Abstract
The protective function of nitric oxide (NO) has been extensively clarified in plant responses to abiotic stresses. However, little is known about the regulation of NO in plants exposed to sulfur dioxide (SO2). In the present study, we found that co-exposure to NO significantly attenuated SO2-induced wheat seedling growth inhibition. Data showed that NO efficiently prevented SO2-triggered oxidative stress, as indicated by decreasing reactive oxygen species production, lipid peroxidation, and electrolyte leakage. This might be attributed to the regulatory role of NO in antioxidative defense, such as increasing the activities of antioxidative enzymes and the contents of non-enzymatic antioxidants. The SO2-caused declines in soluble protein and chlorophyll content were efficiently recovered by NO application. Photosynthetic parameters, such as net photosynthetic rate, maximum photochemical efficiency, and actual photochemical efficiency, were protected by NO. In conclusion, this study demonstrated that during SO2 exposure, co-application of NO can efficiently alleviate plant damage probably by regulating the antioxidative defense, and protecting plant photosynthesis-related process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Sulfur dioxide (SO2) is a major air pollutant produced by burning and processing of sulfur-containing fossil fuels. Although the release of SO2 has been strictly controlled, its atmospheric concentration has increased in many areas of the world during the past few decades due to increased SO2 emissions (Smith et al. 2011). Exposure to high doses of SO2 can induce plant leaf chlorosis and necrosis, growth inhibition, even death (Agrawal et al. 2003). Several mechanisms have been proposed to account for the SO2 phytotoxicity, but the essential mechanism is still unclear. SO2 enters plant leaves mainly through stomata, and fast dissolves in cellular fluid to form sulfite (SO3 2−) and bisulfite (HSO3 −), which have been verified to be toxic to many plant biochemical and physiological processes (Pfanz and Heber 1986). For instance, bisulfite may reduce photosynthesis rate and thereby crop yield through inducing reactive oxygen species (ROS) production probably by plasma membrane NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) oxidase (Li et al. 2007). The inhibition of photosynthesis and the formation of ROS are generally recognized as the first effects of SO2 on plants (Bowler et al. 1992; Nouchi 2002). ROS in excess causes cell membrane and DNA damage, protein and enzyme inactivation (Valko et al. 2007). Therefore, in the present study, the photosynthesis-related performance and the oxidation-antioxidation contest are analyzed to evaluate the NO attenuation in SO2-induced damage to wheat seedlings.
Nitric oxide (NO) is a redox signaling molecule with versatile biological functions. Since the end of the twentieth century when the plant function of NO was suggested, more and more researches have linked NO with a large number of plant phenomena (Wendehenne and Hancock 2011). In plant response to stress conditions, NO can exert a positive or negative regulatory role depending on its local concentration and spatial generation patterns (Mur et al. 2012). Although NO is an uncharged free radical with limited reactivity, it can interact rapidly with other radicals (for a review see Fancy et al. 2017). For example, NO can react with superoxide anion (O2˙ˉ) to produce a more powerful oxidant ONOOˉ. Also, NO2ˉ and NO3ˉ are generated following the O2˙ˉ oxidation of NO. Nitric oxide also interacts with O2 to form NO2. These resultant molecules are much stronger oxidants compared with NO. However, at low concentrations, NO provides a protective role in plant response to abiotic stress such as high salt, heavy metals, drought, excess light, cold, heat, ozone, UV-B, and so on (Fancy et al. 2017). Many mechanisms have been proposed to decipher the plant protection of NO, among which the scavenging of ROS and the regulation of stomatal aperture are generally accepted. Based on the fact that little is known about the NO regulatory role in plant response to SO2, we carried out this study. The aim is to explore plant response mechanisms to SO2 and NO alone or in combination.
Materials and Methods
Wheat (Triticum aestivum L.) seeds were purchased from a local market. Healthy seeds were surface sterilized with sodium hypochlorite (0.1%, w/v) for 5 min, then washed by rinsing in distilled water, and sown in pots containing a mixture of peat + perlite (3:1, v/v) in a growth chamber under 14-h photoperiod (irradiance of 200 μmol m−2 s−1), day/night temperature of 22/18°C and relative humidity of 75%. Plants were randomly divided into control group (control plants), SO2 treatment group (SO2 plants), NO treatment group (NO plants), and SO2 + NO treatment group (SO2 + NO plants). For the exposure of SO2 and NO alone or in combination, 2-week-old plants were transferred to an air tight glass chamber (0.8 × 0.8 × 0.8 m), and SO2 and/or NO was supplied directly from cylinders into a dilution reservoir into which charcoal filtered air was drawn. The SO2 and NO gases are purchased from Dalian Special Gases Co., LTD (Dalian, China). The original concentrations of SO2 and NO gases in cylinders are 2% and 3%, respectively, prepared by charcoal filtered compressed air. The diluted gas mixture was delivered to fumigation chamber equipped with inlet (at the upper portion) and outlet (at the lower portion) tubings. The flow rate was regulated by a mass flow controller (D07-7C; Sevenstar Electronics, Beijing, China). The concentrations of the chamber SO2 and/or NO were continuously monitored using a gas analyzer (SWG 300-1; MRU, Germany), and the detection limit is 0.1 ppm. The outlet was connected to three glass bottles in tandem containing NaOH solution trap for exhaust gases. To ensure the effective concentrations of the tested gases in the fumigation chamber, we measured the gas recovery rate using standard concentrations of gases, with recovery rates of 94.6% and 97.4% for SO2 and NO, respectively. For the control, charcoal-filtered air was supplied. Plants were fumigated during the light period for 6 h d−1 for 3 days. The fumigation concentrations of SO2 and NO (if any) were 20 ± 1 and 10 ± 1 ppm, respectively. Although concentrations greater 2 ppm SO2 are not generally obtained in the environment, higher concentrations of SO2 are the usual doses in an acute exposure experiment such as in the previous studies (Li and Yi 2012; Li et al. 2016) where 30 mg m−3 SO2 (about 10.5 ppm) was used. Except where mentioned in the text, all physiological and biochemical analyses were carried out immediately following the end of fumigation. Each experiment was performed separately three times.
At the end of fumigation, aerial plant parts were harvested, and washed to remove surface residues by rinsing in tap water, then blotted moisture with filter papers and weighed as fresh weight. For dry weight determination, the plant was dried at 80°C in an oven for 48 h. To observe plant recovery after fumigation, the plant growth was prolonged for 7 days at ambient air conditions, and the fresh weight and dry weight were respectively determined with above-mentioned methods. Pictures of aerial plant parts were taken before harvesting.
Total chlorophyll contents were determined using an 80% (v/v) acetone extract of leaves, and soluble protein contents were measured using the Coomassie Brilliant Blue colorimetric method, for a detail description see Zhang and Qu (2003). The leaf levels of hydrogen peroxide were estimated as described by He et al. (2014). Briefly, collected fresh leaves (1.0 g) were homogenized with 10 mL cooled acetone at 10°C using a mortar and pestle. The homogenate was filtered with Whatman No. 1 filter paper, then mixed with 4 mL titanium reagent and 5 mL ammonium solution, and centrifuged at 10,000g for 10 min. The precipitate was dissolved in 10 mL 2 M H2SO4, and recentrifuged. The supernatant was analyzed at 415 nm in a spectrophotometer (UV762, INESA, Shanghai, China). The content of malondialdehyde was assayed according to Shalata and Tal (1998). Electrolyte leakage was determined using conductivity meter (SA29-DDB-11A, Midwest Group, Beijing, China) as described by Hao et al. (2012).
For the determination of antioxidative enzymes, leaves were collected and homogenized using a mortar and pestle under liquid nitrogen. The powder was suspended in 5 mL of ice-cold extract solution consisting of 50 mM phosphate-buffered saline (pH 7.8), 0.1 mM EDTA, 1% (v/v) Triton X-100, and 4% (w/v) polyvinyl pyrroidone. The homogenate was incubated for 10 min on ice, and centrifuged at 12,000g at 4°C for 15 min. The supernatant was used to measure (1) superoxide dismutase (SOD; EC 1.15.1.1) by the nitroblue tetrazolium (NBT) method (Beyer and Fridovich 1987), and one unit was defined as the amount of enzyme required to inhibit the photoreduction of NBT by 50%; (2) peroxidase (POD; EC1.11.1.7) according to Hemeda and Klein (1990). An increase per minute of 0.01 absorbance value at 470 nm was defined as one unit of POD; (3) catalase (CAT; EC 1.11.1.6) by measuring the decomposition of H2O2 at 240 nm for 3 min as described by Aebi (1983), where 0.04% (v/v) of H2O2 was added as the initial concentration. A decrease per minute of 0.01 absorbance value at 240 nm was defined as one unit of CAT. For all these measurements, a spectrophotometer UV762 (INESA, Shanghai, China) was used. For the expression of specific activity, protein content in the crude enzyme extract was quantified by the method of Bradford (1976) using bovine serum albumin as standard.
For the determination of ascorbic acid (AA) content, fresh leaves were ground under liquid nitrogen, and the powder was added to a test tube containing 5 mL of 10% trichloroacetic acid. The mixed solution was incubated for 10 min on ice, centrifuged at 12,000g for 15 min at 4°C, and supernatant was used to assay AA content based on the method of Keller and Schwager (1977). The reduced glutathione (GSH) content was detected as described by Griffith and Meister (1979). Fresh leaves were ground under liquid nitrogen, and the powder was placed in a test tube containing 5 mL of 2% metaphosphoric acid, and incubated for 10 min on ice. After centrifuged at 12,000g for 15 min at 4°C, the supernatant was neutralized by adding 0.6 mL 10% sodium citrate.
Net photosynthetic rate and stomatal conductance were measured using a portable photosynthetic system (LI-6200, LI-COR, Lincoln, NE, USA) at ambient conditions, irradiance of 200 µmol m−2 s−1, and 25°C. Maximum photochemical efficiency (Fv/Fm) and actual photochemical efficiency of PS2 (ΦPS2) were analyzed using a fluorometer (Handy-PEA, Hansatech, Norfolk, UK). Briefly, plants were dark-adapted for 15 min before measurement. Minimal fluorescence yield (F0) was detected with a weak irradiance (0.5 µmol m−2 s−1) from a light-emitting diode. Maximum fluorescence (Fm) was determined by a 1.0-s saturating pulse (1500 µmol m−2 s−1), and steady state fluorescence (Fs) was detected by 15-min actinic light (30 µmol m−2 s−1). Fv/Fm = (Fm − F0)/Fm, and ΦPS2 = (Fm − Fs)/Fm.
Experiment data are expressed as means ± SD. Difference among treatments were analyzed by one-way analysis of variance (ANOVA) using SAS software (SAS Institute, Cary, NC, USA), taking p < 0.05 as significant according to Duncan’s multiple range test.
Results and Discussion
Sulfur dioxide exposure caused leaf wilt and chlorosis, and leaf apex necrosis, especially severe in 7-day-recovery plants (Fig. 1). The phenotype of plants received only NO treatment was similar to control plants. As expected, the SO2-induced damages were dramatically alleviated by co-exposure to NO (Fig. 1). The fresh and dry weight of aerial plant parts at the end of fumigation and after the recovery period also properly reflected SO2-induced plant damage, as well as the repairing function of NO (Fig. 2a–d). Interestingly, although NO did not alter the fresh or dry weight of plants at the end of fumigation (Fig. 2a, b), it moderately promoted plant growth during the recovery period, as compared with control plants (Fig. 2c, d).
Effect of SO2 and NO on plant growth, total chlorophyll and soluble protein content. A and B, fresh and dry weight of aerial plant parts at the end of fumigation, respectively; C and D, fresh and dry weight of aerial plant parts after 7 days recovery, respectively; E, total chlorophyll content; and F, soluble protein content. Means ± SD, n = 3 (different letters indicate significant difference at p < 0.05)
In the previous SO2-related studies, the mean concentration of fumigation chamber SO2 was monitored by colorimetric measurement such as using pararosaniline hydrochloride spectrophotometry (Hao et al. 2005, 2011; Li et al. 2016), or SO2 was supplied by SO2 donors (Wei et al. 2013). In the present study, a gas analyzer was used to continuously monitor the fumigation concentration, thereby greatly improved the experimental accuracy. Our data clearly showed that the effect of SO2 on wheat seedlings, as indicated by leaf morphology, dry weight, especially the fresh weight, was much more prominent during the recovery period than the fumigation period. This suggested that SO2-induced subsequent reactions may be more toxic to plants. The co-exposure to NO almost completely mitigated the SO2 phytotoxicity in the recovery plants (Figs. 1, 2c, d), implying that NO-induced subsequent changes may provide a protective effect even long after the NO is removed. Additionally, NO treatment alone promoted plant growth compared with control plants during the 7-day-recovery period, although to a lesser extent. This further confirmed the NO function inducing a primed state, which benefits plant growth, or renders plants to respond more efficiently to subsequent stress conditions (Conrath 2011). The prolonged effect of exogenous NO might be related to the induction of endogenous NO synthesis (Groß et al. 2013). In addition, NO gas was used in this study instead of NO donors. Although many NO-releasing compounds can be used conveniently in most of the NO-related studies, they have undesired side-effects on plants probably by the remaining products when all NO has been produced. Also, the in planta kinetics of NO generation by various NO donors are different, including NO releasing amount, timing and persistence (Mur et al. 2013). Therefore, NO gas fumigation is an alternative method for the application of NO (Bai et al. 2011; Kovacs et al. 2015).
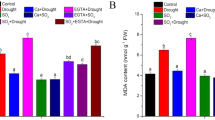
Because of the severe damage of SO2 plants after recovery period, physiological and biochemical parameters were measured only on the plants at the immediate end of fumigation. When compared with the control level, total chlorophyll content in SO2 plants decreased significantly, whereas it was maintained at the same level in NO plants, and just slightly declined in SO2 + NO plants (Fig. 2e). Similarly, the content of soluble protein was comparable to the control level in NO plants, but obviously lowered in SO2 plants, which was largely resumed by NO co-exposure (Fig. 2f). The level of H2O2 dramatically increased in SO2 plants (Fig. 3a), correspondingly, the content of malondialdehyde (MDA) and electrolyte leakage were greatly elevated (Fig. 3b, c), indicating that the SO2 exposure in this study had caused oxidative damage to plants. However, the SO2-induced increases in H2O2, as well as in the oxidative stress as shown by MDA production and electrolyte leakage, were efficiently prevented by NO co-exposure (Fig. 3a–c). The values of all these oxidative stress-related parameters were at lower levels in NO plants than those in control plants (Fig. 3a–c).
The protective role of NO in plant chlorophyll against various stress factors have been repeatedly reported (e.g. Fatma et al. 2016 and references therein). This might be one of the mechanisms responsible for NO-mediated protection in photosynthetic rate under stress conditions. Also, many publications documented that exogenous NO can efficiently reverse stress-induced soluble protein decrease (e.g. Zeng et al. 2011). Several lines of evidence have demonstrated that SO2 can promote ROS production and cause oxidative stress (Dat et al. 2000; Hao et al. 2011). In this study, the SO2-induced oxidative damage may be a major reason causing plant chlorosis, necrosis, and protein degradation. Exogenous NO always improved abiotic stress tolerance associated with a decrease in the levels of H2O2 and MDA (Groß et al. 2013). This held true in the case of SO2 stress. As a direct scavenger of ROS, NO is not just a down-stream signal of H2O2 but also affect ROS generation and degradation (Groß et al. 2013). For instance, NO suppresses ROS accumulation by inhibiting the activity of ROS-producing enzyme NADPH oxidase (Yun et al. 2011).
The activities of antioxidative enzyme SOD, POD and CAT, and the contents of non-enzymatic antioxidant glutathione and ascorbic acid were markedly decreased under SO2 exposure, whereas they were at a comparable level, even further enhanced in CAT activity in SO2 + NO plants relative to control plants. Without exception, the above-mentioned antioxidants increased significantly in NO plants (Figs. 3d–f, 4a, b). This correlated well with the change trends in H2O2, MDA and electrolyte leakage. A large body of evidence has shown that exogenous NO-mediated plant tolerance to stress conditions is associated with NO as a signal molecule stimulating the antioxidant system and/or a primed state of stress defense. For example, exogenous application of NO (as NO donors or NO gas) can efficiently up-regulate the activities of SOD, POD and CAT (for a review see Groß et al. 2013). Apart from promoting the expression level of enzymatic proteins, increasing evidence also showed that NO improved antioxidant enzyme activities via the regulation of antioxidant protein carbonylation and S-nitrosylation statuses (e.g. Bai et al. 2011). Also, many studies have found that exogenous NO might promote the contents of glutathione and ascorbic acid under stress conditions (such as Keyster et al. 2012; Hasanuzzaman and Fujita 2013). The study by Innocenti et al. (2007) demonstrated that exogenous NO stimulated transcription levels of genes involved in glutathione synthesis, thereby resulting in an increase of glutathione.
The exposure to SO2 obviously lowered stomatal conductance regardless of whether NO was present, whereas NO treatment alone markedly increased stomatal conductance (Fig. 4c). Photosynthetic rate (Pn), maximum photochemical efficiency (Fv/Fm) and actual photochemical efficiency (ΦPS2) were significantly decreased by SO2 (Fig. 4d–f), but the reduced magnitudes of Pn and ΦPS2 obviously narrowed in SO2 + NO plants (Fig. 4d, f), and the ratio of Fv/Fm was even at a comparable level to the control (Fig. 4e). Nitric oxide treatment alone did not affect the levels of Fv/Fm and ΦPS2, but enhanced Pn to a lesser extent (Fig. 4d). The inhibited effect of SO2 on stomatal conductance, as well as the photosynthesis-related parameters was also found in Arabidopsis plants (Hao et al. 2011). Many studies have demonstrated that NO is involved in plant stomata movements, either opening or closing depending on NO concentrations tested (for a review see Mur et al. 2013). However, our data showed that NO used alone increased stomatal conductance, but simultaneous use of NO did not influence the SO2-induced inhibitory extent on stomatal conductance, implying that the alleviation effect of NO on SO2 plants was related to other factors rather than to stomatal factors, even though SO2 enters mesophyll mainly via stomata. Many studies have shown that exogenous NO can efficiently prevent stress-induced decrease in chlorophyll fluorescence parameters such as ΦPS2 and Fv/Fm, thereby protecting photosynthesis (e.g. Zhang et al. 2006). In the present study, co-exposure to NO largely counteracted the SO2 damage to ΦPS2 and Fv/Fm, even though NO used alone did not affected these two parameters (Fig. 4e, f).
Effect of SO2 and NO on non-enzymatic antioxidant contents, and photosynthesis-related parameters. A, glutathione content; B, ascorbic acid content; C, stomatal conductance; D, net photosynthetic rate; E, maximum photochemical efficiency; and F, actual photochemical efficiency. Means ± SD, n = 3 (different letters indicate significant difference at p < 0.05)
In conclusion, this short paper demonstrated that co-exposure to NO gas can efficiently alleviate SO2-induced plant damage by enhancing antioxidative capability, reducing oxidative stress, protecting photosynthesis system, therefore, maintaining a moderate photosynthetic rate and plant growth. This extends the list of NO involved in plant response to abiotic stresses.
References
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analyses, Verlag Chemie, Weinheim, pp 273–282
Agrawal M, Singha B, Rajputa M, Marshallb F, Bellb JNB (2003) Effect of air pollution on peri-urban agriculture: a case study. Environ Pollut 126:323–329
Bai X, Yang L, Tian M, Chen J, Shi J, Yang Y, Hu X (2011) Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS ONE 6:e20714
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bowler C, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for thequantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Fancy NN, Bahlmann AK, Loake GJ (2017) Nitric oxide function in plant abiotic stress. Plant Cell Environ 40:462–472
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in Mustard. Front Plant Sci 7:521
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (s-n-butylhomocysteine sulfoximine). J Biol Chem 254:7558–7560
Groß F, Durner J, Gaupels F (2013) Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci 29:419
Hao L, Zhang H, Xu X, Tao S, Yu L (2005) SO2-caused oxidative stress and modulation of some signal molecules in wheat. Chin J Appl Ecol 16:1038–1042
Hao L, Wang Y, Xu J, Feng SD, Ma CY, Liu C, Xu X, Li GZ, Herbert SJ (2011) Role of endogenous salicylic acid in Arabidopsis response to elevated sulfur dioxide concentration. Biol Plant 55:297–304
Hao L, Zhao Y, Jin D, Zhang L, Bi XH, Chen HX, Xu Q, Ma CY, Li GZ (2012) Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil 354:81–95
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596
He QQ, Zhao SY, Ma QF, Zhang YY, Huang LL, Li GZ, Hao L (2014) Endogenous salicylic acid levels and signaling positively regulate Arabidopsis response to polyethylene glycol-simulated drought stress. J Plant Growth Regul 33:871–880
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, De Stefano M, Delledonne M, Puppo A, Baudouin E, Frendo P (2007) Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 225:1597–1602
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J For Pathol 7:338–350
Keyster M, Klein A, Ludidi N (2012) Caspase-like enzymatic activity and the ascorbate-glutathione cycle participate in salt stress tolerance of maize conferred by exogenously applied nitric oxide. Plant Signal Behav 7:349–360
Kovacs I, Durner J, Lindermayr C (2015) Crosstalk between nitric oxide and glutathione is required for NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1)-dependent defense signaling in Arabidopsis thaliana. New Phytol 208:860–872
Li L, Yi H (2012) Differential expression of Arabidopsis defense-related genes in response to sulfur dioxide. Chemosphere 78:718–724
Li B, Xing D, Zhang L (2007) Involvement of NADPH oxidase in sulfur dioxide-induced oxidative stress in plant cells. Photochem Photobiol Sci 6:628–634
Li L, Xue M, Yi H (2016) Uncovering microRNA-mediated response to SO2 stress in Arabidopsis thaliana by deep sequencing. J Hazard Mater 316:178–185
Mur LA, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH (2012) Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot 63:4375–4387
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJM, Hebelstrup KH, Gupta KJ (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052
Nouchi I (2002) Responses of whole plants to air pollutions. In: Omasa K (ed) Air pollution and plant biotechnology, Springer-Verlag, Tokyo, pp 4–8
Pfanz H, Heber U (1986) Buffer capacities of leaves, leaf cells, and leaf cell organelles in relation to fluxes of potentially acidic gases. Plant Physiol 81:597–602
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:167–174
Smith SJ, van Aardenne J, Klimont Z, Andres RJ, Volke A, Delgado Arias S (2011) Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos Chem Phys 11:1101–1116
Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Wei A, Xin X, Wang Y, Zhang C, Cao D (2013) Signal regulation involved in sulfur dioxide-induced guard cell apoptosis in Hemerocallis fulva. Ecotoxicol Environ Saf 98:41–45
Wendehenne D, Hancock JT (2011) New frontiers in nitric oxide biology in plant. Plant Sci 181:507–508
Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, Pallas JA, Loake GJ (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478:264–268
Zeng CL, Liu L, Wang BR, Wu XM, Zhou Y (2011) Physiological effects of exogenous nitric oxide on Brassica juncea seedlings under NaCl stress. Biol Plant 55:345–348
Zhang ZL, Qu WJ (2003) Guide to plant physiology experiment. 3rd edn. Higher Education Press, Beijing, pp 60–160
Zhang L, Wang Y, Zhao L, Shi S, Zhang L (2006) Involvement of nitric oxide in light-mediated greening of barley seedlings. J Plant Physiol 163:818–826
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 31572213, 31570502, 31270446).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yang Liu and Yunfeng Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, Y., Li, L. et al. Attenuation of Sulfur Dioxide Damage to Wheat Seedlings by Co-exposure to Nitric Oxide. Bull Environ Contam Toxicol 99, 146–151 (2017). https://doi.org/10.1007/s00128-017-2103-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2103-9