Abstract
In this study, ambient NH3, NO, NO2, CO and SO2 were measured continuously from February, 2008, to December, 2016. The annual average mixing ratios (mole/mole) of NH3, NO, NO2, CO and SO2 were 17.8 ± 3.4 ppb; 21.2 ± 2.3 ppb, 18.1 ± 3.2 ppb; 1.7 ± 0.3 ppm and 2.0 ± 0.3 ppb, respectively. All the trace gases (NH3, NO, NO2, CO and SO2) showed significant annual variation during the study. A significant increasing trend in mixing ratios of ambient NH3 and NO2 were observed at the observational site of Delhi, whereas, increasing trend were recorded in case of NO, CO and NO2 mixing ratios. The results emphasized that traffic could be one of the significant sources of ambient NH3 at the urban site of Delhi, as illustrated by positive correlations of NH3 with traffic related co-pollutants (NO and CO).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since the gaseous toxic air pollutants are normally associated with acute short-term and chronic long-term human health problems, the study of these pollutants is essential to understand their mechanism of occurrence (Behera et al. 2015; Saraswati et al. 2017). Ammonia (NH3) is the third most abundant nitrogen containing gas in the atmosphere after N2 and N2O (Seinfeld and Pandis 1998; Aneja et al. 2000). As a primary alkaline gas in ambient air, NH3 can neutralize nitric acid and sulphuric acid gases to form ammonium nitrate and sulphate (Pinder et al. 2007; Sharma et al. 2012c, 2014, Saxena et al. 2017), which are important constituents of airborne fine particles or PM2.5 (Chow et al. 2004; Kean et al. 2000; Aneja et al. 2001; Ram et al. 2010; Huang et al. 2011). The most recent consideration for NH3 emissions on the global scale is linked to climate change based on its ability to form PM2.5, specifically ammonium sulphate (Aneja et al. 2001). These aerosols can possibly increase the Earth’s albedo (Sutton et al. 2000). The need to better understand the role of this particular air pollutant has been underscored in recent years as its atmospheric concentration increases. Effective management strategies are essential to mitigate the pollutants over public health concern and to enhance visibility (Brunekreef and Holgate 2002; Wang et al. 2012).
Major anthropogenic sources of ambient NH3 are agricultural practices, livestock, transport and industrial activities (Sutton et al. 2000; Li et al. 2006; Sharma et al. 2010), along with natural sources like forest fires and emissions from soil (Olivier et al. 1998; Lee et al. 2005). Recent studies (Sutton et al. 1995, 2000; Wang et al. 2012) have established that reduction of NO compounds in three-way catalytic converters of automobile exhaust and industrial power stations emissions are significant contributors to atmospheric NH3 in urban environments \((2{\text{NO }} + {\text{ }}2{\text{CO }} + {\text{ }}3{{\text{H}}_2} \to {\text{ }}2{\text{N}}{{\text{H}}_3} + {\text{ }}2{\text{C}}{{\text{O}}_2}{\text{ or }}2{\text{NO }} + {\text{ }}5{{\text{H}}_2} \to {\text{ }}2{\text{N}}{{\text{H}}_3} + {\text{ }}2{{\text{H}}_2}{\text{O}})\) (Gandhi and Shelef 1991). Correlation matrix of ambient NH3 with CO also suggested the dependency of NH3 mixing ratio on the traffic intensity and air temperature (Perrino et al. 2002; Meng et al. 2011; Sharma et al. 2014). Li et al. (2006) observed spikes in the NH3 time-series that was correlated with the spikes in the CO, and inferred that the NH3 came from nearby traffic. In the previous study, we have reported the diurnal, seasonal and temporal variations in mixing ratios of ambient NH3 and its interaction with other trace gases and reference theirein (Sharma et al. 2010a, b, 2012a, b, c, 2014; Saraswati et al. 2017). In this paper, we present the inter-annual variation in mixing ratios of ambient NH3 and other related trace gases (NO, NO2, CO and SO2) in an urban area of Delhi.
Materials and Methods
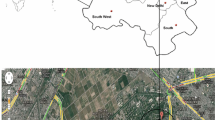
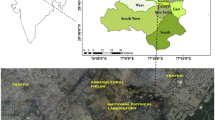
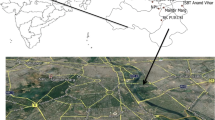
Delhi, the capital of India is surrounded by four different climatic zones (Himalyas in the north, central hot plains in the south, the Thar desert in the west and the Indo Gangetic plain in the east), which influence its semi-arid climate consided as one of the most polluted megacites of the world. The mixing ratios (mole/mole or mole fraction) of ambient NH3, NO, NO2, CO and SO2 were measured, along with meteorological parameters (temperature, relative humidity (RH), wind speed and wind direction) at the CSIR-National Physical Laboratory, New Delhi (28°38′N, 77°10′E; 218 m amsl) from February, 2008, to December, 2016. The observational site is amenable to free wind flow from all directions and represents a typical urban atmosphere, surrounded by huge roadside traffic (~100 m) and agricultural fields in the southwest direction (~500 m). The observational area is under the influence of air mass flow from north-east to north-west in winter and from south-east to south-west in the summer. The temperature of Delhi varied from maximum in summer to minimum in winter. Detailed descriptions of the sampling site including meteorology are available in Sharma et al. (2016a).
Ambient NH3 mixing ratio was measured initially using an NH3-analyzer (CLD88CYp, ECO Physics AG, Duernten, CH) operating (accuracy: ±0.5 ppb) on a chemiluminescence method (February, 2008, to December, 2012). From January, 2012, to December, 2015, the measurement of ambient NH3 was carried out using an NH3-analyzer (AC32M&CNH3, Environment SA, Paris, FR), which also operates (accuracy: ±1.0 ppb) on the chemiluminescence method. NO and NO2 were measured using a NOx-analyzer (CLD 88p, ECO Physics AG, Duernten, CH) with a photocatalytic converter (Model: PLC 860 M/s. ECO Physics AG; accuracy: ±0.5 ppb). Carbon monoxide (CO) was observed continuously using a non-dispersive infrared (NDIR) gas filter correlation analyzer (Model 48 C; Thermo Fisher Scientific, Waltham, MA, USA). Sulphur dioxide was measured using a SO2-analyser (APSA 360 A, Horiba Ltd, Japan). The detailed principle of operations, detection limits, calibration procedures, calibration standards used (traceable to NIST-USA)and repeatability etc., of all the respective analyzers are available in Saraswati et al. (2017). All the analytical instruments showed <2.0% repeatability error during the span calibration. The meteorological parameters (temperature, RH, wind speed and wind directions) were measured simultaneously using sensors at a meteorological tower, which was 100 m away from the observational site within the same campus (Sharma et al. 2010a, b, 2014).
Results and Discussion
The annual average mixing ratios of ambient NH3, NO, NO2, CO and SO2 were 17.8 ± 3.4 ppb; 21.2 ± 2.3 ppb and 18.1 ± 3.2 ppb; 1.7 ± 0.3 ppm and 2.0 ± 0.3 ppb, respectively from February, 2008 to December, 2016 (Table 1). The highest annual average mixing ratio of ambient NH3 was observed in 2014 (24.3 ± 3.6 ppb), whereas the minimum mixing ratio ambient NH3 was recorded in 2010 (13.4 ± 4.2 ppb). Similarly, higher annual average mixing ratios of ambient NO and NO2 were recorded in 2016 (25.6 ± 7.5 ppb) and 2015 (23.9 ± 6.6 ppb), respectively. Figure 1 showed the annual variation alongwith trend in mixing ratios of ambient NH3, NO, NO2, CO and SO2 during 2008–2016. Mixing ratios of all the trace gases varied non-significantly, except for ambient NH3 and NO2 during the study. This may have been due to source strength of respective trace gases and prevailing meteorological conditions at the observational site in Delhi.
Kapoor et al. (1992) reported the average NH3 mixing ratio of NH3 as 47.3 ± 13.6 ppb in Delhi, whereas, Sharma et al. (2010) reported the average mixing ratio of ambient NH3 as 20.23 ± 2.71 ppb. Zutshi et al. (1970) reported the NH3 mixing ratio as 50.7 ppb at Mumbai, whereas, Sharma et al. (2016b) reported the NH3 mixing ratio as 43.4 ± 7.0 ppb at Kolkata, India. The diurnal, seasonal and day to day variations in mixing ratios of ambient NH3 and other trace gases (O3, NO, NO2, CO and SO2) at Delhi and other locations of India are already discussed in our previous studied (Sharma et al. 2010a, b, 2012a, b, c, 2014; Saraswati et al. 2017) and compared with other studies (Sharma et al. 2014).
Annual average mixing ratios and inter-annual variations in mixing ratios of NH3, NO, NO2, CO and SO2 (from the average value of the study period) are summarized in Table 1. The maximum annual variation in mixing ratio of ambient NH3 was in 2014 (6.58 ppb), whereas minumum in 2015 (0.72 ppb). The minimum and maximum inter-annual variations in mixing ratio of ambient NO were −0.35 ppb in 2012 and 4.35 ppb in 2016, respectively. Similarly, maximum (5.78 ppb) significant inter-annual variation in mixing ratio of NO2 was observed during 2015. The mixing ratios of CO and SO2 were also varying inter-annually during the study period (Table 1). The inter-annual variation in mixing ratios of NH3, NO, NO2, CO and SO2 were observed may be due to source strength of respective traces and meteorological condition of the observational site.
Although agriculture has historically been the major source of ambient NH3, however, the contribution of vehicles equipped with three-way catalytic converters has increased considerably at the urban sites (Sutton et al. 2000; Heeb et al. 2008). Therefore, in order to examine the contribution of traffic to NH3, it may be useful to compare/correlate ambient NH3 with those of primary pollutants mainly emitted by motor vehicle exhausts, such as NO and CO. Table 2 summarized the correlations among NH3 versus NO, NH3 versus NO2, NH3 versus CO and NH3 versus SO2 during 2008–2016, along with meteorological parameters at the study site.
The correlation matrix of hourly annual average mixing ratios of NH3 with NO and CO during 2008–2016 showed positive correlation (r = 0.53 NH3 vs. NO; r = 0.66 NH3 vs. CO), supporting the hypothesis that traffic is also an important source of NH3 at the observational site (Table 2). Meng et al. (2011) also reported a strong positive correlation (r = 0.78) between NH3 and NO during winter at Beijing, China, and considered traffic to be one of the sources of ambient NH3 at there. The correlation coefficient of NH3 versus CO was also reported as r = 0.71 during winter in Beijing, China (Meng et al. 2011), further supporting the present study. SO2 also reacts with the hydroxyl radical (OH) to form sulphuric acid (Seinfeld and Pandis 1998). Table 2 shows a positive relationship of between NH3 and NO2 (r = 0.41), which indicates the formation of HNO3 and simultaneously NH4NO3 in the atmosphere (Stockwell et al. 2000). During night time, the increased humidity and lowered temperature, along with a higher mixing ratio of NH3, SO2 and NO2 may facilitate the formation of \({\text{NH}}_{_{\text{4}}}^ +\) aerosol in the atmosphere. Table 2 also shows the positive relationship between NH3 and SO2 during study (r = 0.32) indicationg the possible formation of (NH4)2SO4.
References
Aneja VP, Chauhan JP, Walker JT (2000) Characterization of atmospheric ammonia emissions from swine waste storage and treatment lagoons. J Geophys Atmos 105:11535–11545
Aneja VP, Roelle PA, Murray GC, Southerland J, Erisman JW, Fowler D (2001) Atmospheric nitrogen compounds II: emissions, transport, transformation, deposition and assessment. Atmos Environ 35:1903–1911
Behera SN, Sharma M, Shukla SP (2015) Characterization of gaseous pollutants and water soluble inorganic ions in PM2.5 during summer time at an urban site of North India. J Hazard Toxic Radioact Waste A4015002-1
Brunekreef B, Holgate ST (2002) Air pollution and health. Lancet 360:1233–1242
Chow JC, Watson JG, Chen LWA, Arnott WP, Moosmuller H (2004) Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ Sci Technol 38:4414–4422
Gandhi HS, Shelef M (1991) Effect of sulphur on noble metal automotive catalysts. Appl Catal 77:175–186
Heeb NV, Saxer CJ, Forss AM, Bruhlmann S (2008) Trends of NO−, NO2− and NH3− emissions from gasoline fueled euro-3- to euro-4 passenger cars. Atmos Environ 42:2543–2554
Huang X, Qiu R, Chan CK, Kant PR (2011) Evidence of high PM2.5 strong acidity in ammonia-rich atmosphere of Guangzhou, China, transition in pathways of ambient ammonia to form aerosol ammonium at [NH4 +]/[SO4 2−] = 1.5. Atmos Res 99:488–495
Kapoor RK, Singh G, Tiwari S (1992) Ammonia concentration viš a viš meteorological conditions at Delhi, India. Atmos Res 28:1–9
Kean AJ, Harley RA, Littlejohn D, Kendal GR (2000) On-road measurement of ammonia and other motor exhaust emission. Environ Sci Technol 34:3535–3539
Lee S, Baumann K, Schauer JJ, Sheesley RJ, Nacher LP, Meinardi S, Blake DR (2005) Gaseous and particulate emissions from prescribed burning in Georgia. Environ Sci Technol 39:9049–9056
Li Y, Schwab JJ, Demerjian KL (2006) Measurement of ambient ammonia using a tunable diode laser absorption spectrometer: characteristics of ambient ammonia emissions in an urban area of New York City. J Geophys Res. doi:10.1029/2005JD006275
Meng ZY, Lin WL, Jiang XM, Yan P, Wang Y, Zhang YM, Jia XF, Yu XL (2011) Characteristics of atmospheric ammonia over Beijing, China. Atmos Chem Phys 11:6139–6151
Olivier JGJ, Bouwman AF, Van der Hoek KW, Berdowski JJM (1998) Global air emission inventories for anthropogenic sources of NOx, NH3 and N2O in 1990. Environ Poll 102:135–148
Perrino C, Catrambone M, Bucchianico ADMdi, Allegrini I (2002) Gaseous ammonia in the urban area of Rome, Italy and its relationship with traffic emissions. Atmos Environ 36:5385–5394
Pinder RW, Adams PJ, Pandis SN (2007) Ammonia emission controls as a cost-effective strategy for reducing atmospheric particular matter in the eastern United States. Environ Sci Technol 41:380–386
Ram K, Sarin MM, Tripathi SN (2010) One-year record of carbonaceous aerosols from an urban location (Kanpur) in the Indo-Gangetic Plain: characterization, sources and temporal variability. J Geophy Res. doi:10.1029/2010JD014188
Saraswati, Sharma SK, Mandal TK (2017) Five-year measurement of ambient ammonia and its interaction with other trace gases at an urban site of Delhi, India. Meteorol Atmos Phys. doi:10.1007/s00703-017-0512-2
Saxena M, Sharma A, Sen A, Saxena P, Saraswati, Mandal TK, Sharma SK (2017) Water soluble inorganic species of PM10 and PM2.5 at an urban site of Delhi, India: seasonal variability and sources. Atmos Res 184:112–125
Seinfeld JH, Pandis SN (1998) Atmospheric chemistry and physics: from air pollution to climate change, John Wiley, New York
Sharma SK, Datta A, Saud T, Mandal TK, Ahammed YN, Arya BC, Tiwar MK (2010a) Study on concentration of ambient NH3 and interactions with some other ambient trace gases. Environ Monit Asses 162:225–235
Sharma SK, Datta A, Saud T, Saxena M, Mandal TK, Ahammed YN, Arya BC (2010b) Seasonal variability of ambient NH3, NO, NO2 and SO2 over Delhi. J Environ Sci 22(7):1023–1028
Sharma SK, Saxena M, Saud T, Korpole S, Mandal TK (2012a) Measurement of NH3, NO, NO2 and related particulates at urban sites of Indo Gangetic Plain (IGP) of India. J Sci Indust Res 71(5):360–362
Sharma SK, Singh AK, Saud T, Mandal TK, Saxena M, Singh S, Ghosh S, Raha S (2012b) Measurement of ambient NH3, NO, NO2 and SO2 over Bay of Bengal during W_ICARB campaign. Ann Geophys 30:371–377
Sharma SK, Singh AK, Saud T, Mandal TK, Saxena M, Singh S, Ghosh S, Raha S (2012c) Study on water soluble ionic composition of PM10 and trace gases over Bay of Bengal during W_ICARB campaign. Meteor Atmos Phy 118:37–51
Sharma SK, Kumar M, Rohtash, Gupta NC, Saraswati, Saxena M, Mandal TK (2014) Characteristics of ambient ammonia over Delhi, India. Meteorol Atmos Phys 124:225–230
Sharma SK, Mandal TK, Jain S, Saraswati, Sharma A, Saxena M (2016a) Source apportionment of PM2.5 in Delhi, India using PMF model. Environ Contam Toxicol 97:286–293
Sharma SK, Rohtash, Mandal TK, Deb NC, Pal S (2016b) Measurement of ambient NH3, NO and NO2 at an urban area of Kolkata, India. Mapan 31:75–80
Stockwell WR, Watson JG, Robinson NF, Steiner W, Sylte WW (2000) The ammonium nitrate particle equivalent of NOx emissions for winter time conditions in Central California’s San Joaquin Valley. Atmos Environ 34:4711–4717
Sutton MA, Place CJ, Eagar M, Fowler D, Smith RL (1995) Assessment of the magnitude of ammonia emissions in the United Kingdom. Atmos Environ 29:1393–1411
Sutton MA, Dragostis U, Tang YS, Flower D (2000) Ammonia emissions from non-agricultural sources in the UK. Atmos Environ 34:855–869
Wang XM, Ding X, Fu XX, HE QF, Wang SY, Bernard F, Zhao XY, Wu D (2012) Aerosol scattering coefficients and major chemical compositions of fine particles observed at a rural site in the central Pearl River delta, south China. J Environ Sci 24:72–77
Zutshi PK, Sequaria R, Mahadevan TN, Banerjee T (1970) Environmental concentrations of some of the major pollutants at the BARC site, Trombay. Ind J Meteorol Geophys 21:473–478
Acknowledgements
The authors are thankful to the Director, CSIR-National Physical Laboratory, New Delhi and Head, Environmental Sciences and Biomedical Metrology Division (ES&BMD), CSIR-NPL, New Delhi for their encouragement and support. We also acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi (CSIR-CMM; CSIR-EMPOWER projects) and the Department of Science and Technology, New Delhi (Grant No.: SR/S4/AS:12/2008) for financial support. The authors are thankful to the anonymous reviewers and Editor-in-Chief for their constructive suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S.K., Saraswati, Mandal, T.K. et al. Inter-annual Variation of Ambient Ammonia and Related Trace Gases in Delhi, India. Bull Environ Contam Toxicol 99, 281–285 (2017). https://doi.org/10.1007/s00128-017-2058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2058-x