Abstract
This study evaluated the environmental safety of Igbokoda River, a popular fishing hub in an oil producing area in Nigeria. Biomarkers of oxidative stress and heavy metals were determined in the liver and muscle of Clarias gariepinus from Igbokoda River and also in fish samples from a clean fish farm (control). Water samples from both sites were analysed for physicochemical parameters, heavy metals and bacterial contamination. There was significant increase in the level of heavy metals in water samples and in the organs of fish from Igbokoda River. A significant increase in malondialdehyde level as well as alterations in antioxidant status was observed in the organs of fish samples from Igbokoda River compared with control. Coliforms and salmonella were also visible in Igbokoda River alongside particulate matter. These results show that Igbokoda River is polluted; consumption of aquatic organisms from the River may be unsafe for people in that community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The unfortunate effects of man’s activities on the environment include depletion of natural resources alongside pollution of the natural systems with grave consequences on the ecosystems (Williams et al. 2016). The aquatic system is not left unscathed as discharges from oil spills, domestic sewage, industrial effluents and metallurgical and mining operations continue to compromise the quality of water content (Gavrilescu et al. 2015). Heavy metals when released as a by-product of these processes leach into water bodies and trigger biological processes which are of grave health concern (Eroglu et al. 2015).
Water pollution can be detected by chemical and biological methods. In the former, water samples are collected and analyzed for concentrations of chemicals which they possess (Kong et al. 2015). Such chemicals such as cadmium, nickel, lead, arsenic and other compounds can easily be detected, alongside bacteria load, particulate matter and foreign bodies. Conversely, in biological method which is commonly used today; fishes, insects and other marine organisms are used in assessing the quality of aquatic bodies, serving as bio-indicators of environmental pollution (Okay et al. 2016). When heavy metals amass in their tissues, they generate specific reactive oxygen species (ROS), a major precursor of oxidative stress (Eroglu et al. 2015; Abdel-Gawad et al. 2016). The body acts to counter the effect of these oxidants by activating a series of antioxidant defense systems such as superoxide dismutase (SOD), catalase (CAT), and the glutathione triad: reduced glutathione (GSH), glutathione s-transferase (GST) and glutathione peroxidase (GPx). They all have specific functions in detoxifying the ROS species generated by aquatic pollutants (Farombi et al. 2007). Aquatic contaminants are not easily destroyed through the natural process of biological degradation and therefore have the ability to accumulate in the environment (Liu et al. 2016). This makes these toxicants harmful to the aquatic ecosystem and to humans who depend on aquatic products as sources of food; it raises grave public health concerns.
In Nigeria, increased industrialization has negative impact on water bodies with reports of pollution on the increase (Godwin 2016; Onisokyetu et al. 2016). Petroleum exploration has continued to impact negatively in the Niger Delta region of Nigeria due to incessant and continuous environmental, socioeconomic and physical disasters that have befallen these regions in the last two decades (Achi 2003). Pipelines vandalism has been reported as a common occurrence from different oil producing fields in Nigeria (Olaniyan 1985). This ultimately results into oil spills that grossly contaminate the aquatic and non- aquatic environment, impact negatively Nigerian economy and destroy ecosystem (Nwankwo and Ifeadi 1988). This eventually causes health problems among residents, including cancer and other degenerative diseases to mention but a few (Bayode et al. 2011).
This study evaluated the environmental safety status of Igbokoda River (a popular fishing hub in Ilaje local government area in Ondo State; one of the oil producing states in Nigeria). Environmental pollution of Igbokoda River is multifaceted and exacerbated by the crude oil deposits, rendering the aquatic life unsafe for human consumption. Heavy metals are closely related with crude oil pollution since heavy metals are constituents of crude oil (Osuji and Onajake 2004; Censi et al. 2006; Nie et al. 2010); therefore we evaluated heavy metal content, alongside other indices of pollution: particulate matter, bacteria content in water samples from Igbokoda River. Because these pollutants can be absorbed or ingested by aquatic life (crabs, fishes, etc) from surrounding water, and accumulated in certain tissue and organs (Okay et al. 2016), we assessed the levels of heavy metals and some oxidative stress markers in the liver and tissue, (which is consumed by humans) of Clarias gariepinus from the River as biomarkers of aquatic pollution. Since the harmful effects of aquatic pollutants on aquatic organisms may eventually get to humans through the food chain, this study is a necessity at this time when there is increase in reported cases of infertility, cancer and other diseases associated with environmental pollution in the Niger delta area of the country (Ordinioha and Brisibe 2013).
Materials and Methods
Ten C. gariepinus with average weight of 220 g were caught from Igbokoda River located at latitude 6°17′3.1794″N and longitude 4°49′6.303″E coordinates in Ilaje community in Ondo State, Nigeria. The fish were caught with fishing nets from different points in the River. Clarias gariepinus from Ilesanmi fish farm in Ondo State were used as control. According to our knowledge, the fish farm is devoid of any industrial discharge or any other contaminants that could cause pollution (Fig. 1).
Map of study area (Igbokoda). (Iyun and Oke 2000)
The fishes were dissected and liver and muscle were removed, rinsed in 1.15% KCl and homogenized in four volumes of homogenizing buffer (50 mM Tris–HCl mixed with 1.15% KCl and pH adjusted to 7.4). The resulting homogenate was centrifuged at 12,500×g for 10 min to obtain the post mitochondrial fraction.
Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Farombi et al. (2000). The muscle and hepatic reduced glutathione (GSH) concentration was determined according to the method of Jollow et al. (1974) modified by Giustarini et al. (2013). The superoxide dismutase (SOD) activity was evaluated by the method of Misra and Fridovich (1972) modified by Oyagbemi et al. (2017). Glutathione-S-transferase (GST) activity was determined according to the method described by Farombi et al. (2008). Catalase activity was estimated according to the method of Sinha (1972) modified by Mahmoud (2016). The pH of the water sample was determined using pH meter Hanna H8921 model. The total suspended solids (TSS) and total dissolved solids (TDS) were determined using tritimetric method according to the guidelines of A.O.A.C. 2005; conductivity of the water sample was determined using conductivity meter Hanna H8921 model. The heavy metals (Lead, cadmium, copper, arsenic and nickel) contents in the water samples were determined by atomic absorption spectrophotometry using atomic absorption spectrophotometer (AAS) Buck 211 model. The Cl− (mg/L), SO42− (mg/L), NO3− (mg/L), Na+ (mg/L), Ca2+ (mg/L), and K+ (mg/L) in the water sample were determined by flame photometry in accordance with the guidelines of A.O.A.C. 2005. The coliform and salmonella count counts were determined by colony counter Model 2510 Jenway using macConkey agar and salmonella-Shigella agar.
All values for the results are expressed as the mean ± S.D. Parameters obtained from the experimental groups were compared with the control. The data obtained were analyzed using repeated measures one-way analysis of variance (ANOVA) with the Tukey post-hoc analysis for the analysis of scientific data using GraphPad Prism® 5.01 Values were considered statistically significant at p < 0.05.
Results and Discussion
The concentration of heavy metals in water samples from Igbokoda River was found to be extremely higher than that of the control and also exceeded the acceptable threshold for drinking water. There were about 180, 544, 129, 361 and sevenfold increases in lead, cadmium, copper, arsenite and nickel respectively in water samples from Igbokoda River when compared with control (Table 1). The trend of heavy metal concentration was found as follows: As > Cu > Ni > Pb > Cd. Lead is toxic to human beings even at low concentrations, and has been shown to cause irreversible damage to the hepatic, renal and erythrocyte systems (Omobowale et al. 2014; Oyagbemi et al. 2015). Copper is required in trace amounts for biochemical and physiological processes as well as co-factor for several oxidative stress enzymes such as catalase and superoxide dismutase (Stern 2010), while cadmium and arsenic are of no importance and are actually considered deleterious (Chang et al. 1996). Excessive concentrations of these heavy metals trigger reactive oxygen species (ROS) production and induction of oxidative stress (Jomova et al. 2011). In extreme cases, these manifest in DNA damage, cell cycle disruption, cancer and cell death (Stevens et al. 2010).
Also, most of the physicochemical parameters of Igbokoda water samples are not within the acceptable limits set by regulatory bodies for drinking water. There were high concentrations of chloride, sulphate, nitrate, sodium, calcium and potassium ions in water samples from Igbokoda River when compared with control. There was about threefold increase in total solids and 346% and 360% increases in total suspended solids (TSS) and total dissolved solids (TDS) in water samples from Igbokoda River when compared with the control sample. Also, there were 16.6 and 8.7 fold increases in total hardness and conductivity (Table 1).
The total number of active bacteria in Igbokoda water sample was extremely higher than that of the control water sample. Furthermore, coliforms and salmonella were extremely visible in Igbokoda water sample but these bacteria species were not found in the control water sample (Table 2).
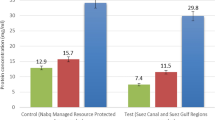
The high levels of the heavy metals in Igbokoda water sample reflected in the tissues of fish from the River as the metals bioaccumulated in the liver and muscle of the fish. There was a significant increase (p < 0.001) in the concentration of heavy metals in both the liver and muscle of C. gariepinus from Igbokoda River compared with control (Table 3). Heavy metals trigger biological processes which are of grave health concern (Eroglu et al. 2015). Of special interest is the fact that when compared to the muscle, the liver had higher levels of all heavy metals assayed. This agrees with (El-Moselhy et al. 2014) who showed that the liver had consistently higher concentrations of these heavy metals when compared with the muscle. This probably reflects the capacity of the liver for storage and as a target center for metabolism of drugs and foreign compounds. Metallothionein, a natural binding protein found in the liver bind to such metals e.g. copper to retain it within the liver as an enzymatic co-factor for physiological processes (Gorur et. al. 2012). Also, because cadmium has the ability to displace metallothionein bound metals; it is usually found in higher concentrations in hepatic tissue (Capaldo et al. 2016).
Unsurprisingly, the accumulation of heavy metals correlated with elevated lipid peroxidation in both the hepatic and muscular tissues (Table 4). The observed increase in lipid peroxidation is similar to the reports of Huang et al. (2000), Farombi et al. (2007), Sanchez et al. (2007), and Falfushynska and Stolyar (2009). Heavy metals are known to catalyze the formation of ROS within tissues which then cause damage to nucleic acids, disrupt cellular structures and components, while damaging proteins and lipids (Tchounwou et al. 2012).
Antioxidant enzymes are biomarkers of oxidative stress in aquatic organisms and they could be induced in response to pollutants (Borković 2005). Our findings also reveal that the activities of the antioxidant enzymes SOD, GST and CAT were increased in both hepatic and muscular tissues in the test samples compared with control, there was also an increase in glutathione (GSH) concentration in the liver and muscle of C. gariepinus from Igbokoda River when compared with control (Table 4). This may also be attributable to the significant deposition of heavy metals within these tissues. Superoxide anions must have been produced in response to these metals leading to elevated SOD levels as it seeks to mop them up, converting them to the less harmful hydrogen peroxide (H2O2). The rise in the activity of GST and its substrate, GSH was an indication of an adaptive response against oxidative stress. Increased ROS generation has been shown to up regulate antioxidant enzyme activity via the Nrf2-Keap 1 pathway (Baird and Dinkova-Kostova 2011). Because the CAT-SOD system works in tandem as a first line of defense against oxidative stress, both enzymes are usually expected to increase in response to ROS stressors. This study demonstrates a rise in catalase activity in both hepatic and muscular tissues of fish harvested from Igbokoda River.
While this study showed high concentrations of heavy metals in both water samples and tissues of C. gariepinus from Igbokoda River, it also highlights the fact that Igbokoda River not only has an oil effluence problem, but a wider pollution issue as indicated by the other indices of pollution measured. Particularly, bacteria load and particulate content were markedly elevated when compared with the reference site. This could be due to sewage and domestic waste discharge from settlements located in and around the River region (Kress et al. 2004). As the region is primarily an artisanal community with livelihood based on fishing, homesteads are often built on or around the River bodies. Inevitably, wastes are at times discharged directly into the River (Abdus-Salam et al. 2010). Agricultural practices by local farmers along the river route or even further inland poses another major source of pollution when organic fertilizers and manure used to enrich the farmlands leach into the underground water and drain into the river bed (Murray et al. 2004; Quilbé et al. 2004). In addition, the region is also known for the mining and transportation of silica sand. Such activities increase the probability of industrial effluents being discharged into water bodies. At the Durogbe park, a hub for transport activities on the River, commonly seen activities include washing of clothes, bathing, deposition of fecal waste and agricultural waste (Olaniyan et al. 2016). The natural location of the Igbokoda along the Niger delta coastline of Nigeria means that the intrusion of saline water from the Atlantic Ocean and transboundary activities might propagate the spread of these pollutants (Adebowale et al. 2008). The presence of atmospheric precipitates and coastal waves increase the rate of pollutant transfer and mixing making the environment largely unsuitable for human use.
This study confirms existing models of fishes as biomarkers of aquatic pollution and also raises health concerns for people using water from Igbokoda River for domestic purposes and those who consume fishes and other aquatic animals from the contaminated River.
References
Abdel-Gawad FK, Guerriero G, Khalil WKB, Abbas HH (2016) Evaluation of oxidative stress, genotoxicity and gene expression alterations as oil pollution markers in solea vulgaris from Suez Canal. Quantum Matter 5(2):291–296
Abdus-Salam N, Adekola FA, Apata AO (2010) A physicochemical assessment of water quality of oil producing areas of Ilaje, Nigeria. Adv Nat Appl Sci 4(3):333–344
Achi C (2003) Hydrocarbon exploitation, environmental degradation and poverty: the Niger Delta experience. In: Proceedings of the Diffuse Pollution Conference, Dublin
Adebowale KO, Foluso OA, Bamidele IO (2008) Impact of natural and anthropogenic multiple sources of pollution on the environmental conditions of Ondo state coastal water, Nigeria. Electron J Environ Agric Food Chem 7:2797–2811
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1–Nrf2 pathway. Arch Toxicol. 85:241–272
Bayode OJA, Adewunmi EA, Odunwole S (2011) Environmental implications of oil exploration and exploitation in the coastal region of Ondo State, Nigeria: a regional planning appraisal. J Geogr Reg Plan 4(3):110–121
Borković SS, Saponjić JS, Pavlović SZ, Blagojević DP, Milosević SM, Kovacević TB, Radojicić RM, Spasić MB, Zikić RV, Saicić ZS (2005) The activity of antioxidant defence enzymes in the mussel Mytilus galloprovincialis from the Adriatic Sea. Comp Biochem Physiol 141(4):366–374
Capaldo A, Gay F, Scudiero R, Trinchella F, Caputo I, Lepretti M, Marabotti A, Esposito C, Laforgia V (2016) Histological changes, apoptosis and metallothionein levels in Triturus carnifex (Amphibia, Urodela) exposed to environmental cadmium concentrations. Aquat Toxicol 173:63–73
Censi P, Spoto SE, Saiano F, Sprovieri M, Mazzola S, Nardone G, Ottonello D (2006) Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere 64:1167–1176
Chang LW, Magos L, Suzuki T (eds) (1996) Toxicology of metals. CRC Press, Boca Raton, FL
El-Moselhy KM, Othman AI, Abd El-Azem H, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J Basic Appl Sci 1(2):97–105
Eroglu A, Dogan Z, Kanak EG, Atli G, Canli M (2015) Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ Sci Pollut Res Int 22(5):3229–3237
Falfushynska H, Stolyar O (2009) Responses of biochemical markers in carp Cyprinus carpio from two field sites in Western Ukraine. Ecotoxicol Environ Saf 72(3):729–736
Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO (2000) Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 38(6):353–541
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Publ Health 4(2):158–165
Farombi EO, Ajimoko YR, Adelowo OA (2008) Effect of butachlor on antioxidant enzyme status and lipid peroxidation in fresh water African Catfish, (Clarias gariepinus). Int J Environ Res Publ Health 5(5):423–427
Gavrilescu M, Demnerová K, Aamand J, Agathos S. Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol 32(1):147–156
Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R (2013) Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc 8(9):1660–1669
Godwin AI (2016) Assessment of heavy metals associated with bottom sediment of Asejire Reservoir, Southwest Nigeria. Int Lett Nat Sci 55:9–16
Gorur FK, Keser R, Akcay N, Dizman S (2012) Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 87:356. https://doi.org/10.1016/j.chemosphere.2011.12.022
Huang DJ, Zhang YM, Song G, Long J, Liu JH, Ji WH (2000) Contaminants-induced oxidative damage on the Carp Cyprinus carpio collected from the upper Yellow River, China. J Environ Monit Assess 128(1–3):483–488
Iyun BI, Oke EA (2000) Ecological and cultural barriers to treatment of childhood diarrhea in riverine areas of Ondo State. Nigeria Soc Sci Med 50(7–8):953–964
Jollow DJ, Michell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver Necrosis: protective role of GSH and evidence for 3,4-Bromobenzene oxide as the Hepatotoxic metabolite. Pharmacology 11(3):151–169
Kong L, Kadokami K, Wang S, Duong HT, Chau HTC (2015) Monitoring of 1300 organic micro-pollutants in surface waters from Tianjin, North China. Chemosphere 122:125–130
Kress N, Herut B, Galil BS (2004) Sewage sludge impact on the sediment quality and benthic assemblage off the Mediterranean coast of Israel—a long term study. Marine Environ Res 57:213–233
Liu W-X, Wang Y, He W, Qin N, Kong XZ, He QS, Yang B, Yang C, Jiang YJ, Jorgensen SE, Xu FL (2016) Aquatic biota as potential biological indicators of the contamination, bioaccumulation and health risks caused by organochlorine pesticides in a large, shallow Chinese lake (Lake Chaohu). Ecol Indic 60:335–345
Mahmoud HH (2016) New method for assessment of serum catalase activity. Indian J Sci Technol 9(4):1–5
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Murray KS, Rogers DT, Kaufman MM (2004) Heavy metals in an Urban Watershed in Southeastern Michigan. J Environ Qual 33:163–172
Nie M, Xian N, Fu X, Chen X, Li B (2010) The interactive effects of petrolem-hydrocarbon spillage and plant rhizosphere on concentrations and distribution of heavy metals in sediments in the Yellow River Delta, China. J Hazard Mater 174(1):156–161
Nwankwo N, Ifeadi CN (1988) Case studies on the environmental impact of oil production and marketing in Nigeria. In: Sada PO, Odemerho FO (eds) Environmental issues and management in Nigerian Development. Ibadan.: Evans Brothers (Nigeria Publishers) Limited, Ibadan, pp 208–223
Okay OS, Ozmen M, Güngördü A, Yilmaz A, Yakan SD, Karacik B, Tutak B, Schramm KW (2016) Heavy metal pollution in sediments and mussels: assessment by using pollution indices and metallothionein levels. Environ Monit Assess 188(6):352
Olaniyan CO. (1985). The balance of nature: live and let live. 1986 Foundation Day lecture of the Federal University of Technology, Akure, Nigeria, Held on 23 April, 1986
Olaniyan RF, Ugwumba AO, Ayoade AA (2016) Physicochemical properties of Igbokoda River, Ondo State, Nigeria. Int J Sci Edu 6(1):16–20
Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, Ogunpolu BS, Olopade JO (2014) Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharm 37(3):1202–1211
Onisokyetu A, Godwin M, Chinenye NG (2016) Bioaccumulation of selected heavy metals in water, sediment and Blue Crab (Callinectes amnicola) from Bodo Creek, Niger Delta, Nigeria. J Fish Sci 10(3):77–83
Ordinioha B, Brisibe S (2013) The human health implications of crude oil spills in the Niger delta, Nigeria: an interpretation of published studies. Niger Med J 54(1):10–16. https://doi.org/10.4103/0300-1652.108887
Osuji LC, Onojake CM (2004) Trace heavy metals associated with crude oil: a case study of Ebocha-8 oil-spill-polluted site in Niger Delta, Nigeria. Chem Biodivers 1(11):1708–1715
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243
Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM et al (2017) Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol 32:1089–1101
Quilbé R, Pieri I, Wicherek S, Dugas N, Tasteyre A, Thomas Y, Oudinet J (2004) Combinatory chemical and biological approaches to investigate metal elements in agricultural runoff water. J Environ Qual 33:149–153
Sanchez W, Selim A, Olivier P, Jean-Maxence D, Jean-Marc P (2007), Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasteros-teus aculeatus L.) sampled in contaminated streams. Ecotoxicology 16(2), 279–287
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A 73(2):114–127
Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C (2010) The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health 7(5):2018–2032
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular clinical and environmental toxicology, vol 3. Springer Basel, Basel, pp 133–164
WHO (1984) Guidelines for drinking water quality. Health criteria and other supporting information, vol 2, WHO, Geneva
Williams CJ, Frost PC, Morales-Williams AM, Larson JH, Richardson WB, Chiandet AS, Xenopoulos MA (2016) Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Glob Change Biol 22(2):613–626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arojojoye, O.A., Oyagbemi, A.A. & Afolabi, J.M. Toxicological Assessment of Heavy Metal Bioaccumulation and Oxidative Stress Biomarkers In Clarias gariepinus from Igbokoda River of South Western Nigeria. Bull Environ Contam Toxicol 100, 765–771 (2018). https://doi.org/10.1007/s00128-018-2341-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2341-5