Abstract
The study was to identify the potential tolerance of Crotalaria juncea to diclosulam uptake and translocation and its effects on the physiological metabolism of plants. Two experiments were carried out; I—Evaluation of uptake and translocation of 14C-diclosulam (35 g a.i. ha−1) in C. juncea, at seven and 14 days after emergence. II—Evaluation of chlorophyll a transient fluorescence of dark-adapted C. juncea leaves when applied diclosulam in pre-emergence. Plants of C. juncea presented an anatomical/metabolic barrier to diclosulam translocation in the stem, which may confer tolerance to this herbicidal, besides reduced translocation due to low accumulation in the cotyledons. In addition, plants can maintain photosynthetic metabolism active when growing in soil with diclosulam by not changing the dynamics of energy dissipation. Thus, when cultivated in soil with residual of diclosulam, C. juncea can tolerate the herbicide to maintain plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Herbicides are an integral component of global agriculture used to control weeds, though herbicide applications may impose stress on registered crops (Kopsell et al. 2011). Among the herbicides, diclosulam is used in pre-emergence for the control of weeds in commercial crops (Braz et al. 2017). Diclosulam, from the triazolopyrimidine sulfonanilide chemical family, is a selective herbicide recommended for the control of dicot weeds in soybean, which acts by inhibiting the enzyme acetolactate synthase (ALS) (Hanley and Billington 2001). However, this herbicide, when applied in soybean, under a rotation system, can cause injury to subsequent crops, such as maize, due to its residual activity in soil (Karavangeli et al. 2005).
Understanding the movement and fate of herbicides in soils is an important step in limiting their environmental impacts (Carabias-Martínez et al. 2000) and, therefore, allows the use of techniques that promote the rapid degradation of herbicides in agricultural soils after cultivation, such as plants with potential to survive in between the crops rotation system. The use of tolerant plants in a context of agricultural production reduces the persistence of high residual herbicides in the soil, minimizing damage to subsequent crops, soil and groundwater (Ramborger et al. 2017), besides reducing the area release time for planting, in succession with plant species that are not tolerant to persistent compounds.
Herbicide after uptake can be metabolized in plants through conversion, conjugation, and compartmentalization which decreases the phytotoxic effects in plants capable to deal with them (Siminszky 2006). The phytotoxic effect of diclosulam is due to ALS inhibition which causes branched-chain amino acids deficiency (valine, leucine, and isoleucine), and lead to a decrease in DNA and protein synthesis (Tan et al. 2005), which adversely affects the dynamic dissipation of the photosynthetic energy (Dayan and Zaccaro 2012). Moreover, chlorophyll fluorescence measurements have become an effective and widely adapted technique to access herbicide-induced photosynthetic stress on different crop species (McCurdy et al. 2008; Kocurek et al. 2009; Silva et al. 2009; Dayan and Zaccaro 2012).
Crotalaria juncea (L.) is commonly cultivated as fodder or for fiber production (Sadhukhan and Sarkar 2016). Characterized as green manure due to its ability to return large amounts of nitrogen to the soil through a symbiotic association with bacteria (Sadhukhan et al. 2018), makes this plant desirable for cultivation. In this view, no studies have been reported regarding uptake and translocation of diclosulam herbicide by C. juncea in plant tissues. Therefore, the aim of this study was to identify the diclosulam herbicide uptake and translocation and its effects on biochemical and physiological metabolism of C. juncea cultivated in soil with residual of diclosulam herbicide.
Materials and Methods
Two experiments were carried out twice to evaluate: uptake and translocation of diclosulam herbicide in C. juncea (I); and physiological responses of C. juncea in response to diclosulam herbicide application (II). Experiment I: 14C-diclosulam herbicide uptake and translocation experiment: The experiment I was carried out with C. juncea plants grown in a greenhouse under natural light (~ 1000 µmol photons m−2 s−1) and temperature conditions (30 ± 5°C). Seeds of C. juncea were sowed in 1 dm3 pot containing soil [Planosol soil (Santos et al. 2015), containing 67% clay, 9% silt, 24% sand)], without a history of herbicide use and taken from a depth of 0–20 cm for the plantings.
14C-diclosulam (> 99% radiochemical purity) preparation was performed according to Nandula and Vencill (2015), by using the labeled herbicide based on the commercial recommended doses of non-labeled herbicide (35 g a.i. ha−1), to get final radioactivity in the work solution of 4.489.333 dpm. Each pot was constituted by two layers of soil (above described) as following: the lower layer was composed of 500 g of soil (without herbicide) and the upper layer by 30 g of soil containing the 14C-diclosulam. The seeds were sowed in the middle of both layers and afterward, water was added sufficiently to reach the field capacity to homogeneously redistribute the herbicide in the whole pot. After seedling emergence, one plant was kept and watered daily to keep the field capacity of the soil at 80%. At seventh and 14th days after the emergence, the seedlings were harvested to analyze the uptake and translocation of the labeled diclosulam.
Autoradiography image analysis: To perform autoradiography, the plants were removed from the pots, washed and dried in a forced circulation oven at 40°C until reaching a constant mass. After drying, plants were placed in an X-ray film and analyzed in an autoradiographic detector (Cyclone Plus PerkinElmer’s Storage Phosphor System with the OptiQuantTM program), according to (Crafts and Yamaguchi 1964). Radioactivity measurements: To quantify the total herbicide that was not uptake by C. juncea plants, the water, and soil remaining after the harvesting of plants were analyzed. Water was kept resting to collect the solid and after that, water was centrifuged (4000×g for 10 min). The solids of both, resting water and centrifugation were added to the remaining soil portion and the supernatant was saved for analysis. An aliquot from the supernatant quantified in a liquid scintillation spectrometer (Liquid Scintillation Analyzer 1600 TR from Packard Instruments Company Inc).
The soil portion was dried at room temperature and ground. Three replicates of 0.2 g were removed from each soil sample and burned in the biological oxidizer OX 500 to quantify the remaining herbicide. The samples were burned for 3 min in the presence of oxygen, resulting in 14CO2, which was captured by a scintillation solution containing ethanolamide (CO2 fixing solution). The radioactivity of the scintillation solution was quantified in a liquid scintillation spectrometer (Liquid Scintillation Analyzer 1600 TR from Packard Instruments Company Inc).
To quantify the total herbicide that was absorbed by C. juncea, plants were divided into groups of leaf, stem, and root to quantify the radioactivity present in the tissues. These parts were previously weighed and then burned in the biological oxidizer OX 500. As described above, the scintillation solution resulting from the combustion of the plants was quantified in a liquid scintillation spectrometer (Liquid Scintillation Analyzer 1600 TR from Packard Instruments Company Inc). The radioactivity present throughout the plant was considered absorbed and the radioactivity present in each tissue of the plant (leaf, stem or root) compared to the total absorbed radiation was considered translocated radiation (Fadin et al. 2018).
Experiment II: Physiological responses of C. juncea to diclosulam: The experiment II was carried out with C. juncea plants grown in a greenhouse under natural light (~ 1000 µmol photons m−2 s−1) and temperature conditions (30 ± 5°C). Seeds of C. juncea were sowed in 1 dm3 pot containing soil [Planosol soil (Santos et al. 2015), containing 29% clay, 7% silt, 64% sand)], without a history of herbicide use and taken from a depth of 0–20 cm for the plantings.
The seeds were sowed, and, before emergence, the herbicide was applied (pre-emergence application) using a CO2 pressurized backpack sprayer equipped with four XR-110.015 flat-fan nozzles at a pressure of 280 kPa and a speed of 3 km h−1, delivering an application volume of 150 L ha−1. The treatments consisted of doses of diclosulam herbicide: 35 g a.i. ha−1 (recommended dose); 70 g a.i. ha−1 and untreated plants (without herbicide). After seedling emergence, one plant was kept per pot and watered daily to keep the field capacity of the soil at 80%. The experimental design consisted of four replicates in a fully randomized design.
Chlorophyll a fluorescence transients measurements: Chlorophyll a fluorescence transients were measured in a dark-adapted leaf of C. juncea plants with a Handy-PEA fluorimeter (Plant Efficiency Analyser, Hansatech Instruments Ltd, UK). Eight measurements were done in each treatment of intact young leaves (fully expanded the first leaf) still attached to the plant and kept in the dark for at least 30 min. The polyphasic fluorescence rise, OJIP, was induced by one saturating red-light flash (peak at 650 nm) with 3.000 μmol photons m−2 s−1 and measured during the first second of illumination (10 µs to 1 s). The OJIP fluorescence transients were calculated as described by Strasser et al. (2004) and Tsimilli-Michael and Strasser (2008). The JIP-test was also applied for the analysis and comparison of the OJIP transients by using normalizations and subtractions. In addition, difference kinetics from relative variable fluorescence data were also calculated according to Yusuf et al. (2010).
Results and Discussion
The radioactivity image and digital image of C. juncea cultivated in the soil for 7 and 14 days with 14C-diclosulam herbicide are shown in Fig. 1. The darkness regions in plants (Fig. 1c, d), indicate the presence of radiolabelled diclosulam. Most of the absorbed diclosulam is highly accumulated in the crown area of the stem (lower portion of the stem). In addition, part of the uptake diclosulam is translocated to the shoot and accumulated in the cotyledons. The translocation increases over the time from stem to the cotyledons leading to a more accumulation in the cotyledons at 14th day (darker tissue) as shown by the radiolabelled image.
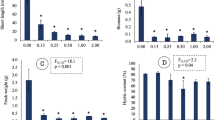
The uptake of diclosulam by C. juncea is low (less than 1%) compared to the total of the recovered radioactivity (~ 99%), being the most part of it (95%) remaining in the soil (Fig. 1e). When the herbicide is applied to the soil, it is usually obtained high recovery values in the medium (Florido et al. 2014). Walls et al. (1993), investigated the effect of the application of radiolabelled imazaquin in pre-planting (PPI) and directly on the leaf in the absorption of diclosulam in tobacco and get recovery of imazaquin in plant around 7% to 8%. However, when the herbicide is applied to the leaf, up to 90% of the herbicide is recovered in the plant. The low differential uptake shows that plants may exhibit a different mechanism of uptake and translocation, as observed in C. juncea.
After absorbed, the most part of the herbicide is translocated from root to the shoot and accumulated mainly in the stem and leaf (~ 50% and 30%, respectively; Fig. 1f, g). From the total absorbed herbicide (Fig. 2e), the most part of the diclosulam is absorbed at the initial development stage, within seven days after emergence (90%), being the uptake highly reduced from 7th to the 14th day after emergence (Fig. 2f), with the increase in the translocation from the root to the shoot over the time (Salihu et al. 1998). Besides that, after absorbed the herbicide translocation can be affected by several conditions, such as growth stage, photosynthesis rate and environmental conditions (Pester et al. 2001).
Chl a fluorescence transients of dark-adapted leaves of C. juncea grown in soil with diclosulam herbicide for 7 and 14 days. a, b Photosynthetic parameters deduced by the JIP-test analysis of fluorescence transients normalized using as reference the control. c, d Relative variable fluorescence between the steps O and P (Wt) on logarithmic time scale; e, f Relative variable fluorescence between the steps O and I (WOI; on logarithmic time scale); g, h relative variable fluorescence between the steps I and P (WIP) and WOI in the insert; i, j relative variable fluorescence between the steps O and J (WOJ; left vertical axis and graph with symbols) and average kinetics (right vertical axis and graph without symbols) depicted between the steps O and J (ΔWOJ), revealing the K-band; k, l relative variable fluorescence between the steps O and K (WOK; left vertical axis and graph with symbols) and average kinetics (right vertical axis and graph without symbols) depicted between the steps O and K (ΔWOK), revealing the L-band. ms milliseconds
Several works have reported differential translocation of herbicides throughout the plant tissues, such as glyphosate (Nandula and Vencill 2015; Fadin et al. 2018), atrazine (Wehtje et al. 2007), and imazaquin (Florido et al. 2014), a pre-emergence herbicide ALS-inhibitors, showing to have differential translocation in Canavalia ensiformis. However, no report has demonstrated the translocation and concentration of any herbicide on plant crown area of the stem (Fig. 1), which might be a mechanism of C. juncea tolerance to diclosulam, though further investigation is needed. Diclosulam tolerance mechanism was showed in soybean and peanut as a limitation in translocation from its site of action in the apical meristem of plants (Senseman 2007).
ALS-inhibiting herbicides are widely used because of their low dose rate, sound environmental properties, low mammalian toxicity, wide crop selectivity and high efficacy (Tan et al. 2005). The enzyme ALS is present in chloroplasts, mainly in young tissues of the plant, where meristematic tissues predominate (Senseman 2007). The autoradiographs of C. juncea demonstrate that translocation of diclosulam is impeded to reach the shoot where the apical meristem is located. In view of this, the presence of an anatomical/metabolic barrier to translocation of diclosulam in the crown area of the stem may operate to give tolerance to diclosulam (Fig. 1). It is noteworthy to emphasize that the tolerance of this species is not related to phytoremediation properties since low uptake from the total isotopes was showed in plant tissues. However, phytostimulation might induce metabolic alterations, since radioactivity was recovered in each tissue (Fig. 2f, g), and no reduction in the amount of the herbicide in the soil (radioactivity), was uptake showing no phytoextraction capacity.
The biophysical fluorescence transients depicted in Fig. 2a and b show the JIP-test to deduce functional and structural parameters of the photosynthetic behavior of C. juncea subjected to different doses of diclosulam herbicide (energy distribution in the photosynthetic apparatus) (Strasser et al. 2004). All parameters (doses: 35 and 70) were normalized to their respective untreated one. The sequence of parameters, referring to the sequential energy transduction indicated per RC (Reaction Centre), showed an increase in the reduction of the end electron acceptors at the PSI acceptor side (RE0/RC) increased mainly in the dose 70 while the energy dissipated as heat per RC (DI0/RC) decreased, leading to an increase in the performance indexes (PI) upon 7 days. The increase in both, the performance index (potential) for energy conservation from exciton to the reduction of intersystem electron acceptors (PIABS) and performance index (potential) for energy conservation from exciton to the reduction of PSI (photosystem I) end acceptors (PItotal), indicate an increase in the functionality of the electron transport chain of plants. In an opposite way, the performance index decreased in plants upon 14 days (Fig. 2b). Increases were in the energy fluxes for (light) absorption [ABS/RC; which measures the apparent antenna size (total absorption or total Chl per active RC)], electron trapping (ET0/RC; which measures the energy trapping flux per active RC, able to lead a quinone A (QA) reduction), electron transport flux (further than Q−A) per RC (EC0/RC) and electron flux reducing end electron acceptors at the PSI acceptor side per RC (ET0/RC). Regarding quantum yields and efficiencies, no significant differences in maximum quantum yield for primary photochemistry (φPo), was observed in C. juncea plants in all doses of diclosulam herbicide and neither upon the period of analysis. On the other hand, on the 7th day, and on the 14th day, increase and decrease, respectively, was observed for quantum yield for reduction of end electron acceptors at the PSI acceptor side (RE) (φRo), in a similar way of performance indexes.
Chl a fluorescence transients in Fig. 2c–l, exhibited a typical polyphasic Chl a fluorescence OJIP (Wt) transients (the O50µs, J2ms, I30ms, and P1s steps are marked in the plot), rising from initial fluorescence (FO) to maximum fluorescence (FM). However, a slight decrease in fluorescence was observed in plants subjected to both doses of diclosulam herbicide (35 and 70), showed by a decrease in the curves of relative variable fluorescence at the J- and I-steps (2 and 30 ms, respectively) upon 7 days (Fig. 2c). The normalization between the steps O and I (WOI), did not affect the events from exciton trapping by PSII up to plastoquinone (PQ) reduction (Fig. 2e, f). On the other hand, Fig. 2g and h (with its respective insert graphs) shows an increase in the sequence of events from the PSI-driven electron transfer to the end electron acceptors on the PSI acceptor side, starting at PQH2 (plastoquinol) (WIP), upon 7 days all doses compared to untreated plants while, a decrease in WIP was showed by plants after 14 days. The kinetics evaluations revealed the presence of L-band (ΔWOK) and K-band (ΔWOJ). Positive K-band after 14 days of emergence (Fig. 2j), could mean that the oxygen-evolving complex (OEC) becomes leaky and offers access to non-water electron donors evidenced by a decreased reduction rate of quinone (QA), the primary electron acceptor of PSII, from QA to Q−A. The absence of positive L-band (Fig. 2k, l), indicates the maintenance of the energetic connectivity and efficient consumption of the excitation energy and stability of the system upon diclosulam treatments.
Diclosulam is a soil-applied acetolactate synthase (ALS)-inhibiting herbicide which results in a blockage of the synthesis of branched-chain amino acids (valine, leucine, and isoleucine). The phytotoxic effect of diclosulam is caused by a deficiency of these amino acids, leading to a decrease in DNA and protein synthesis, which adversely affects cellular division and photosynthate translocation to growing points (Tan et al. 2005).
The high photosynthetic performance indexes (PI, Fig. 2a) evidenced at the 7th day after emergence in leaves of C. juncea may be the result of high uptake and translocation of diclosulam in the tissues (Fig. 1), over this time to metabolize the herbicide molecule. The following decline in the uptake and translocation of the diclosulam from root to shoot up to 14 d after emergence also coincides with a decline in photosynthetic performance indexes of plants and with an increase in energy dissipation as heat (DI0/RC), changing the dynamic dissipation of the photosynthetic energy.
To support the hypothesis that high initial uptake and translocation of diclosulam is associated with high photosynthetic efficiency to metabolize and transport the herbicide through the tissues of C. juncea, fluorescence data were normalized between the steps O (50 μs) and I (30 ms) and presented as relative variable fluorescence (WOI), where was evidenced an increase in the sequence of events from exciton trapping by (photosystem II) PSII up to plastoquinone (PQ) reduction (O–I phase; WOI from 0 to 1; Fig. 2f). No decline in ET0/RC helps to maintain the PSI-driven electron transfer to the end electron acceptors on the PSI acceptor side, starting at PQH2 (plastoquinol) (I–P phases; WOI ≥ 1, insert graph in the Fig. 2h). In the main plot (Fig. 2h), fluorescence normalized as I-P phase, the reduction of the end electron acceptor was higher in plants treated with diclosulam compared to untreated one, which may lead to an increase in NADPH and ATP production (the insert graph), to keep the metabolism of C. juncea functioning.
Maintenance of the energy connectivity of PSII [indicated by the absence of a marked positive L-band; normalized between the steps O (50 μs) and K (300 μs), as WOK; Fig. 2k, l], implies in keeping the system stability and energy utilization efficiently with absorbed energy dissipation between PSII units (Tsimilli-Michael and Strasser 2008). The presence of positive K-band [normalized between the steps O and J (2 ms), as WOJ] in C. juncea plants (Fig. 2i, j), reflects either an increase of the functional PSII antenna size, and/or an inactivation of the oxygen-evolving complex (Yan et al., 2013), as evidenced by an increase in ABS/RC upon 14 days of diclosulam treatment.
Damage to the oxygen-evolving complex leads to an imbalance in the energy flux in PSII, increasing the time of excited states of chlorophyll molecules from the reaction center of the photosystem, which increases the probability of singlet oxygen formation and, consequently photooxidative damage (Foyer et al. 2017). The increase in ET0/RC associated to low PI is due to the blockage in electron transport chain (ETC), which allows electron leakage and may allow reactive oxygen species (ROS) production, once there is an increase in TR0/RC (Fig. 2). Although K-band showed problems in the oxygen-evolving complex, connection and stability between the PSII units indicated that the functioning of the photosystems was maintained.
Therefore, plants of C. juncea have a barrier to the translocation of diclosulam herbicide in the crown area of the stem, which may give to the plant tolerance to this molecule. In addition, plants can maintain photosynthetic metabolism active when growing in soil with diclosulam by not changing the dynamics of energy dissipation. Thus, when cultivated in soil with residual of diclosulam, C. juncea can tolerate the herbicide to maintain plant growth.
References
Braz GBP, Oliveira RS Jr, Zobiole LHS, Rubin RS, Voglewede C, Constantin J, Takano HK (2017) Sumatran Fleabane (Conyza sumatrensis) control in no-tillage soybean with diclosulam plus halauxifen-methyl. Weed Technol 31:184–192
Carabias-Martínez R, Rodríguez Gonzalo E, Fernández-Laespada ME, Sánchez-San Román FJ (2000) Evaluation of surface and ground-water pollution due to herbicides in agricultural areas of Zamora and Salamanca (Spain). J Chromatogr A 869:471–480
Crafts AS, Yamaguchi S (1964) The autoradiography of plant materials. California Agricultural Experiment Station, California
Dayan FE, Zaccaro MLM (2012) Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pest Biochem Physiol 102:189–197
Fadin DA, Tornisielo VL, Barroso AAM, Ramos S, Dos Reis FC, Monquero PA (2018) Absorption and translocation of glyphosate in Spermacoce verticillata and alternative herbicide control. Weed Res 58:389–396
Florido FG, Monquero PA, Dias ACR, Tornisielo VL (2014) The absorption and translocation of imazaquin in green manures. Acta Sci-Agron 36:291–300
Foyer CH, Ruban AV, Noctor G (2017) Viewing oxidative stress through the lens of oxidative signaling rather than damage. Biochem J 474:877–883
Hanley TR, Billington R (2001) Toxicology of triazolopyrimidine herbicides. In: Krieger RI, Krieger WC (eds) Handbook of pesticide toxicology. Academic Press, California, pp 1653–1665
Karavangeli M, Labrou NE, Clonis YD, Tsaftaris A (2005) Development of transgenic tobacco plants overexpressing maize glutathione S-transferase I for chloroacetanilide herbicides phytoremediation. Biomol Eng 22:121–128
Kocurek V, Smutny V, Filova J (2009) Chlorophyll fluorescence as an instrument for the assessment of herbicide efficacy. Cereal Res Commun 37:289–292
Kopsell DA, Armel GR, Abney KR, Vargas JJ, Brosnan JT, Kopsell DE (2011) Leaf tissue pigments and chlorophyll fluorescence parameters vary among sweet corn genotypes of differential herbicide sensitivity. Pest Biochem Physiol 99:194–199
McCurdy JD, McElroy JS, Kopsell DA, Sams CE (2008) Mesotrione control and pigment concentrations of large crabgrass (Digitaria sanguinalis) under varying environmental conditions. Pest Manag Sci 65:640–644
Nandula VK, Vencill WK (2015) Herbicide absorption and translocation in plants using radioisotopes. Weed Sci 63:140–151
Pester TA, Nissen SJ, Westra P (2001) Absorption, translocation, and metabolism of imazamox in jointed goatgrass and feral rye. Weed Sci 49:607–612
Ramborger BP, Gularte CAO, Rodrigues DT, Gayer MC, Carriço MRS, Bianchini MC, Puntel RL, Denardin ELG, Roehrs R (2017) The phytoremediation potential of Plectranthus neochilus on 2,4-dichlorophenoxyacetic acid and the role of antioxidant capacity in herbicide tolerance. Chemosphere 188:231–240
Sadhukhan S, Sarkar U (2016) Production of biodiesel from Crotalaria juncea (Sunn-Hemp) oil using catalytic trans-esterification: process optimisation using a factorial and box-Behnken design. Waste Biomass Valor 7:343–355
Sadhukhan S, Bhattacharjee A, Sarkar U, Baidya PK, Baksi S (2018) Simultaneous degumming and production of a natural gum from Crotalaria juncea seeds: physicochemical and rheological characterization. Int J Biol Macromol 111:967–975
Salihu S, Hatzios KK, Derr JF (1998) Comparative uptake, translocation, and metabolism of root-applied isoxaben in ajuga (Ajuga reptans) and two ornamental euonymus species. Pest Biochem Physiol 60:119–131
Santos FM, Vargas L, Christoffoleti PJ, Martin TN, Mariani F, Silva DRO (2015) Alternative herbicides to control Conyza sumatrensis (Retz.) E. H. Walker resistant to and EPSPs inhibitors. (In Portuguese, with English abstract.). Ceres 62:531–538
Senseman SA (2007) Herbicide handbook, 9th edn. Weed Science Society of America, Lawrence
Silva CMM, Gomes MMA, Freitas SP (2009) Effects of herbicides associated to a brassinosteroid analogue on the photosynthetic apparatus of Eucalyptus grandis seedlings. Planta Daninha 27:789–797
Siminszky B (2006) Plant cytochrome P450-mediated herbicide metabolism. Phytochem Rev 5:445–458
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC (ed) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 321–362
Tan S, Evans RR, Dahmer ML, Singh BK, Shander DL (2005) Imidazolinone tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
Tssimilli-Michael M, Strasser RJ (2008) In vivo assessment of plants vitality: applications in detecting and evaluating the impact of Mycorrhization on host plants. In: Varma A (ed) Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics, 3rd edn. Springer, Dordrecht, pp 679–703
Walls FR, Corbin FT, Collins WK, Worsham AD, Bradley JR (1993) Imazaquin absorption, translocation, and metabolism in flue-cured tobaco. Weed Technol 7:370–375
Wehtje G, Miller ME, Grey TL, Brawner WR Jr (2007) Comparisons between X-ray film- and phosphorescence imaging-based autoradiography for the visualization of herbicide translocation. Weed Technol 21:1109–1114
Yan K, Chen P, Shao H, Shao C, Zhao S, Brestic M (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 8:62100
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee Sarin NB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviate abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta Bioenergy 1797:428–1438
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil (CAPES)- Finance Code 001, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Associação Pró-Gestão das Águas da Bacia Hidrográfica do Rio Paraíba do Sul (AGEVAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, C.d.C.B., Borella, J., Leal, J.F.L. et al. Limited Diclosulam Herbicide Uptake and Translocation-Induced Tolerance in Crotalaria juncea. Bull Environ Contam Toxicol 104, 114–120 (2020). https://doi.org/10.1007/s00128-019-02742-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02742-7