Abstract
In this study, the genotoxic effect of contaminants was assessed through detection of DNA damage using the micronucleus (MNs) test in erythrocytes from 149 flounder fish collected in two regions of the Gulf of Mexico (GoM). The frequency of microcytes (MCs) was also evaluated in the same group of fish collected from the Perdido Foldbelt (PF) and the Yucatan Platform (YP). The MCs frequency was different among locations of the YP (p = 0.011), while MNs frequency varied among locations of PF (p = 0.024). MCs and MNs values correlated with heavy metals from fish muscle, fish species and localities. Mean number, prevalence, and intensity of MCs and MNs correlated with Al, PAHs, depth, and locality. MNs frequency showed a species-specific association (p = 0.004). MNs and MCs were associated with heavy metals and PAHs from fish muscle and sediments, and the MNs frequency was species dependent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Crude oil is one of the most widespread pollutant released into the marine environment causing a wide range of biological effects in native species (Bejarano 2018). In the Gulf of Mexico (GoM) there is always a constant menace of oil spill events because crude oil production activities are very important in the region (Allan et al. 2012; Ward and Tunnell 2017). In 2010, around 4.4 × 106 oil barrels were released into the sea for 87 days in the Deepwater Horizon oil spill blowout (Perez et al. 2017). Offshore fish was used to detect the accumulation of polycyclic aromatic hydrocarbons (PAHs) after the DWH disaster (Murawski et al. 2020).
Flounder fish have been used as bioindicators of habitat disturbance because they are in direct contact with the marine sediments (Bolognesi et al. 2006; Conti and Iacobucci 2008; Holt and Miller 2010). And some components of oil are highly persistent (Gregson et al. 2021). Sediments act as sinkholes for hydrocarbons, heavy metals, and other contaminants. These substances become bioavailable to flatfish and are accumulated in their tissues, altering their physiology and reproduction (Kirby et al. 2000; van der Oost et al. 2003; Holt and Miller 2010; Rahmanpour et al. 2016; Yu et al. 2019).
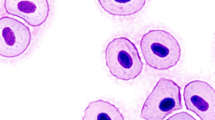
The effect of oil pollutants can be assessed by in vivo and in vitro assays where cellular and molecular biomarker responses are implemented as indicators of environmental disturbances (Gold-Bouchot et al. 2017; Praveen Kumar et al. 2017). The presence of abnormal cells in blood smears of fish are useful to detect chromosomic damage produced by a wide range of toxic compounds, specifically by detecting binucleated or polynucleated cells containing micronucleus (MNs) (Ayanda et al. 2018). The MNs are extranuclear bodies that contain damaged chromosome fragments and/or whole chromosomes that were not incorporated into the nucleus after cell division. While microcytes (MCs) are small mature erythrocytes characterized by having a smaller diameter when compared to normal erythrocytes and their presence has been associated to a deficiency of hemoglobin formation linked to anemia and malnourishment (Alkaladi et al. 2015). The MNs assay are biomarkers used to detect chromosomic damage induced by a variety of genotoxic agents (Sommer et al. 2020). Fish challenged with Cd showed a time-dependent increase in the frequency of MNs and nuclear abnormalities that had high correlation with cytoplasmic cell alterations including MCs (Jindal and Verma 2015).
The objectives of this study were to report the presence of MNs and MCs in seven species of fish flounder from two areas of the GoM as well as to associate these values with oil pollutants from sediments and fish muscle.
Methods and Materials
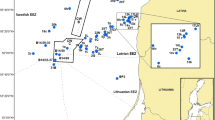
A permit for collection (PPF/DGOPA-070/16) was issued by Comisión Nacional de Acuacultura y Pesca. Flounder fish were collected from two areas of the GoM; 41 fish from eight locations (4 to 6 fish per location) from the Perdido Foldbelt (PF). Sampling was done aboard the oceanographic cruise “Justo Sierra”-UNAM using benthic sled trawlers with 2.40 m of width, 0.90 m of height, 5 m of coded large and mesh size of 0.0254 m. Trawling of approximately one mile was performed at depths of 44 to 107 m between the 12th to 19th in May 2016. In the Yucatan Platform (YP), 108 fishes were collected using a boat from the commercial shrimp fleet implemented with net shrimp trawls of 18.3 m and 0.034 m of mesh size. Sampling was done on two occasions: In 2015 (November 17th to 18th) five locations were surveyed and in 2016 (April 13th to 16th), 13 locations were surveyed. In both cases four to 10 organisms per location were collected in trawlers of approximately one mile done at depths of 40 to 200 m (Fig. 1) (Supplementary Table 1).
After capture, ≈ 20 µL of blood was collected from the caudal vein of each fish using a hypodermic syringe and dropped on glass-slides to perform two blood smears from each fish. Smears were air dried and fixed in absolute methanol (Hycel®) for 10 min (Al-Sabti and Metcalfe 1995). Subsequently, fishes were euthanized using a sharp scalpel on the fish head. After that, they were dissected, and their sex was identified by visual inspection. Muscle tissue was collected above the lateral line from both sides of the fish avoiding the abdominal cavity. Muscle samples were stored at − 20°C for pollutants analyses: PAHs (polycyclic aromatic hydrocarbons) and heavy metals (V, Ni, Cd and Pb). Also, approximately 200 g of sediments from nine locations (G34, H39, I43, J48, L59, O73, P78, P79 and P80) from the YP and from each location of the PF (B1, B2, C1, C2, D1, D2, F1 and F2) were collected for contaminant (PAHs, Al, V, Ni, Cd and Pb) analyses. For heavy metals quantifications, sediments samples were placed in plastic bags previously washed with a 1 M HNO3 (Sigma-Aldrich pure grade) solution and deionized water, while for PAHs quantification, they were placed in glass containers previously washed with hexane and acetone (both Sigma-Aldrich chromatographic grade). Both samples were kept at 4ºC until further analysis.
For the MNs and MCs assay, glass slides were stained with 10% Giemsa solution for 8 min, air dried, and then analysed under bright-field microscopes Olympus BX51 at 100× (Baršiene et al. 2004). The MNs and MCs were scored from 3000 mature erythrocytes analysed in each sample. MNs were identified as small nuclei separated from the main nucleus. MCs were identified from normal erythrocytes by having sizes less than one-third of the normal erythrocyte. Results were expressed as frequency (MNs/3000 mature erythrocytes and MCs/3000 mature erythrocytes), mean value (an average of n numbers computed by adding some function of the numbers and dividing by some function of n), prevalence (%) (the number of organisms with MNs or MCs, divided by the total number of organisms in the sample), and mean intensity (the mean number of MNs or MCs found in infected hosts in a particular population (see Jindal and Verma 2015).
PAHs concentration in muscle was measured in the dry-freeze tissue following the procedures described by MacLeod et al. (1985) and Wade et al. (1988), and in the dry-freeze sediments following Wade et al. (1988). Previous to column chromatographic extraction, samples were fortified using a surrogate solution of deuterated PAHs (1–3 dimethyl-2 nitrobenzene; acenaphthene δ10, phenanthrene δ10, pyrene δ10, triphenyl phosphate, chrysene δ10 and perylene δ10 from UltraScientific). Detection limits for PAHs ranged from 0.0152 (benzo[a]anthracene) to 0.4039 (benzo[a]pyrene) and all recovery percentages were above 60%. Compounds were identified and quantified using a Clarus 500 Perkin Elmer gas chromatographer coupled to a mass spectrometer detector by the full scan and the ion selected methods (Wang et al. 1994).
For heavy metals analysis, samples were acid-digested (MARS 6-CEM, EPA method 3052) with nitric acid trace metal grade. Cd, Ni, V and Pb quantification was done using an ICP-MS iCAP Q Thermo Scientific using the ions 51V, 60Ni, 112Cd and 208Pb. Multielement solutions in several concentrations were prepared from a multistandard solution according to manufacturer instructions. PACS3 and MESS4 (National Research Council Canada) certified reference material was used to determine precision and accuracy of the analysis. Recovery percentages (RP) were 89.5% for 51V, 86% for 60Ni, 111% for 112Cd, and 118% for 208Pb; the coefficient of variation (CV) for V = 20.4%, for Ni = 21.1%, for Cd = 23.7% and for Pb = 24.5% in PACS3. In CRM MESS4, RP were 83% for 51 V, 81% for 60Ni, 95% for 112Cd, and 112% for 208Pb with a CV of 15.2% for V, for Ni = 22.1%, 8.9% for Cd and for Pb = 22.5%. Detection limits (DL) were 0.0043 µg g−1 for V, 0.0049 µg g−1 for Ni, 0.0039 µg g−1 for Cd, and 0.0053 µg g−1 for Pb, calculated as three times the standard deviation of the measured concentrations of 12 blanks.
The data mapping of all locations was geopositioned with Ocean Data View software version 4.7.9. The variability of MNs and MCs frequencies between localities and fish species were compared with Fisher´s exact test using the Statistica 8.0 software. The association of variables like frequency, mean number, prevalence and intensity of MNs and MCs with sex, fish species, depth and pollutants (PAHs, heavy metals) from fish tissues and sediments were established using the generalised additive models for location of scale and shape (GAMLSS) (Rigby and Stasinopoulos 2005). Previously, the multicollinearity of variables was evaluated through a variance inflation factor index (VIF) and Spearman’s correlation, for which the usdm package of R was used (Naimi et al. 2014). To decide which variables were discarded, a threshold of VIF < 4 was established and considered in the GAMLSS analysis (Zuur et al. 2010). The Akaike information criterion (AIC) value in the model setting GAMLSS package in R was used to fit the models. The best statistical model was selected by performing a forward procedure using the stepGAIC (generalised Akaike information criterion) function in the GAMLSS package, as this assessed the contribution of each variable and their combinations in the final model through an iterative process. This function chooses the best model based on the lowest AIC value. In addition, the goodness of fit of each model was evaluated through the explained deviance (ED), expressed as a percentage (Rigby and Stasinopoulos 2005). Finally, similitude of mean MNs for the species was tested with MVSP 3.1 software.
Results and Discussion
This study represents the first approach of using MNs and MCs from flounder fish collected from the YP and PF of the GoM. The values of MNs and MCs were associated with data of pollutants obtained from fish muscle and sediments. Fish collected from location C1 of PF were the most affected. Three to nine MNs/fish were observed, 100% of prevalence and mean intensity of 5. Also 60% of sampled fish harbored MCs. The location M63 from YP was the least affected because MNs were not detected there (Table 1). Likewise, we found significant differences in MCs frequencies between locations in the YP (F17;108 = 32.952; p = 0.011) but not in locations from the PF (F7;41 = 0.976; p = 0.995). No significant differences of MNs frequencies were found among locations from the YP (F17;108 = 24.939; p = 0.096), but significant differences were found in locations from the PF (F7;41 = 16.17; p = 0.024). MNs are useful biomarkers associated to genotoxicity (Jindal and Verma 2015; Shah et al. 2020), and the MCs are more related to health status of fish, but in controlled studies the frequency of MCs showed a high time-dependent correlation to the frequency of MNs (Jindal and Verma 2015). Based in the results of this study, we could speculate that MCs can be also useful biomarkers, but this topic needs further considerations in laboratory and field studies. For instance, MNs are used in monitoring studies and the variation on their frequencies is associated with anthropogenic pressure and pollution like the presence of heavy metals in each location (Rebok et al. 2017). Our statistical analysis suggested that MNs and MCs frequencies could be subjected to the environmental condition of localities sampled, in special, because the PF areas are used for petroleum exploration and exploitation.
Heavy metal values detected herein in fish muscle (except for Cd) were higher in the PF than in the YP, however PAHs values were higher in the YP than in PF (Table 2). Our data values differ with previous reports in the GoM. During 2012–2014 the muscle tissues of flounder fish Syacium gunteri presented lower values of heavy metals and PAHs in YP than in PF (Quintanilla-Mena et al. 2019). Whereas in 2016, higher PAHs concentrations were detected in tissues of the fish Lopholatilus chamaeleonticeps from the YP than the ones from the PF (Snyder et al. 2020). Probably the higher values of PAHs and heavy metals found in PF were related to remains of the Macondo oil spill occurred in 2010. Fish have a high capacity to metabolize PAHs through a well-developed enzymatic system (Baali et al. 2016), thus, the PAHs values decrease in fish muscle. Nevertheless, the higher values observed in this study in the YP, could be related to bioaccumulation of contaminants from different sources.
In sediments the heavy metals (except for Cd) and PAHs concentration were higher in PF than in YP (Table 2). PAHs concentration for the YP (18.59 ± 15.72 µg kg−1) and the PF (82.16 ± 60.38 µg kg−1) are considered low according to the classification suggested by Recabarren-Villalón et al. (2019) (PAHs low values from 10 to 100 µg kg−1). The variations of contaminants levels in sediments between YP and PF could be reflecting remains of contaminants from the oil spill occurred in 2010 in the northern GoM (McNutt et al. 2012) although, local inputs such as extraction, production, and transport of petroleum, among others (Murawski and Hogarth 2013), and contaminant transport related with the hydrology and the hydrodynamics, could also be contributing to the contaminant load (Botello et al. 2015).
The GAMLSS models of MCs and MNs frequency correlated with heavy metals from fish muscle, fish species and the locality, with a 20.37% and 25.15% contribution to the explained deviance, respectively (Table 3). Heavy metals are bioaccumulated in the muscle of fish and it is widely known that Cd, V, Pb and Ni induce the formation of MNs and MCs and that the values are different between species (Singh et al. 2019; Shah et al. 2020).
The variability of metals levels found in fish tissue can be subjected to feeding habits, habitats (location) and behaviour of species (Ergene et al. 2007). In this sense, our results indicate that the MNs and MCs have a multifactorial induction and can be closely related to the biology of the species. The MNs and MCs prevalence, intensity and mean values correlated with Al, PAHs, depth, and locality (31.08%, 59.13% and 33.19% explained deviance to MNs, and 43.22%, 60.45% and 76.37% to MCs respectively) (Table 3). The contaminants detected in sediments from this study could persist for decades in the subsurface layers and organisms could bioaccumulate and incorporate them into their food chains, increasing the risk for humans through fish and seafood consumption (Bianchini and Morrissey 2018). Likewise, the PAHs and heavy metals mutagenicity affect directly on DNA damage, and also induce MCs formation through cytoplasmic alterations (Shah et al. 2020). PAHs are considered the most toxic components of crude oil and have genotoxic effects including DNA damage and DNA mutation (Santana et al. 2018). The exposure of fish to PAHs of oil-contaminated sediment can cause severe adverse effects like metabolic changes, energy imbalance, alterations in organs structure, altered intestinal microbiome structures and increased mortality (Brown-Peterson et al. 2015). Our results suggest that the variation of PAHs and heavy metals in the location samples at different depths are important factors in the variation of the prevalence, mean and intensity of the MNs and MCs because they are associated with the habitat and fish behaviour (Ergene et al. 2007).
From the 149 flounders collected we detected 7 species: Syacium papillosum (n = 81), Cyclopsetta chittendeni (n = 18), Cyclopsetta fimbriata (n = 11), Syacium micrurum (n = 4), Ancyclopsetta dilecta (n = 8), Syacium gunteri (n = 22), and Trichopsetta ventralis (n = 5). In all species MNs were detected (range = 0–9 MNs per fish), as well as MCs (range = 0–15 MCs per fish), but the highest number of MNs was found in C. chittendeni (F6;149 = 18.99; p = 0.004). In this sense, fish response to xenobiotics as the PAHs differs among the species and stages (Recabarren-Villalón et al. 2019). In contrast, the MCs frequency variation was not significant for the different species (F6;149 = 5.23; p = 0.515). The MVPS analysis showed 45.68% of similitude of the mean MNs among S. papillosum and C. chittendeni. These results might indicate different susceptibility among species. Therefore, the selection of sentinel species to assess xenobiotics impact in aquatic environments is a key factor that needs to be evaluated deeply to obtain more accurate information. The presence of environmental contaminants has different accumulation rates that depend on their type, lifetime, and toxicity.
In conclusion, this study represents the first effort to evaluate the presence of MNs and MC in flounders from two important areas of the GoM. Their values were related to PAHs and heavy metals exposition and showed a negative association to health or fitness of fish. MNs and MCs frequencies were more evident in the PF than in the YP. PAHs found in fish muscle were higher than the values found in sediments. MNs frequency showed a species-specific association being C. chittendeni the most affected organism. Also, we consider that MNs and MCs inductions are multifactorial with a probable relevant influence of the fish biology. Our results support the potential use of MNs and MCs as biomarkers in environmental pollution studies.
References
Alkaladi A, El-Deen NAMN, Afifi M, Zinadah OAA (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563. https://doi.org/10.1016/j.sjbs.2015.02.012
Allan SE, Smith BW, Anderson KA (2012) Impact of the Deepwater Horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol 46(4):2033–2039. https://doi.org/10.1021/es202942q
Al-Sabti K, Metcalfe CD (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res Toxicol 343:121–135. https://doi.org/10.1016/0165-1218(95)90078-0
Ayanda IO, Yang M, Yu Z, Zha J (2018) Cytotoxic and genotoxic effects of perfluorododecanoic acid (PFDoA) in Japanese medaka. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2017058
Baali A, Kammann U, Hanel R et al (2016) Bile metabolites of polycyclic aromatic hydrocarbons (PAHs) in three species of fish from Morocco. Environ Sci Eur 28:25. https://doi.org/10.1186/s12302-016-0093-6
Baršiene J, Lazutka J, Šyvokiene J et al (2004) Analysis of micronuclei in blue mussels and fish from the Baltic and North Seas. Environ Toxicol 19:365–371. https://doi.org/10.1002/tox.20031
Bejarano AC (2018) Critical review and analysis of aquatic toxicity data on oil spill dispersants. Environ Toxicol Chem 37(12):2989–3001. https://doi.org/10.1002/etc.4254
Bianchini K, Morrissey CA (2018) Assessment of shorebird migratory fueling physiology and departure timing in relation to polycyclic aromatic hydrocarbon contamination in the Gulf of Mexico. Environ Sci Technol 52:13562–13573. https://doi.org/10.1021/acs.est.8b04571
Bolognesi C, Perrone E, Roggieri P, Sciutto A (2006) Bioindicators in monitoring long term genotoxic impact of oil spill: Haven case study. Mar Environ Res 62:S287–S291. https://doi.org/10.1016/j.marenvres.2006.04.047
Botello AV, Soto LA, Ponce-Velez G, Villanueva-F S (2015) Baseline for PAHs and metals in NW Gulf of Mexico related to the Deepwater Horizon oil spill. Estuar Coast Shelf Sci 156:124–133. https://doi.org/10.1016/j.ecss.2014.11.010
Brown-Peterson NJ, Krasnec M, Takeshita R et al (2015) A multiple endpoint analysis of the effects of chronic exposure to sediment contaminated with Deepwater Horizon oil on juvenile Southern flounder and their associated microbiomes. Aquat Toxicol 165:197–209. https://doi.org/10.1016/j.aquatox.2015.06.001
Conti ME, Iacobucci M (2008) Marine organisms as biomonitors. . WIT Transact State-of-the-art Sci Eng 30:81–110. https://doi.org/10.2495/978-1-84564-002-6/04
Ergene S, Cavas T, Celik A et al (2007) Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology 16:385–391. https://doi.org/10.1007/s10646-007-0142-4
Gold-Bouchot G, Rubio-Piña J, Montero-Muñoz J et al (2017) Pollutants and biomarker responses in two reef fish species (Haemulon aurolineatum and Ocyurus chrysurus) in the Southern Gulf of Mexico. Mar Pollut Bull 116:249–257. https://doi.org/10.1016/j.marpolbul.2016.12.073
Gregson BH, McKew BA, Holland RD, Nedwed TJ, Prince RC, McGenity TJ (2021) Marine oil snow, a microbial perspective. Front Mar Sci 8:619484. https://doi.org/10.3389/fmars.2021.619484
Holt EA, Miller SW (2010) Bioindicators: using organisms to measure environmental impacts. Nature 3(10):8–13
Jindal R, Verma S (2015) In vivo genotoxicity and cytotoxicity assessment of cadmium chloride in peripheral erythrocytes of Labeo rohita (Hamilton). Ecotoxicol Environ Saf 118:1–10. https://doi.org/10.1016/j.ecoenv.2015.04.005
Kirby MF, Morris S, Hurst M, Kirby SJ, Neall P, Tylor T, Fagg A (2000) The use of cholinesterase activity in flounder (Platichthys flesus) muscle tissue as a biomarker of neurotoxic contamination in UK estuaries. Mar Pollut Bull 40(9):780–791. https://doi.org/10.1016/S0025-326X(00)00069-2
MacLeod WD, Brown DD, Friedman AJ et al (1985) Standard analytical procedures of the NOAA (National Oceanic and Atmospheric Administration) national analytical facility, 1985–1986: extractable toxic organic compounds, 2nd edn. S. Department of Commerce, NOAA/NMFS, NOAA Tech Memo NMFS F/NWRC-92
McNutt MK, Chu S, Lubchenco J et al (2012) Applications of science and engineering to quantify and control the Deepwater Horizon oil spill. Proc Natl Acad Sci USA 109:20222–20228. https://doi.org/10.1073/pnas.1214389109
Murawski SA, Hogarth WT (2013) Enhancing the ocean observing system to meet restoration challenges in the Gulf of Mexico. Oceanography 26(1):10–16. https://doi.org/10.5670/oceanog.2013.12
Murawski SA, Hollander DJ, Gilbert S, Gracia A (2020) Deep-water oil and gas production in the Gulf of Mexico, and related global trends. In: Murawski SA, Ainsworth C, Gilbert S, Hollander D, Paris CB, Schlüter M et al (eds) Scenarios and responses to future deep oil spills—fighting the next war. Springer, Cham
Naimi B, Hamm NAS, Groen TA et al (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37:191–203
Perez CR, Moye JK, Cacela D et al (2017) Low level exposure to crude oil impacts avian flight performance: The Deepwater Horizon oil spill effect on migratory birds. Ecotoxicol Environ Saf 146:98–103. https://doi.org/10.1016/j.ecoenv.2017.05.028
Praveen Kumar MK, Shyama SK, D’Costa A et al (2017) Evaluation of DNA damage induced by gamma radiation in gill and muscle tissues of Cyprinus carpio and their relative sensitivity. Ecotoxicol Environ Saf 144:166–170. https://doi.org/10.1016/j.ecoenv.2017.06.022
Quintanilla-Mena M, Gold-Bouchot G, Zapata-Pérez O et al (2019) Biological responses of shoal flounder (Syacium gunteri) to toxic environmental pollutants from the southern Gulf of Mexico. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113669
Rahmanpour S, Ashtiyani SML, Ghorghani NF (2016) Biomonitoring of heavy metals using bottom fish and crab as bioindicator species, the Arvand River. Toxicol Ind Health 32:1208–1214. https://doi.org/10.1177/0748233714554410
Rebok K, Jordanova M, Slavevska-Stamenković V et al (2017) Frequencies of erythrocyte nuclear abnormalities and of leucocytes in the fish Barbus peloponnesius correlate with a pollution gradient in the River Bregalnica (Macedonia). Environ Sci Pollut Res 24:10493–10509. https://doi.org/10.1007/s11356-017-8665-6
Recabarren-Villalón T, Ronda AC, Arias AH (2019) Polycyclic aromatic hydrocarbons levels and potential biomarkers in a native South American marine fish. Reg Stud Mar Sci 29:100695. https://doi.org/10.1016/j.rsma.2019.100695
Rigby RA, Stasinopoulos DM (2005) Generalized additive models for location, scale and shape. J R Stat Soc Ser C Appl Stat 54:507–554
Santana MS, Sandrini-Neto L, Filipak Neto F et al (2018) Biomarker responses in fish exposed to polycyclic aromatic hydrocarbons (PAHs): systematic review and meta-analysis. Environ Pollut 242:449–461. https://doi.org/10.1016/j.envpol.2018.07.004
Shah N, Khan A, Ali R, Marimuthu K et al (2020) Monitoring Bioaccumulation (in Gills and Muscle Tissues), Hematology, and Genotoxic Alteration in Ctenopharyngodon idella Exposed to Selected Heavy Metals. Biomed Res Int. https://doi.org/10.1155/2020/6185231
Singh M, Khan H, Verma Y, Rana SVS (2019) Distinctive fingerprints of genotoxicity induced by As, Cr, Cd, and Ni in a freshwater fish. Environ Sci Pollut Res 26:19445–19452. https://doi.org/10.1007/s11356-019-05274-z
Snyder SM, Olin JA, Pulster EL, Murawski SA (2020) Spatial contrasts in hepatic and biliary PAHs in Tilefish (Lopholatilus chamaeleonticeps) throughout the Gulf of Mexico, with comparison to the Northwest Atlantic. Environ Pollut 258:113775. https://doi.org/10.1016/j.envpol.2019.113775
Sommer S, Buraczewska I, Kruszewski M (2020) Micronucleus assay: the state of art, and future directions. Int J Mol Sci 21:1–19. https://doi.org/10.3390/ijms21041534
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Wade TL, Atlas EL, Brooks JM et al (1988) NOAA Gulf of Mexico status and trends program: Trace organic contaminant distribution in sediments and oysters. Estuaries 11:171–179. https://doi.org/10.2307/1351969
Wang Z, Fingas M, Li K (1994) Fractionation of a light crude oil and identification and quantitation of aliphatic, aromatic, and biomarker compounds by GC-FID and GC-MS, part I. J Chromatogr Sci 32:361–366. https://doi.org/10.1093/chromsci/32.9.361
Ward CH, Tunnell JW (2017) Habitats and Biota of the Gulf of Mexico: An Overview. In: Ward C (ed) Habitats and biota of the Gulf of Mexico: before the Deepwater Horizon oil spill. Springer, New York, pp 1–54
Yu Z, Lin Q, Gu Y, Du F, Wang X, Shi F, Ke Ch, Xiang M, Yu Y (2019) Bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in wild marine fish from the coastal waters of the northern South China Sea: risk assessment for human health. Ecotoxicol Environ Saf 180:742–748. https://doi.org/10.1016/j.ecoenv.2019.05.065
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
Special thanks are conveyed to the personnel of the laboratory of Geochemistry (CINVESTAV-Unidad Merida) for processing samples. Research funded by the National Council of Science and Technology of Mexico – Mexican Ministry of Energy – Hydrocarbon Trust, project 201441. This is a contribution of the Gulf of Mexico Research Consortium (CIGoM) and PEMEX. We acknowledge PEMEX´s specific request to the Hydrocarbon Fund to address the environmental effects of oil spills in the Gulf of Mexico. The authors are grateful to the comments of the anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ek-Huchim, J.P., Árcega-Cabrera, F., May-Tec, A.L. et al. Red Blood Cell Cytotoxicity Associated to Heavy Metals and Hydrocarbons Exposure in Flounder Fish from Two Regions of the Gulf of Mexico. Bull Environ Contam Toxicol 108, 78–84 (2022). https://doi.org/10.1007/s00128-021-03176-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03176-w