Abstract
Biomarkers are applied as early warning indicators of organisms’ exposure to pollutants. The aim of this study was to utilise a multi-biomarker approach in the freshwater shrimp Caridina nilotica (Decapoda: Atyidae) as indicators of persistent pollutant exposure. A suite of biomarkers was selected to cover oxidative stress and damage, and energetics of the organisms. Five sites, representing an agricultural and pesticide application gradient, were sampled during two flow related hydro-periods in rivers of the Phongolo floodplain, north-eastern South Africa. Cytochrome P450 (CYP) activity was significantly higher in shrimp at sites directly adjacent to regions of increased human activity. Increased oxidative responses, i.e. catalase (CAT; p < 0.01) and protein carbonyl (PC, p < 0.01) were also found at these sites. The energetics biomarker did not show any influence of increased contaminant exposure. We demonstrated that the biomarkers of exposure (CYP) and effect (CAT, PC) were suitable to detect effects of stressors, probably persistent pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Phongolo River and associated floodplain is one of southern Africa’s largest floodplain systems and the area’s sub-tropical climate supports high biodiversity. Located in Maputaland, KwaZulu-Natal province of South Africa, the most northern section of the system within the country is situated in a conservation area, the Ndumo Game Reserve (NGR). In 1991 the area was proclaimed a Ramsar accredited wetland of international importance (Malherbe 2018). This area is an endemic malaria area and malaria vector control is achieved using, amongst other pesticides, dichlorodiphenyltrichloroethane (DDT, Smit et al. 2016). Although the area is protected, conservation authorities cannot control water quality as this originates upstream of the protected areas’ borders. The catchment upstream of NGR is subjected to large-scale commercial and small-scale subsistence agriculture. The extensive irrigation along the Phongolo River and application of malaria vector control has resulted in high levels of organochlorine pesticide (OCP) exposure of aquatic (Wepener et al. 2012) and terrestrial (Thompson et al. 2017) organisms in the area.
In a recent study upstream of and in the NGR, Wolmarans et al. (2018) demonstrated a clear relationship between OCP exposure in the African clawed toad (Xenopus laevis) and biochemical responses. Biomarkers are used as “early warning” signs indicating potential risks to organisms following exposure to toxicants. Toxicants impact aquatic systems in a hierarchical manner, affecting individual organisms at the subcellular and cellular level before progressing to alterations in populations or ecosystem structures (van der Oost et al. 2003). Biomarkers of exposure can provide insight into the specific type of pollutant that an organism is exposed to, e.g. Cytochrome P450 induction in response to organic pollutant exposure (Han et al. 2017). Biomarkers of effect indicate some form of biochemical/physiological alterations in the form of anti-oxidant (Hong et al. 2018) or energetic (Goodchild et al. 2019) responses. For this study a standard suite of biomarkers was selected to cover a broad range of responses such as Phase I and II biotransformation pathways, oxidative stress and changes in energy reserves due to oxidative damage.
The freshwater shrimp Caridina nilotica is an important component of food webs in tropical African freshwater systems by providing food for predators and cycling organic matter (Mensah et al. 2012). Over the years it has been shown that there is persistent organic pollutant (POP) contamination of the Phongolo River. Most of these chemicals have been banned but are still detected due to their long half-life, including hexachlorobenzenes (HCBs), hexachlorocyclohexanes (HCHs) and chlordanes, while DDT is still being used for ongoing malaria vector control (Wepener et al. 2012; Smit et al. 2016; Wolmarans et al. 2018; Volschenk et al. 2019). The aim of this study was to evaluate whether biomarker responses in C. nilotica reflect the known POP exposure as shown by the recent surveys. We test the hypotheses that (i) biomarker responses in C. nilotica will reflect the higher organic pollutant exposure outside of the NGR conservation area and that (ii) there are increased responses to greater persistent pollutant exposure during the high flow sampling period due to a combination of increased pesticide use through agricultural activities, malaria vector control and run-off from the catchment.
Materials and Methods
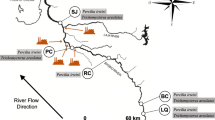
Surveys were conducted at five sites during high flow (April 2014, HF) and low flow (October 2014, LF) hydrological conditions. Organisms were only found at three out of the five sites during the HF survey. Site 1 is situated along the Usuthu River, site 2 along the Ngwavuma River, a tributary of the Phongolo River, and three sites are situated along the Phongolo River (Sites 3, 4 and 5, Fig. 1). Sites were selected to represent a gradient of human habitation and agricultural activities as reported by Smit et al. (2016). Sites 3 and 4 are situated upstream of the NGR (site 5) at the Jozini Town and Ndumo rural area respectively. Sites 1 and 5 in the NGR represent lower-impacted sites, which have also been proposed as a potential refuge for aquatic biota (Smit et al. 2016).
Organisms were collected by agitating peripheral vegetation with a 1 mm mesh size net. Whole organisms were placed in cryovials, inundated with Henriksson’s stabilising buffer and flash frozen/stored in liquid nitrogen. Samples were kept at − 80°C until analysis when they were thawed, weighed (± 0.01 mg) and placed into the appropriate homogenizing buffer (at the designated ratio, w/v) related to the protocols described below. Samples were pooled due to their small size, with approximately three cephalothorax sections used for hepatopancreas based biomarkers and two abdomen samples used for energetic biomarkers. Final replicate numbers are indicated in Table 1.
Acetylcholinesterase activity (AChE, Ellman et al. 1961) and metallothionein content (MT, Viarengo et al. 1997) were analysed as biomarkers of exposure. Phase I and Phase II metabolism of xenobiotics were analysed through the determination of cytochrome P450 (CYP, DetectX P450 demethylating fluorescent activity kit, Arbor Assays, K011-F1) and reduced glutathione (GSH, Cohn and Lyle 1966) activity. Insight into the anti-oxidant responses of the organisms was generated using superoxide dismutase (SOD, Del Maestro and McDonald 1985), catalase (CAT, Cohen et al. 1970) activity, protein carbonyl (PC, Floor and Wetzel 1998) and malondialdehyde (MDA, Ohkawa et al. 1979) content as indicators of oxidative stress. All aforementioned biomarkers were determined in hepatopancreas tissue while cellular energy allocation (CEA, De Coen and Janssen 2003) was determined in muscle tissue. The CEA is a calculation-based biomarker, using quantified components of energy stores and energy consumption. The components used in the calculation are protein (Bradford 1976), lipids (Bligh and Dyer 1959) and carbohydrates (Roche, GOD-PAP 1448668), and energy consumption (De Coen and Janssen 2003). Cellular energy allocation was calculated as CEA = Ea – Ec where Ea (energy allocation) is the sum of protein, lipids and carbohydrates, and Ec is energy consumption. The Ea components were converted into energy equivalents by using enthalpy of combustion of 24 J/g, 39.5 J/g and 17.5 J/g for protein, lipids and carbohydrates respectively. The Ec was calculated using the theoretical stoichiometric relationship that for every 2 µmol of formazan formed, 1 µmol O2 was used by the electron transport system in the mitochondria.
All colorimetric and fluorometric analyses were carried out using an ELx 800 and an FLx 800 universal microplate reader, respectively (Biotek Instruments Corp). Biomarker activities are expressed per milligram protein, to allow for standardisation. Protein content for each biomarker (except CEA) was determined using the protein-dye binding method (Bradford 1976), with bovine serum albumin as a standard. All biomarker measurements were done in triplicate.
A log10 transformation was applied after biomarker data failed Shapiro-Wilk and Levene’s tests for parametric assumptions. The data then met parametric assumptions and a two-way ANOVA with Sidak’s multiple comparison test (site and hydrological period as independent variables) was applied and statistical significance was regarded as p < 0.05. All statistical analyses were done using SPSS version 21 (IBM Software Group). Discriminant function analysis (DFA) was used to investigate the differences among dependent variables (sites) on the basis of the independent variables (biomarkers) and indicates how much the independent variables have contributed to group separation (Quinn and Keough 2002). The DFA discriminates sites on a bi-plot and indicates which variable is the strongest driver for discrimination on each axis. Box-and-whisker plots were produced in GraphPad Prism version 5 and maps were made using QGIS version 3.8.
Results and Discussion
It is standard to apply a suite of biomarkers to provide an integrated reflection of biological exposure and effects of aquatic organisms to xenobiotics (Wepener 2008). Lipophilic pollutants such as OCPs induce defence mechanisms in organisms, such as CYP, that results in the formation of polar compounds to ease excretion (Han et al. 2017). There was a significant interaction between spatial and temporal CYP activities (F = 5.74; p = 0.008). The significant increase in CYP activity in shrimp from sites 2, 3 and 4 (low flow) are possibly indicative of stimulation of Phase I biotransformation in response to higher organic pollutant exposure than at the sites with lower direct human influence, i.e. sites 1 and 5 (Fig. 2a). The exposure of C. nilotica to DDTs and HCHs is highly likely since these persistent compounds were measured in five fish species from different trophic levels such as the predatory Tigerfish (Hydrocynus vittatus) and herbivorous Mozambique Tilapia (Oreochromis mossambicus), collected during the same hydro-period from the lower Phongolo River system (Volschenk et al. 2019). The induction is also significant during the high flow period, which coincides with the summer months when OCPs are applied both for agricultural pest and malaria vector control. The clear separation on the DFA biplot (63% of variation explained by function 1) between biomarkers of exposure in shrimp inside the NGR and at sites associated with increased human activities outside the reserve is driven primarily by CYP activity (Fig. 3a). Similarly, there were significant spatial and temporal interactions for GSH (F = 3.35; p = 0.048) and MTs (F = 8.66; p = 0.001). The reduced response from high to low flow was also noted, at site 1, in GSH activity (Fig. 2b) which is regarded as a biomarker involved in Phase II biotransformation through conjugation (Gunderson et al. 2018). Considered a good biomarker of metal exposure, MTs are involved with the homeostasis, and removal, of metals in organisms’ tissues (Kemp et al. 2017). It is therefore likely that the MT induction (Fig. 2c) observed in samples from sites 1 and 5 during low flow survey was in response to the higher metal exposure at these sites. Significantly lower levels of MT expression at sites 2 and 4 during the low flow survey may be attributed organic pollutants inhibiting the synthesis of MT (Gerpe et al. 2000). The temporal differences in MT activities are reflected on Function 2 (30% of the variation explained) of the DFA bi-plot (Fig. 3a). There were no significant spatial and temporal interactions (F = 1.21; p = 0.31) in AChE activity found in C. nilotica from the study area (Fig. 2d) possibly indicating the low use and subsequent exposure to organophosphate and carbamate pesticides. Similar results were also found by (Wolmarans et al. 2018) for Müller’s clawed frog (Xenopus muelleri) that were sampled from the same sites.
Box-and-whisker plots representing median and 5–95 percentile range of all biomarker measurements in Caridina nilotica. Grey fill indicates high flow and no fill indicates low flow hydro-periods. Spatially significant differences indicated with common letter superscripts while temporally significant differences indicated with common symbol superscripts (p < 0.05). Sites that are significantly different spatially and temporally are indicated with an asterisk (*). a cytochrome P450; b reduced glutathione; c metallothionein content; d acetylcholinesterase activity; e superoxide dismutase; f catalase activity; g protein carbonyl content; h malondialdehyde content; i cellular energy allocation; j energy consumption; k energy allocation; l protein; m lipids; n carbohydrates
Discriminant function analysis, a biomarkers of exposure (CYP, GSH, MT and AChE) and b effect and energetics (SOD, CAT, PC, MDA, CEA and its components Ec, Ea, proteins, lipids and carbohydrates). High flow indicated by solid symbols while low flow is indicated by symbols filled with grey. Group centroids marked with an X. The first two canonical discriminant functions are shown
According to Hong et al. (2018) the exposure of crustaceans to organic pollutants results in a disturbance of reactive oxygen species (ROS) elimination due to a decrease in the efficiency of the antioxidant defence mechanisms. Both CAT (F = 9.36; p < 0.0001) and SOD (F = 8.03; p = 0.0004) displayed significant spatial and temporal interactions. The antioxidant defence consists of a cascade of enzymatic reactions in which SOD and CAT are major enzymes in the first line of defence by converting superoxide anion radicals to hydrogen peroxide and molecular oxygen, and hydrogen peroxide to water and molecular oxygen, respectively. The activity of SOD (Fig. 2e) was particularly elevated at site 2 (high flow) and site 4 (low flow). Catalase levels (Fig. 2f) were more variable during the low flow survey, the levels of both antioxidants were lowest within the NGR at sites 1 and 5. This is reflected on Function 2 (8% of the total variation explained) of the DFA ordination for biomarkers of effect (Fig. 3b). Similar increases in SOD and CAT activity were reported by Touaylia et al. (2018) and Hong et al. (2018) in freshwater crustaceans following exposure to glyphosate and pyrethroid pesticides. Increased levels of MDA and PCs (both also displaying significant—p < 0.005—spatial and temporal interactions) are used as indicators of oxidative cellular damage to lipids and proteins respectively (Hong et al. 2018). Even though there were increases in the levels of SOD and CAT, there was still an increase in cellular damage with particularly high PC levels at the sites outside the NGR (sites 2, 3 and 4) during both surveys (Fig. 2g). Site 2 and 5 showed the only significant difference for MDA (Fig. 2h).
Changes in energy acquisition and allocation may provide an indication of energetic homeostasis disruption due to exposure to multiple stressors (Goodchild et al. 2019). Only energy consumption (Ec) and carbohydrate energy contribution in C. nilotica did not have any significant spatial and temporal interactions. Cellular energy allocation (Fig. 2i) is calculated using energy consumption (Ec, Fig. 2j) and energy allocation (Ea, Fig. 2k) allowing for a more ecologically relevant interpretation (De Coen and Janssen 2003). There were no clear influences of environmental toxicant exposure on the CEA and its components as was the case for the biomarkers of oxidative stress. The protein content (Fig. 2l) and therefore the Ea and CEA of samples from site 4 during the high flow were significantly higher (p < 0.05) than any of the other sites. The significant contribution of protein energy content at site 4 may be related to increased nutrient input into the Phongolo River from the human activities in Ndumo rural area. Smolders et al. (2004) found similar increases in CEA of bivalves downstream of a municipal wastewater treatment plant. They attributed this increase to eutrophic conditions brought about by the nutrient release into the receiving stream. Cellular energy allocation showed limited responses to possible environmental stressors exposure in C. nilotica. These findings are similar to those found by Ferreira et al. (2015) following exposure of terrestrial isopods to an organophosphate pesticide. They attributed this to the limited response of the energy consumption parameter at the different sites to influence the CEA. Spatial differences in the Ea components may be the result of age, sex or physiological requirements such as breeding and moulting cycles within the separate systems, causing different reserves to be prioritised. Crustaceans undergo moulting cycles, before which they starve themselves and utilise energy reserves while in a starvation period. The first to be mobilised, is the lipid reserves, hence storage of lipids for times of starvation is a crucial biological process for all crustaceans (Sánchez-Paz et al. 2006).
The biomarker responses from the conservation area (NGR) indicate relatively lower responses indicating possibly lower persistent organic pollutant exposure during the LF winter months when there is no vector control and lower agrochemical use. This corroborates biodiversity studies that indicate that the NGR acts as a refuge for birds, amphibians and fish on the Phongolo floodplain (Smit et al. 2016). This study further supports findings by Volschenk et al. (2019) that aquatic organisms in the lower Phongolo River are exposed to diffuse sources of contaminant exposure. The suite of biomarker responses in the freshwater shrimp, C. nilotica, reflected both the spatial and temporal responses to potential environmental contamination of persistent organic pollutant exposure in the absence of chemical residue data.
References
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38. https://doi.org/10.1016/0003-2697(70)90083-7
Cohn VH, Lyle J (1966) A fluorometric assay for glutathione. Anal Biochem 14:434–440. https://doi.org/10.1016/0003-2697(66)90286-7
De Coen WM, Janssen CR (2003) The missing biomarker link: relationship between effects on the cellular energy allocation biomarker of toxicant-tressed Daphnia Magna and corresponding population characteristics. Environ Toxicol Chem 22:1632–1641. https://doi.org/10.1002/etc.5620220727
Del Maestro RF, McDonald W (1985) Oxidative enzymes in tissue homogenates. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 291–296
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Ferreira NGC, Morgado R, Santos MJG et al (2015) Biomarkers and energy reserves in the isopod Porcellionides pruinosus: the effects of long-term exposure to dimethoate. Sci Total Environ 502:91–102. https://doi.org/10.1016/j.scitotenv.2014.08.062
Floor E, Wetzel MG (1998) Increased protein oxidation in human Substantia Nigra Pars Compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem 70:268–275. https://doi.org/10.1046/j.1471-4159.1998.70010268.x
Gerpe M, Kling P, Berg AH, Olsson P-E (2000) Arctic char (Salvelinus alpinus) metallothionein: cDNA sequence, expression, and tissue-specific inhibition of cadmium‐mediated metallothionein induction by 17β‐estradiol, 4‐OH‐PCB 30, and PCB 104. Environ Toxicol Chem 19:638. https://doi.org/10.1897/1551-5028(2000)019%3C0638:ACSAMC%3E2.3.CO;2
Goodchild CG, Simpson AM, Minghetti M, DuRant SE (2019) Bioenergetics-adverse outcome pathway: Linking organismal and suborganismal energetic endpoints to adverse outcomes. Environ Toxicol Chem 38:27–45. https://doi.org/10.1002/etc.4280
Gunderson MP, Nguyen BT, Cervantes Reyes JC et al (2018) Response of phase I and II detoxification enzymes, glutathione, metallothionein and acetylcholine esterase to mercury and dimethoate in signal crayfish (Pacifastacus leniusculus). Chemosphere 208:749–756. https://doi.org/10.1016/j.chemosphere.2018.05.183
Han J, Won E-J, Kang H-M et al (2017) Marine copepod cytochrome P450 genes and their applications for molecular ecotoxicological studies in response to oil pollution. Mar Pollut Bull 124:953–961. https://doi.org/10.1016/j.marpolbul.2016.09.048
Hong Y, Yang X, Huang Y et al (2018) Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis Chemosphere 210:896–906. https://doi.org/10.1016/j.chemosphere.2018.07.069
Kemp M, Wepener V, de Kock KN, Wolmarans CT (2017) Metallothionein induction as indicator of low level metal exposure to aquatic macroinvertebrates from a relatively unimpacted river system in South Africa. Bull Environ Contam Toxicol 99:662–667. https://doi.org/10.1007/s00128-017-2201-8
Malherbe W (2018) Ramsar wetlands in South Africa: Historic and current aquatic research Ramsar wetlands in South Africa. South Afr J Sci Technol 37:1–13
Mensah PK, Palmer CG, Muller WJ (2012) Lipid peroxidation in the freshwater shrimp Caridina nilotica as a biomarker of Roundup® herbicide pollution of freshwater systems in South Africa. Water Sci Technol 65:1660–1666. https://doi.org/10.2166/wst.2012.060
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Quinn GP, Keough MJ (2002) Multivariate analysis of variance and discriminant analysis. Experimental design and data analysis for biologists. Cambridge University Press, New York, pp 425–441
Sánchez-Paz A, García-Carreño F, Muhlia-Almazán A et al (2006) Usage of energy reserves in crustaceans during starvation: status and future directions. Insect Biochem Mol Biol 36:241–249. https://doi.org/10.1016/j.ibmb.2006.01.002
Smit NJ, Vlok W, Van Vuren JHJ et al (2016) Socio-ecological system management of the lower Phongolo River and floodplain using relative risk methodology. Water Research Commission, Pretoria
Smolders R, Bervoets L, De Coen W, Blust R (2004) Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ Pollut 129:99–112. https://doi.org/10.1016/j.envpol.2003.09.027
Thompson LA, Ikenaka Y, Yohannes YB et al (2017) Concentrations and human health risk assessment of DDT and its metabolites in free-range and commercial chicken products from KwaZulu-Natal, South Africa. Food Addit Contam Part A 34:1959–1969. https://doi.org/10.1080/19440049.2017.1357209
Touaylia S, Khazri A, Mezni A, Bejaoui M (2018) Effect of permethrin (pyrethroid insecticide) on the biochemical response of the freshwater amphipod Echinogammarus tacapensis (Chevreux and Gauthier, 1924). Mar Freshw Behav Physiol 51:57–66. https://doi.org/10.1080/10236244.2018.1473711
van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Volschenk CM, Gerber R, Mkhonto MT et al (2019) Bioaccumulation of persistent organic pollutants and their trophic transfer through the food web: human health risks to the rural communities reliant on fish from South Africa’s largest floodplain. Sci Total Environ 685:1116–1126. https://doi.org/10.1016/j.scitotenv.2019.06.144
Wepener V (2008) Application of active biomonitoring within an integrated water resources management framework in South Africa. S Afr J Sci 104:367–373
Wepener V, Smit N, Covaci A et al (2012) Seasonal bioaccumulation of organohalogens in Tigerfish, Hydrocynus vittatus Castelnau, from Lake Pongolapoort, South Africa. Bull Environ Contam Toxicol 88:277–282. https://doi.org/10.1007/s00128-011-0439-0
Wolmarans NJ, Du Preez LH, Yohannes YB et al (2018) Linking organochlorine exposure to biomarker response patterns in Anurans: a case study of Müller’s clawed frog (Xenopus muelleri) from a tropical malaria vector control region. Ecotoxicology 27:1203–1216. https://doi.org/10.1007/s10646-018-1972-y
Acknowledgements
We would like to acknowledge the collaborators and team members from the Flemish Interuniversity Council (VLIR-UOS Project Number ZIEN21013PR396) and Water Research Commission of South Africa (WRC Project Number K5-2185, NJ Smit PI) that made this project possible. This work is also based on research supported in part by the National Research Foundation (NRF) of South Africa (NRF project CPRR160429163437, Grant 105979, NJ Smit, PI and NRF scarce skills scholarship Grant 89629 to G Jansen van Rensburg). Opinions expressed, and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF. We thank Ezemvelo KZN Wildlife for research permits OP 526/2014, and OP 839/2014. This is contribution number 334 of the North-West University (NWU) Water Research Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in this study were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Jansen van Rensburg, G., Bervoets, L., Smit, N.J. et al. Biomarker Responses in the Freshwater Shrimp Caridina nilotica as Indicators of Persistent Pollutant Exposure. Bull Environ Contam Toxicol 104, 193–199 (2020). https://doi.org/10.1007/s00128-019-02773-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02773-0