone Miner. Metab., 2004, vol. 22, p. 439. https://doi.org/10.1007/s00774-004-0505-3
Viarengo, A., Bettella, E., Fabbri, R., et al., Heavy metal inhibition of EROD activity in liver microsomes from the bass Dicentrarchus fabrax exposed to organic xenobiotics: role of GSH in the reduction of heavy metal effects, Mar. Environ. Res., 1997, vol. 44, no. 1, p. 1. https://doi.org/10.1016/S0141-1136(96)00097-9
Vlahogianni, T.H. and Valavanidis, A., Heavy-metal effects on lipid peroxidation and antioxidant defence enzymes in mussels Mytilus galloprovincialis,Chem. Ecol., 2007, vol. 23, no. 5, p. 361. https://doi.org/10.1080/02757540701653285
Vlastov, B.V. and Kachanova, A.A., Sex observations of living D. polymorpha and some data on the sexual cycle in this mollusk, Russ. Zool. J., 1959, vol. 38, no. 7, p. 991.
Winston, G.W. and di Giulio, R.T., Prooxidant and antioxidant mechanisms in aquatic organisms, Aquat. Toxicol., 1991, vol. 19, p. 137. https://doi.org/10.1016/0166-445X(91)90033-6
Funding
This work was carried out under research program no. АААА-А18-118012690123-4, with partial financial support from the priority project Volga Rehabilitation no. AAAA-A18-118052590015-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by D. Pavlov
Rights and permissions
About this article
Cite this article
Klimova, Y.S., Chuiko, G.M., Gapeeva, M.V. et al. The Use of Oxidative Stress Parameters of Bivalve Mollusks Dreissena polymorpha (Pallas, 1771) as Biomarkers for Ecotoxicological Assessment of Environment. Inland Water Biol 12 (Suppl 2), 88–95 (2019). https://doi.org/10.1134/S1995082919060063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082919060063