Abstract
Water pollution indices and oxidative stress biomarkers in African sharp-toothed catfish, Clarias gariepinus were used to evaluate the health status of Abereke River on the Ilaje coastal zone of Ondo’s Niger Delta region. Muscle and liver tissues from fishes harvested from the river and from a clean fish farm were comparatively subjected to heavy metal status and redox stress biomarker analyses. Water samples from the river and fish farm were also subjected to water quality tests. Our findings showed significant elevations in bacterial contaminants, heavy metal pollutants, and particulate matter deposits in Abereke River. In addition, heavy metal deposits were found in tissues of fish harvested from the river correlated with increases in markers of oxidative stress and depletions in antioxidant defense systems. Taken together, water from Abereke River is unsafe for human consumption, and consumption of aquatic organisms from the river may be harmful to health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic interferences in the balance of local aquatic ecosystems have been reported to lead to high levels of pollution in marine and seawater ecosystems (Machado et al. 2015). The dependence on and growth of the petroleum hydrocarbon industry in Nigeria have been correlated with increased aquatic pollution (Allison et al. 2018). Further, the Nigerian petroleum industry has played a huge role in supplying global energy demand while contributing to local economic development, but this has come at a huge cost (Ite et al. 2018; Mogaji et al. 2018). Moreover, one of the largest oil-producing regions in the world, the Niger Delta, is reported to experience large volumes of oil spill–related pollution with little quantification of the negative impacts on human beings (Obida et al. 2018). Pipeline vandalism, accidental spillages, oil theft, and poor transportation network for crude are major culprits leading to crude and its products being routinely discharged onto farmlands and waterways, with attendant irreversible damage on the eco-system. Over an 8-year period, at least 90 million liters of oil were released into the Niger Delta with approximately 29% of humans living in close proximity to the pipe networks affected (Obida et al. 2018).
These damages from aquatic pollution are cumulative due to several factors including the depletion of dissolved oxygen required by aquatic life for survival (Venturino et al. 2017), the buildup of by-products of pollution, heavy metals, and hydrocarbons in aquatic and plant life (Eroglu et al. 2015), the generation of reactive oxygen and nitrogen species (ROS/RNS) causing oxidative stress-related damage (Abdel-Gawad et al. 2016), the resultant deaths and decomposition of wildlife, and the reduction in general wellness of the water bodies (Jarvela Rosenberger et al. 2017). These all coalesce to make such environments unsuitable for life. For those who depend on coastal bodies for economic and domestic activities, pollution completes a cycle, affecting their health and livelihood (Nwankwo and Ifeadi 1988; Ordinioha and Brisibe 2013). Routine biomonitoring of aquatic bodies is crucial in identifying and predicting environmental impacts of anthropogenic and industrial activities (Zhou et al. 2008).
Abereke, one of the sub-towns under the Ilaje local government area of Ondo state is a prime spot for fishmongers and traders due to its location along the coast. It is also known as an oil prospecting community, contributing to Ondo state’s status as an oil-producing region in the Niger Delta. We assessed the water quality levels using established markers of aquatic pollution and analyzed fish tissue harvested from the river for health status. We worked with the premise that the Abereke River would contain evidence of chemical and heavy metal leachates from the oil prospection going on inland which would be above standard levels proposed by the World health organization (WHO) as safe for human health. We also hypothesize that aquatic life when evaluated, would also contain such harmful levels of chemicals making them unfit for human consumption.
Materials and methods
Sampling area
The Ilaje River is a delta-like region which borders the Atlantic Ocean, lying on latitude 5° 50′ N–6° 09′ N and on longitude 4° 45′ E–5° 05′ E and is a part of the broader Ondo coastal area. Abereke is one of the areas located along the river which is known for fish farming as a domestic and economic means of livelihood (Fig. 1).
Map of the Ilaje coastal area showing the study area (Akinnawo et al. 2016)
Animals
Ten Clarias gariepinus, averaging 220 g, were caught from Abereke area on river Ilaje along the Ilaje coastal community of Ondo State, Nigeria by means of local fishing nets at random points along the river. For reference/control, Clarias gariepinus, were similarly harvested from a “clean” fish farm: Ilesanmi fish farm in Ondo State. To the best of our knowledge, this farm was free of industrial effluents or any other pollutants.
Experimental procedure
Fishes were dissected with their target organs (liver and muscle) removed, rinsed in 1.15% KCl and homogenized in 4 volumes of homogenizing buffer (50 mM Tris—HCl mixed with 1.15% KCl and pH adjusted to 7.4). The resulting homogenate was centrifuged at 12,500 g for 10 min to obtain the post-mitochondrial fraction.
Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Farombi et al. (2000). The muscle and hepatic-reduced glutathione (GSH) concentrations were determined according to the method of Jollow et al. (1974) modified by Justarini et al. (2013). The superoxide dismutase (SOD) activity was evaluated by the method of Misra and Fridovich (1972) modified by Oyagbemi et al. (2017). Glutathione-S-transferase (GST) activity was determined according to the method described by Farombi et al. (2008). Catalase activity was estimated according to the method of Sinha (1972) modified by Mahmoud (2016). The pH of the water sample was determined using pH meter Hanna H8921 model. The total suspended solids (TSS) and total dissolved solids (TDS) were determined using the titrimetric method according to the guidelines of A.O.A.C. (2005); conductivity of the water sample was determined using conductivity meter Hanna H8921 model. The heavy metals (lead, cadmium, copper, arsenic, and nickel) contents in the water samples were determined by atomic absorption spectrophotometry using atomic absorption spectrophotometer (AAS) Buck 211 model. The Cl− (mg/L), SO42− (mg/L), NO3- (mg/L), Na + (mg/L), Ca2+ (mg/L), and K+ (mg/L) in the water sample were determined by flame photometry in accordance with the guidelines of A.O.A.C. (2005). MacConkey agar and salmonella-shigella agar were used for the isolation of coliform and salmonella in the water samples and were counted using colony counter Model 2510 Jenway.
All values for the results are expressed as the mean ± S.D. Parameters obtained from the experimental groups were compared with the control. The data obtained were analyzed using repeated measures one-way analysis of variance (ANOVA) with the Tukey post hoc analysis for the analysis of scientific data using GraphPad Prism® 5.01. Values were considered statistically significant at p < 0.05.
Results
From the results in Table 1, the physicochemical parameters and heavy metal concentrations of water sample from Abereke River were significantly higher than that of the control sites and WHO standard.
From the results in Table 2, total viable count was higher in water sample from Abereke River compared with control, while coliform and salmonella were found in Abereke water sample but absent in water sample from the control site.
From the results in Table 3, the concentrations of lead, cadmium, arsenite, copper, and nickel in the liver and muscle of Clarias gariepinus from Abereke River were significantly higher than that of the control.
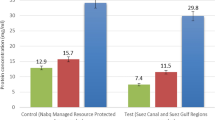
From the results in Fig. 2, malondialdehyde and glutathione concentration were significantly higher in the liver and muscle of Clarias gariepinus from Abereke River compared with control.
From the results in Fig. 3, the activity of the antioxidant enzyme superoxide dismutase (SOD) was significantly lower in the liver and muscle of Clarias gariepinus from Abereke River compared with control. The activity of GST was also significantly lower in the liver of Clarias gariepinus from Abereke River compared with control, but GST activity was significantly higher in the muscle. Catalase activity was significantly higher in both the liver and muscle.
Antioxidant profile in the liver and muscle of Clarias gariepinus from Abereke River compared with control. SOD (superoxide dismutase, units/mg protein); GST (glutathione-S-transferase, mmole1-chloro-2, 4-dinitrobenzene-GSH complex formed/min/mg protein); CAT (μmole H2O2/min/mg protein). Asterisks indicate significant difference from control at p < 0.05
Discussion
Anthropogenic influences are responsible for dwindling health and life forms in water bodies (Machado et al. 2015). In the Niger Delta region of Nigeria, this manifests primarily as oil production in local communities (Obida et al. 2018). An unfortunate consequence of this is the contamination of aquatic bodies with polycyclic aromatic hydrocarbons, heavy metals, and radioactive isotopes which have deleterious effects on human health (Okogbue et al. 2017; Ite et al. 2018). The concentration of heavy metals in Abereke River water samples was significantly higher than the acceptable limits for use as drinking water (Table 1). The concentrations of lead, cadmium, copper, and arsenite were significantly higher by 217, 689, 16, and 428-folds respectively in water samples from Abereke River when compared with control (Table 1). The trend of heavy metal concentration was found as follows: Cd > As > Pb > Cu. Cadmium and arsenic are of no importance to the human body and are actually considered extremely harmful at very small concentrations (Chang et al. 1996). The heavy metal lead has been demonstrated to cause irreversible damage to the liver, kidney, and erythrocytes at low doses (Omobowale et al. 2014; Oyagbemi et al. 2015). Copper as a metal is an important co-factor in redox processes involving catalase and superoxide dismutase as well as being required in several other physiological processes (Stern 2010). Repeated exposure to these heavy metals has been reported to activate ROS production and initiate oxidative stress (Jomova et al. 2011). This can progress to DNA damage, cell cycle disruption, cancer, and cell death (Stevens et al. 2010).
Other physicochemical parameters evaluated from Abereke water sample were well above the acceptable limits for potable water (WHO 1984). High concentrations of chloride, sulphate, nitrate, sodium, calcium, and potassium ions were recorded in water samples when compared with control. When consumed frequently, these ions could precipitate electrolyte imbalances in the body with attendant metabolic risks. Furthermore, there were increases in particulate matter in Abereke River sample when compared with the control. There were about 349% increase in total solids and 337% and 368% increases in total suspended solids (TSS) and total dissolved solids (TDS), respectively, in water samples from Abereke River when compared with the control sample (Table 2).
Bacterial analyses revealed active bacteria to be higher in Abereke water sample when compared with those found in the control water sample. Coliforms and salmonella visible in Abereke water sample were not detected in the control water sample (Table 2). This further confirms that water from Abereke River should not be used for domestic purposes, it could pose additional health hazard of gastrointestinal discomforts and diseases such as typhoid and diarrhea if consumed.
Just like the heavy metal pollution seen in the Abereke water sample, a similar trend was observed in tissues of Clarias gariepinus harvested from the Abereke River. The principle of biomonitoring presumes that the presence of aquatic organisms in a polluted environment can lead to bioaccumulation of pollutants such as heavy metals, bacteria, etc. from the surrounding water, making them bio-indicators of environmental pollution (Zhou et al. 2008; Okay et al. 2016). Our results seem to confirm this finding. Hepatic and muscle tissues of C. gariepinus harvested from Abereke River showed significant increases (p < 0.001) in the concentration of heavy metals in both the liver and muscle samples when compared with the control (Table 3). In both tissues, however, the liver had higher levels of heavy metal accumulation. This probably reflects the functions of the liver as a detoxification organ with storage and metabolism properties (El-Moselhy et al. 2014). Metallothionein is a natural binding protein found in the liver which acts by binding to copper and retaining it within the liver to serve as an enzymatic co-factor for physiological processes (Gorur et al. 2012). However, cadmium displaces metallothionein bound metals making it more commonly found in hepatic tissue (Capaldo et al. 2016).
Bioaccumulation of heavy metals led to increased malondialdehyde deposits, products of lipid peroxidation in both hepatic and muscular tissues (Fig. 2). Alongside, increases were observed in reduced glutathione, a non-enzymic antioxidant. Elevated malondialdehyde contents agree with previous works which showed such as a response to oxidative stress from heavy metals (Farombi et al. 2007; Sanchez et al. 2007). Heavy metals induce the generation of ROS/RNS within tissues which then destroy nucleic acids, proteins, and lipid membranes (Tchounwou et al. 2012).
In this study, the activity of the antioxidant enzyme superoxide dismutase (SOD) was significantly lower in the liver and muscle of Clarias gariepinus from Abereke River compared with control.
The activity of GST was also significantly lower in the liver of Clarias gariepinus from Abereke River compared with control, but this was not so in the muscle as the activity of GST was significantly higher in the muscle, this might be in response to redox stressors. A similar trend was observed in catalase (CAT) activity for both hepatic and muscle tissues (Fig. 3).
Antioxidant enzymes are bio-indicators of oxidative stress in aquatic organisms, and they could be induced in response to pollutants (Borković et al. 2005). The initial depletion in GST and SOD of hepatic and muscular tissues could be due to heavy bioaccumulation of heavy metals within these tissues. This leads to increased production of superoxide anions to convert the harmful radicals to the less harmful form: hydrogen peroxide (H2O2). The enzymes are then mopped up in the antioxidant response, leading to the observed decrease (Fig. 2). Conversely, the rise in CAT activity is probably an adaptive response against ROS-mediated oxidative stress. Oxidative stress has been known to mediate an upregulation in antioxidant system response through the Nrf2-Keap 1 pathway. This increases antioxidant mobilization and facilitates an increased response to clear out the ROS stressors (Baird and Dinkova-Kostova 2011),
Conclusively, there were increases in markers of oxidative stress coupled with alterations in antioxidant status in fish samples from Abereke River compared with control. There were also high concentrations of heavy metals in both water samples and tissues of C. gariepinus from Abereke River, confirming existing models of fishes as biomarkers of aquatic pollution and raising valid health concern issues for inhabitants of these communities who rely on the river for livelihood and consume aquatic products from the river.
References
Abdel-Gawad FK, Guerriero G, Khalil WKB, Abbas HH (2016) Evaluation of oxidative stress, genotoxicity and gene expression alterations as oil pollution markers in Solea vulgaris from Suez Canal. Quant Mat 5:291–296. https://doi.org/10.1166/qm.2016.1303
Akinnawo S, Kolawole R, Edward O (2016) Spatial distribution and speciation of heavy metals in sediment of river Ilaje, Nigeria. International Research Journal of Pure and Applied Chemistry 10(2):1–10. https://doi.org/10.9734/IRJPAC/2016/22031
Allison C, Oriabure G, Ndimele PE, Shittu JA (2018) Dealing with oil spill scenarios in the Niger Delta: lessons from the past. The political ecology of oil and gas activities in the Nigerian aquatic ecosystem. 351–368. https://doi.org/10.1016/B978-0-12-809399-3.00023-9
A.O.A.C. (2005) Official method of Analysis. 18th edn, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24
Baird L, Dinkova-Kostova AT (2011) The cytoprotective role of the Keap1–Nrf2 pathway. Arch Toxicol 85:241–272
Borković SS, Saponjić JS, Pavlović SZ, Blagojević DP, Milosević SM, Kovacević TB, Radojicić RM, Spasić MB, Zikić RV, Saicić ZS (2005) The activity of antioxidant defence enzymes in the mussel Mytilus galloprovincialis from the Adriatic Sea. Comp Biochem Physiol 141:366–374
Capaldo A, Gay F, Scudiero R, Trinchella F, Caputo I, Lepretti M, Marabotti A, Esposito C, Laforgia V (2016) Histological changes, apoptosis and metallothionein levels in Triturus carnifex (Amphibia, Urodela) exposed to environmental cadmium concentrations. Aquat Toxicol 173:63–73
Chang LW, Magos L, Suzuki T (eds) (1996) Toxicology of metals. CRC press, Boca Raton, FL Clairborne a: catalase activity. In: Handbook of methods for oxygen radical research (Greewald AR). CRC Press, Florida, 1995, 237–242
El-Moselhy KM, Othman AI, Abd El-Azem H, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J Basic Appl Sci 1:97–105
Eroglu A, Dogan Z, Kanak EG, Atli G, Canli M (2015) Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ Sci Pollut Res 22:3229–3237. https://doi.org/10.1007/s11356-014-2972-y
Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO (2000) Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a garcinia kola seed extract. Food Chem Toxicol 38:353–541
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4:158–165
Farombi EO, Ajimoko YP, Adelowo OA (2008) Effect of butachlor on antioxidant enzyme status and lipid peroxidation in fresh water African catfish, (Clarias gariepinus). Int J Environ Res Public Health 5:423–427
Gorur FK, Keser R, Akcay N, Dizman S (2012) Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea region of Turkey. Chemosphere 87:356–361. https://doi.org/10.1016/j.chemosphere.2011.12.022
Ite A, Harry T, Obadimu C, Asuaiko E, Inim I (2018) Petroleum hydrocarbons contamination of surface water and groundwater in the Niger Delta region of Nigeria. J Environ Pollut Hum Health 6. https://doi.org/10.12691/jephh-6-2-2
Jarvela Rosenberger AL, MacDuffee M, Rosenberger AGJ, Ross PS (2017) Oil spills and marine mammals in British Columbia, Canada: development and application of a risk-based conceptual framework. Arch Environ Contam Toxicol 73:131–153. https://doi.org/10.1007/s00244-017-0408-7
Jollow DJ, Michell JR, Zampaglione N, Gillete JR (1974) Bromobenzene induced liver necrosis: protective role of GSH and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharm 11:151–169
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko MJ (2011) Arsenic: toxicity, oxidative stress and human disease. Appl Toxicol 31:95–107
Machado NG, Nassarden DCS, Dos Santos F, Boaventura ICG, Perrier G, Souza FSC, de Biudes MS (2015) Chironomus larvae (Chironomidae: Diptera) as water quality indicators along an environmental gradient in a neotropical urban stream. Ambiente e Agua - An Interdisciplinary J App Sci 10:298–309. https://doi.org/10.4136/ambi-agua.1533
Mahmoud HH (2016) New method for assessment of serum catalase activity. Indian J Sci Technol 9(4):1–5
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mogaji OY, Sotolu AO, Wilfred-Ekprikpo PC, Green BM (2018) The effects of crude oil exploration on fish and fisheries of Nigerian aquatic ecosystems. The political ecology of oil and gas activities in the Nigerian aquatic ecosystem:111–124. https://doi.org/10.1016/B978-0-12-809399-3.00008-2
Nwankwo N, Ifeadi CN (1988) Case studies on the environmental impact of oil production and marketing in Nigeria. In: Sada PO, Odemerho FO (eds) Environmental issues and management in Nigerian Development. Evans Brothers (Nigeria Publishers) Limited, Ibadan, Ibadan, pp 208–223
Obida CB, Alan BG, Duncan WJ, Semple KT (2018) Quantifying the exposure of humans and the environment to oil pollution in the Niger Delta using advanced geostatistical techniques. Environ Int 111:32–42. https://doi.org/10.1016/J.ENVINT.2017.11.009
Okay OS, Ozmen M, Güngördü A, Yilmaz A, Yakan SD, Karacik B, Tutak B, Schramm KW (2016) Heavy metal pollution in sediments and mussels: assessment by using pollution indices and metallothionein levels. Environ Monit Assess 188:352
Okogbue CO, Oyesanya OU, Anyiam OA, Omonona VO (2017) Assessment of pollution from produced water discharges in seawater and sediments in offshore, Niger Delta. Environ Earth Sci 76:359. https://doi.org/10.1007/s12665-017-6682-x
Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, Ogunpolu BS, Olopade JO (2014) Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharm 37:1202–1211
Ordinioha B, Brisibe S (2013) The human health implications of crude oil spills in the Niger delta, Nigeria: an interpretation of published studies. Nig Med J 54:10. https://doi.org/10.4103/0300-1652.108887
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30:1235–1243
Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM et al (2017) Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol 32:1089–1101
Sanchez W, Selim A, Olivier P, Jean-Maxence D, Jean-Marc P (2007) Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasterosteus aculeatus L.) sampled in contaminated streams. Ecotoxicol, Springer Verlag (Germany) 16:279–287
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A 73(2):114–127
Stevens JJ, Graham B, Walker AM, Tchounwou PB, Rogers C (2010) The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int J Environ Res Public Health 7:2018–2032
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular Clinical and Environmental Toxicology
Venturino E, Tiwari PK, Misra AK (2017) Modeling the depletion of dissolved oxygen in a water body located near a city. Math Methods Appl Sci 40:1081–1094. https://doi.org/10.1002/mma.4037
WHO (1984) Guidelines for drinking water quality. Health criteria and other supporting information, vol 2. WHO, Geneva
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arojojoye, O.A., Oyagbemi, A.A. & Gbemisola, O.M. Biomarkers of oxidative stress in Clarias gariepinus for assessing toxicological effects of heavy metal pollution of Abereke river in southwest Nigeria. Comp Clin Pathol 28, 1675–1680 (2019). https://doi.org/10.1007/s00580-019-02999-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-02999-8