Abstract
The objective of this study was to investigate effects of tetrabromobisphenol A (TBBPA) on soybean seedlings. Growth and physiological parameters of soybean seedlings were measured by exposing seedlings to different concentrations of TBBPA (0, 5, 10, 20, 40, 80, and 100 mg kg−1 dry weight soil) in a greenhouse. Results showed 5 to 100 mg kg−1 TBBPA treatment reduced the shoot height, stem diameter, dry weight, and fresh weight, while 5 to 100 mg kg−1 TBBPA treatment reduced the chlorophyll content and induced production of malondialdehyde (MDA) in soybean leaves after 7 and 14 days of exposure. TBBPA treatment at low concentrations enhanced soluble sugar and soluble protein content, and it activated superoxide dismutase (SOD; EC:1.15.1.1), catalase (CAT; EC:1.11.1.6), and peroxidase (POD; EC:1.11.1.7); however, high concentrations of TBBPA inhibited activities of antioxidant enzymes and generation of soluble sugar and soluble protein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Tetrabromobisphenol A (TBBPA) is one of the most widely used brominated flame retardants globally. TBBPA enters the environment during the production, consumption, and disposal of TBBPA-containing products such as electronic equipment, plastics, and textiles (Alaee et al. 2003; Eriksson et al. 2004; Ali et al. 2011). Thus far, TBBPA has been detected in air, soil, surface water, sediment, plants and animals (Abdallah et al. 2008; Roosens et al. 2008; Li et al. 2011). The chemical structure of TBBPA is similar to that of thyroxin, characterized by cytotoxicity, immune toxicity, and estrogen (Covaci et al. 2009). Therefore, TBBPA is considered a potential endocrine disruptor. Several studies have demonstrated TBBPA induces immune toxicity and cytotoxicity and interferes with the functioning of the endocrine system (Pullen et al. 2003; Hamers et al. 2006; Van der Ven et al. 2008). TBBPA has been shown to be highly toxic to aquatic animals and mammals, including humans (Ronisz et al. 2004; Shi et al. 2005). It can accumulate in the human body through the food chain and impair brain function and bone development, even causing cancer (Reistad et al. 2005).
Previous studies reported effects of TBBPA on plant growth. Li et al. (2008) and Dogan et al. (2010) demonstrated TBBPA inhibited seed germination and induced oxidative stress in wheat and chickpea. However, the mechanisms of damage of TBBPA on plants are not well understood. Therefore, we determined the damage of TBBPA on soybean seedlings and analysed the mechanisms by measuring the growth index, chlorophyll content, soluble sugar and soluble protein content, as well as antioxidant enzyme activities and malondialdehyde (MDA) content in soybean seedlings under TBBPA stress. Our study will provide a theoretical basis for understanding TBBPA toxicity mechanisms to plants as well as means to protect plants from damage caused by TBBPA in the future.
Materials and Methods
TBBPA was purchased from the Aladdin Chemistry Co. Ltd in Fengxian district, Shanghai, China. Soybean cultivar Zhoudou22 was provided by Zhoukou City Academy of Agricultural Sciences.
Soybean seeds were soaked in 70% ethanol for 1 to 2 min followed by treatment with 0.05% sodium hypochlorite for 10 min, then rinsed with sterile water. Seed germination was induced on moist filter paper placed in a petri dish kept in an incubator at 25°C without light for 72 h. After that, air-dried soil was placed into pots of 20 cm height and 25 cm diameter. TBBPA solution was mixed with soil, and the final concentrations of TBBPA reached 0, 5, 10, 20, 40, 80, and 100 mg kg−1 dry soil weight (DW), respectively. A total of 20 seeds were sowed in each culture pot and covered. Each treatment had three replicates, and each replicate consisted of 10 pots. Soybean seedlings without TBBPA treatment served as controls. Then, soybean seedlings were grown in a climate chamber with temperature 30°C/25°C over day/night 12 h /day light regime and air humidity between 60% and 70%. Fourteen days after TBBPA treatment, shoot height, stem diameter, and fresh and dry weight of soybean seedlings were measured for every TBBPA treatment according to the procedure described in Lutts et al. (1996). Soybean seedlings were dried at 110°C in a hot-air oven for 2 days.
After 7 and 14 days of TBBPA treatment, soil samples were collected and ground to pass through a 2.0 mm sieve before use. Soil (10 g) was soxhlet extracted for 48 h with 250 mL of 4:1 hexane-dichloromethane. The extract solution was concentrated into 1 mL using a rotary evaporator and then passed through a C18 solid extraction column (1.5 mL, 100 mg, Alletch Corporation, USA). The extract solution was then eluted with 6 mL of methanol. The elute was concentrated with clean N2 flow at 28°C, then reconstituted to 1 mL with methanol, and stored at 4°C prior to analysis. Concentration of TBBPA was determined using high performance liquid chromatography (Shimadzu LC-20AT) equipped with an elite hypersil BDS C18 column (25 µm, 4.6 × 250 mm) for separation at 40°C and a diode array detector (SPD-M20A, Japan) for measurement at 290 nm. The mobile phase was methanol: acetic acid (80:20) at a flow rate of 1 mL min−1. Quality control was measured following the method described by Li et al. (2011). Quantitative analysis was conducted on a five-point linear calibration of TBBPA solution obtained by dilution of the certified standard of TBBPA. Soil (10 g) having no TBBPA was spiked by adding various concentrations of TBBPA standard. Samples were analyzed according to the procedure described above. Blank samples were processed together with samples, and limits of detection (LODs) were estimated using a threefold signal-to-noise ratio. A 10-fold signal-to noise ratio served as the limit of quantification (LOQs).
Physiological and biochemical parameters were determined at the seedling stage (7 and 14 days after TBBPA treatment) using leaves at the same position on the soybean stem. Superoxide dismutase (SOD) activity was assayed using the Nitro blue tetrazolium chloride test according to the method described in Wu and Von Tiedemann (2002). Catalase (CAT) activity was estimated using the UV absorption method following the method described in Wu and Von Tiedemann (2002). Peroxidase (POD) activity was also analyzed using the guaiacol assay according to Wu and Von Tiedemann (2002). Chlorophyll content was estimated by the spectrophotometry method according to Hegedüs et al. (2001). Malondialdehyde (MDA) content was detected using the thiobarbituric acid assay (TBA assay) according to Hegedüs et al. (2001). Soluble sugar content was measured using the anthrone assay as described in Zarco-Tejada et al. (2005). Soluble protein content was analyzed by the Coomassie brilliant method according to Bradford (1976).
Mean values of all parameters were calculated from the three replicates. Microsoft Excel 2003 was used to calculate the mean values and standard deviations. Analysis of variance (ANOVA) from the statistical software SPSS16.0 was used to test differences among groups with factor of TBBPA concentration.
Results and Discussion
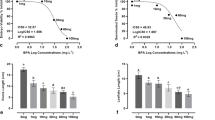
Recovery of TBBPA was 96.87% –99.71%. The relative standard deviations (RSD) were 0.16%–1%. LODs were 0.01 ng/g. LOQs were 0.05 ng/g. Satisfactory linearity was obtained with a correlation coefficient R = 0.9987. Residual concentrations of TBBPA in different treatments on days 0, 7, 14 during the exposure period are shown in Fig. 1. After 14 days of stress, TBBPA concentrations in different treatments decreased 3.68%, 4.40%, 6.52%, 8.16%, 5.66%, and 9.26% in the 5, 10, 20, 40, 80 and 100 mg kg−1 treatment, respectively, compared to that of 0 day. This suggested TBBPA concentrations were stable during the treatment period.
Dogan et al. (2010) reported TBBPA significantly inhibited seed germination and root length of chickpea at a concentration of 100 mg L−1. However, Sverdrup et al. (2006) demonstrated 1–1000 mg kg−1 TBBPA treatment had no significant effects on seed germination and growth of red clover. Current studies showed 5–100 mg kg−1 TBBPA significantly inhibited the growth of soybean seedlings (Table 1) (p < 0.05). Inhibition effects were enhanced with the increase of TBBPA concentration. TBBPA treatment of 100 mg kg−1 produced the strongest inhibitory effect on the seedling growth, as shoot height, stem diameter as well as fresh and dry weight decreased by 36.07%, 28.57%, 49.06% and 58.82%, respectively, compared to control plants (p < 0.05).
Chlorophyll, the main pigment involved in photosynthesis, is used as a key indicator of damage to plant growth and development caused by soil organics and metals (Wang and Zhou 2006). Various stresses often result in chlorophyll content decreasing. This could be attributed to the inhibition of chlorophyll biosynthesis, disturbance of light-harvesting complex formation, and photooxidation breakdown of free chlorophyll (Horváth et al. 1996). Li et al. (2008) demonstrated that 50–5000 mg kg−1 TBBPA significantly decreased chlorophyll content of wheat seedlings, while below 50 mg kg−1, it’s inhibition effect was not significant. Current studies showed 5–100 mg kg−1 TBBPA significantly decreased chlorophyll content of soybean seedlings (Fig. 2), suggesting that much lower TBBPA concentrations brought damage on soybean seedlings. The current study demonstrated 5–100 mg kg−1 TBBPA stress significantly decreased the chlorophyll content of soybean seedlings after 7 d and 14 d of stress compared to control plants (p < 0.05) (Fig. 2). Chlorophyll content reached the lowest level at 100 mg kg−1 TBBPA after 7 and 14 days of exposure (p < 0.05).
Under adverse environmental conditions, plants accumulate soluble sugar to adjust the osmotic potential and to maintain water needed for keeping normal growth (Gill et al. 2003). Therefore, soluble sugar content can be considered an indicator of plant stress resistance. Cha-um et al. (2009) reported that indica rice accumulated soluble sugars to cope with salt stress. In addition, Mishra et al. (2014) thought that Ashwagandha (Withania somnifera Dunal) accumulated soluble sugars to maintain osmotic regulation, protect cell membranes from dissociating, improve the water retention ability of plant cells and stabilize cell structure under cadmium stress. However, Roychoudhury et al. (2012) reported high concentrations of toxic substances inhibited generation of soluble sugars and impaired the stress resistance of plants. Current results found soluble sugar content of soybean seedlings increased at low concentrations with improving TBBPA. However, soluble sugar content decreased when further increasing TBBPA concentration (Fig. 3a). After 7 days of stress, 5 to 80 mg kg−1 TBBPA significantly increased the soluble sugar content of soybean seedlings (p < 0.05). Following an increase in TBBPA concentration, soluble sugar content decreased and reached the lowest level at TBBPA concentration of 100 mg kg−1. Soluble sugar content of soybean seedlings treated with 5 mg kg−1 TBBPA was significantly higher (26.39%) than that of control after 14 days of TBBPA stress (p < 0.05). When treated with 20–100 mg kg−1 TBBPA, soluble sugar content tended to be lower compared to the control.
Changes in soluble sugar content (a) and soluble protein content (b) in soybean leaves under different concentrations TBBPA stress after 7 and 14 days of TBBPA treatment, respectively. Different lowercase letters indicate significant differences in soluble sugar and soluble protein content among TBBPA concentrations at the same treatment time at p < 0.05 level
Soluble proteins can improve plant tolerance to environmental stress by inducing antioxidant enzymes, detoxification enzymes, metabolic enzymes, and several function factors eliminating free radicals in plants (Naeem et al. 2011). Therefore, the change of soluble proteins content can reflect the total metabolism level and the extent of plant damage under both abiotic and biotic stress. In this study, soluble protein content of soybean seedlings first showed an increase followed by a decrease with the rising of TBBPA concentration (Fig. 3b), which is consistent with the findings of Shu et al. (2012) who found soluble protein content first ascended and then declined in Jatropha curcas under Pb stress. Soluble protein content of soybean seedlings under 5–40 mg kg−1TBBPA was higher than that in the control plants after 7 days of stress (p < 0.05), whereas 100 mg kg−1 TBBPA inhibited production of soluble proteins. TBBPA stress (5–10 mg kg−1) for 14 days significantly increased soluble protein content compared to that in control plants (p < 0.05). With the increase of TBBPA concentration, soluble protein content significantly decreased (p < 0.05) and reached the lowest level at 100 mg kg−1 TBBPA. Current results suggest higher concentrations of TBBPA inhibited generation of soluble proteins, aggravated the damage on soybean seedlings, and impaired stress resistance of plants.
Antioxidant enzymes, such as SOD, POD and CAT, play a vital role in scavenging active oxygen free radicals induced by pollutants to protect plant cells from damage (Sun and Zhou 2008). SOD catalyzes superoxide radicals into H2O2, while CAT and POD reduce H2O2 to H2O. Under stress conditions, excessive reactive oxygen species (ROS) accumulate and then damage the plant cell. In order to keep the balance of ROS in plants, antioxidant enzyme activities, such as SOD, CAT, and POD, increase to scavenge excessive ROS and improve stress resistance in plants (Cui et al. 2015). However, when stress is higher than the tolerance threshold of the plant, ROS cannot be eliminated, leading to the decrease in SOD, POD and CAT activities, further inhibiting plant growth (Li et al. 2008). Current results showed SOD, CAT and POD activities of soybean seedlings increased with the increase of TBBPA concentration, followed by a decrease after 7 and 14 days of TBBPA stress (Fig. 4). SOD and CAT activities of soybean seedlings after 7 and 14 days of 5 mg kg−1 TBBPA stress reached the maximum level, while the highest activity of POD appeared under 40 mg kg−1 TBBPA stress (p < 0.05). However, SOD, CAT and POD activities declined following a further increase in TBBPA concentration. These results suggested higher concentrations of TBBPA inhibited the antioxidant enzyme activity, and therefore, the damage of soybean seedlings caused by TBBPA was aggravated.
Changes of the SOD (a), CAT (b), POD (c) activities and MDA content (d) in soybean leaves after 7 and 14 days of TBBPA treatment. Different lowercase letters indicate significant differences in SOD; POD, CAT activities and MDA content among TBBPA concentrations at the same treatment time at p < 0.05 level
After 7 d of TBBPA stress, SOD activity of soybean seedlings treated with 5 to 80 mg kg−1 TBBPA was significantly higher than that in the control (p < 0.05) (Fig. 4a). However, SOD activity declined following a further increase in TBBPA concentration. Seedlings treated with 100 mg kg−1 TBBPA showed a significant decrease in SOD activity compared to the control (p < 0.05). After 14 days of treatment, SOD activity of soybean seedlings treated with 5, 10, and 40 mg kg−1 TBBPA was significantly higher than the control (p < 0.05). When treated with 100 mg kg−1 TBBPA, SOD activity of soybean seedlings reached the lowest level (p < 0.05).
After 7 days of 5 to 20 mg kg−1 TBBPA stress, CAT activity of soybean seedlings increased by 40.87%, 35.35%, and 26.92%, respectively, compared to the control (p < 0.05) (Fig. 4b). After that, CAT activity of soybean seedling decreased with the increase of TBBPA concentration by 18.86% and 31.07% under 80 and 100 mg kg−1 TBBPA stress, respectively (p < 0.05). However, CAT activity in soybean leaves treated with 10 to 20 mg kg−1 TBBPA was not significantly different from that of the control after 14 days of treatment (p > 0.05). With the increase of TBBPA concentration, CAT activity in soybean leaves treated with 40 to 100 mg kg−1 TBBPA tended to be lower than the control (p < 0.05).
After 7 days of stress, the POD activity of soybean seedling increased by 36.0%, 97.47%, 124.0% and 128.0% at TBBPA concentration of 5, 10, 20 and 40 mg kg−1 than that in the control (p < 0.05) (Fig. 4c). However, with the increase of TBBPA concentration, POD activity decreased by 7.99% and reached the lowest level at the concentration of 100 mg kg−1. Changes in POD activity of soybean seedlings after 14 days of TBBPA stress were consistent with that after 7 days of TBBPA exposure.
MDA is an important product of membrane lipid peroxidation in plants under stress conditions and is often used to assess plant cell membrane lipid peroxidation (Bailly et al. 1996). Compared to the control, soybean seedlings treated with 5, 10, 20, 40, 80 and 100 mg kg−1 TBBPA for 7 days showed a 95.88%, 119.46%, 149.71%, 178.43%, 181.50%, and 222.52% increase (p < 0.05), respectively, in the MDA content (Fig. 4d). Changes in the MDA content of soybean seedlings after 14 days of TBBPA exposure were consistent with that after 7 days of TBBPA exposure, suggesting that TBBPA caused lipid peroxidation in soybean seedlings, and lipid peroxidation continued with increasing TBBPA concentration. This is supported by the results of Dogan et al. (2010) and Li et al. (2008) who found that TBBPA stress increased MDA content of wheat and chickpea seedling, respectively.
The present study demonstrated TBBPA at concentrations 5 to 100 mg kg−1 inhibited the growth of soybean seedlings, reduced the chlorophyll content and induced production of malondialdehyde (MDA) in soybean leaves. TBBPA treatment at low concentrations enhanced soluble sugar and soluble protein content and activated SOD, CAT, and POD; however, high concentrations of TBBPA inhibited activities of antioxidant enzymes and generation of soluble sugar and soluble protein. Though there are reports about damage caused by TBBPA on plants, mechanistic research of TBBPA effects are few. Current results provide a theoretical basis to understand TBBPA toxicity mechanisms to plants and further protect them from damage caused by TBBPA in the future. However, owing to the complexity of the underlying mechanisms, future studies are needed to investigate potential interactions.
References
Abdallah MAE, Harrad S, Covaci A (2008) Hexabromocyclododecanes and tetrabromobisphenol A in indoor air and dust in Birmingham, UK: implications for human exposure. Environ Sci Technol 42:6855–6861
Alaee M, Arias P, Sjödin A, Bergman A (2003) An overview of commercially used brominated flame retardants their applications, their use patterns in different countries/regions and possible modes of release. Environ Int 29:683–689
Ali N, Harrad S, Goosey E, Neels H, Covaci A (2011) “Novel” brominated flame retardants in Belgian and UK indoor dust: implications for human exposure. Chemosphere 83:1360–1365
Bailly C, Benamar A, Corbineau F, Come D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plantarum 97:104–110
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cha-um S, Charoenpanich S, Roytrakul S, Kirdmanee C (2009) Sugar accumulation, photosynthesis and growth of two indica rice varieties in response to salt stress. Acta. Physiol Plant 31:477–486
Covaci A, Voorspoels S, Abdallah MA, Geens T, Harrad S, Law RJ (2009) Analytical and environmental aspects of the flame retardant tetrabromobisphenol A and its derivatives. J Chromatogr A 1216:346–363
Cui MG, Lin YC, Zu YG, Efferth T, Li DW, Tang ZH (2015) Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J Plant Biol 58:193–201
Dogan M, Yumrutas O, Saygideger SD, Korkunc M, Gulnaz O, Sokmen A (2010) Effects of bisphenol A and tetrabromobisphenol A on chickpea roots in germination stage. American-Eurasian J Agric Environ Sci 9:186–192
Eriksson J, Rahm S, Green N, Bergman A, Jakobsson E (2004) Photochemical transformations of tetrabromobisphenol A and related phenols in water. Chemosphere 54:117–126
Gill PK, Sharma AD, Singh P, Bhullar SS (2003) Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regul 40:157–162
Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MHA, Andersson PL, Legler J, Brouwer A (2006) In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci 92:157–173
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Horváth G, Droppa M, Oravecz Á, Raskin VL, Marder JB (1996) Formation of the photosynthetic apparatus during greening of cadmium-poisoned barley leaves. Planta 199:238–243
Li Y, Zhou Q, Li F, Liu X, Luo Y (2008) Effects of tetrabromobisphenol A as an emerging pollutant on wheat (Triticum aestivum) at biochemical levels. Chemosphere 74:119–124
Li Y, Zhou Q, Wang Y, Xie X (2011) Fate of tetrabromobisphenol A and hexabromocyclododecane brominated flame retardants in soil and uptake by plants. Chemosphere 82:204–209
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot (Lond) 78:389–398
Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS (2014) Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 251:1031–1045
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011) 5-Aminolevulinic acid ameliorates salinity-induced metabolic, water-related and biochemical changes in Brassica napus L. Acta. Physiol Plant 33:517–528
Pullen S, Boecker R, Tiegs G (2003) The flame retardants tetrabromobisphenol A and tetrabromobisphenol A-bisallylether suppress the induction of interleukin-2–receptor alpha chain (CD25) in murine splenocytes. Toxicology 184:11–22
Reistad T, Mariussen E, Fonnum F (2005) The effect of a brominated flame retardant, tetrabromobisphenol A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci 83:89–100
Ronisz D, Finne EF, Karlsson H, Förlin L (2004) Effects of the brominated flame retardants hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA) on hepatic enzymes and other biomarkers in juvenile rainbow trout and feral eelpout. Aquat Toxicol 69:229–245
Roosens L, Dirtu AC, Goemans G, Belpaire C, Gheorghe A, Neels H, Blust R, Covaci A (2008) Brominated flame retardants and polychlorinated biphenyls in fish from the river Scheldt, Belgium. Environ Int 34:976–983
Roychoudhury A, Basu S, Sengupta DN (2012) Antioxidant and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta. Physiol Plant 34:835–847
Shi H, Wang X, Luo Y, Su Y (2005) Electron paramagnetic resonance evidence of hydroxyl radical generation and oxidative damage induced by tetrabromobisphenol A in Carassius auratus. Aquat Toxicol 74:365–371
Shu X, Yin LY, Zhang QF, Wang WB (2012) Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ Sci Pollut Res 19:893–902
Sun FH, Zhou QX (2008) Oxidative stress biomarkers of the polychaete Nereis diversicolor exposed to cadmium and petroleum hydrocarbons. Ecotoxicol Environ Saf 70:106–114
Sverdrup LE, Hartnik T, Mariussen E, Jensen J (2006) Toxicity of three halogenated flame retardants to nitrifying bacteria, red clover (Trifolium pratense), and a soil invertebrate (Enchytraeus crypticus). Chemosphere 64:96–103
Van der Ven LT, Van de Kuil T, Verhoef A, Verwer CM, Lilienthal H, Leonards PE, Schauer UM, Cantón RF, Litens S (2008) Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology 245:76–89
Wang ME, Zhou QX (2006) Joint stress of chlorimuronethyl and cadmium on wheat Triticum aestivum at biochemical levels. Environ Pollut 144:572–580
Wu YX, Von Tiedemann A (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116:37–47
Zarco-Tejada PJ, Berjón A, López-Lozano R, Miller JR, Martín P, Cachorro V, González MR, de Frutos A (2005) Assessing vineyard condition with hyperspectral indices: leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens Environ 99:271–287
Acknowledgements
This study was supported by the Science and Technology Planning Project of Science and Technology Commission of Henan Province, China (Grant No. 162102310588), by the Middle-aged Backbone Teacher Foundation of Universities and Colleges in Hena of China (2013GGJS-176) and by the School-based Program of Zhoukou Normal University (Grant number ZKNUB215210).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, H., Zhang, F. Effects of Tetrabromobisphenol A Stress on Growth and Physiological Characteristics of Soybean Seedling. Bull Environ Contam Toxicol 98, 141–146 (2017). https://doi.org/10.1007/s00128-016-1974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1974-5