Abstract
The inhibitory effect of plants on algae offers a new and promising alternative method for controlling harmful algal blooms. Previous studies showed that anti-algal effects might be obvious from extracts of fallen leaves from terrestrial plants, which had great potential for cyanobacterial control in field tests. To investigate the anti-algal activities and main algicidal mechanisms of Ginkgo biloba fallen leaves extracts (GBE) on Microcystis flos-aquae, the cell density, photosynthetic fluorescence, and gene expression under different concentrations of GBE treatments were tested. GBE (3.00 g L−1) showed a strong inhibitory effect against M. flos-aquae with an IC50 (96h) of 0.79 g L−1. All the inhibition rates of maximal quantum yield (Fv/Fm), effective quantum yield (Fq’/Fm’), and maximal relative electron transfer rate (rETRmax) were more than 70% at 96 h at 3.00 g L−1 and more than 90% at 6.00 g L−1. Further results of gene expression of the core proteins of PSII (psbD), limiting enzyme in carbon assimilation (rbcL), and phycobilisome degradation protein (nblA) were downregulated after exposure. These findings emphasized that photosynthetic damage is one of the main toxic mechanisms of GBE on M. flos-aquae. When exposed to 12.00 g L−1 GBE, no significant influence on the death rate of zebrafish or photosynthetic activity of the three submerged plants was found. Therefore, appropriate use of GBE could control the expansion of M. flos-aquae colonies without potential risks to the ecological safety of aquatic environments, which means that GBE could actually be used to regulate cyanobacterial blooms in natural waters.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication, one of the key triggers for cyanobacterial bloom occurrences, has been identified as a major water quality management issue worldwide (Lürling et al. 2016). Cyanobacteria blooms are recognized as the greatest threat to water quality by representing the odor and malodorous metabolites associated with bloom-forming cyanobacteria (Brooks et al. 2016; Bukowska et al. 2017). Therefore, controlling cyanobacterial blooms is a crucial step for the safety of water supplies, other water users, and aquatic organisms.
Among the diverse physical (Rajasekhar et al. 2012; Visser et al. 2016), chemical (Crafton et al. 2018; Sinha et al. 2018), and biological methods (Li et al. 2016; Hua et al. 2018) explored for cyanobacterial bloom management, natural plant agents have been proven to be effective, degraded in nature, and environmentally friendly (Yakefu et al. 2018; Patino et al. 2018). Numerous plants have been reported to exhibit algal inhibition effects (Yi et al. 2012; Tazart et al. 2018; Qian et al. 2019; Xu et al. 2020). Our previous study also demonstrated that biologically derived substances that can significantly inhibit the growth of algae are common in different kinds of plants, including woody plants, herbaceous plants, and aquatic plants (Shi et al. 2020).
It is necessary to understand the inhibition mechanisms by obtaining more experimental evidence for better development of this novel method. To date, many researchers have observed that biologically derived substances can interfere with Chl-a synthesis in algae by blocking the photosystem II (PSII) electron transport chain (Huang et al. 2015; Zhao et al. 2019; Xu et al. 2020). For example, pyrogallic acid which can be extracted from various plants, such as Myriophyllum spicatum (Zhang et al. 2010), can damage the oxidative and photosynthetic systems of cyanobacteria (Wu et al. 2013). Measuring gene expression is also been proved as one of the useful tools to investigate the response of algae to some stress (Shao et al. 2013; Lu et al. 2014; Wu et al. 2018). However, to the best of our knowledge, no research has provided a comprehensive evaluation of the potential mechanism in photosynthesis and gene expression of Ginkgo biloba extract (GBE) on Microcystis flos-aquae.
Ginkgo biloba is one of the oldest living tree species, and extracts from G. biloba leaves have been widely used as herbal supplements (Zhou et al. 2004). For example, studies have shown growth-inhibitory activity on the weed species ryegrass (Kato-Noguchi and Takeshita 2013) and seeding (Song et al. 2002) through adding G. biloba extracts. Zheng et al. (2015) reported that the major bioactivities of G. biloba leaves should be flavonoids, which can interrupt electron transport in the PSII reaction center and decrease the effective quantum yield, resulting in impairment of photosynthesis (Huang et al. 2015). These observations suggest that biologically derived substances in G. biloba could serve as the basis for the development of relatively effective tools for algal bloom control. However, to the best of our knowledge, the effects of G. biloba fallen leaves extract on cyanobacteria have not yet been elucidated.
Based on the above description, we proposed several hypotheses: (1) GBE can effectively inhibit the growth of M. flos-aquae; (2) the potential inhibition mechanism is that GBE affects photosynthetic system, causing M. flos-aquae damage and death; and (3) the biologically derived substances of GBE may be eco-friendly. To test these hypotheses, growth was monitored when M. flos-aquae coexisted with different doses of GBE. In addition, photosynthetic fluorescence parameters and gene expression profiles (photosynthesis-related genes of psbD, rbcL, and nblA) were derived to analyze the damage mechanisms of the photosystem. The toxicity of GBE to other species in the aquatic ecosystem was identified by a toxicity test involving zebrafish and several submerged plants as the target organisms. This work not only provided some insights into employing GBE to suppress the growth and photosynthetic system of M. flos-aquae, but also supported that using GBE to control toxic cyanobacterial blooms may be a promising approach in the field. Besides, in our opinion, using G. biloba fallen leaves extract as an algaecide also provide an alternative and promising way to reuse plant litter.
Materials and methods
Algal culture and preparation of GBE
The cyanobacteria species M. flos-aquae were obtained from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China). The algae were cultured at (25 ± 1) °C and a photoperiod of 12 L:12 D with 60 μmol photons m−2 s−1 in the laboratory. Culture flasks were gently swirled twice daily. Stock cultures in the exponential growth phase were used in the following experiments.
The fallen leaves of G. biloba were collected from Shanghai Ocean University in November 2019. Leaves were processed for extracts according to Shi et al. (2020) with minor modifications. Briefly, the leaves were rinsed with tap water, oven-dried at 60 °C for 48 h, and then ground into powder (approximately 50 mesh). Thirty grams dried powder and DI water were mixed at a mass/volume ration of 1/20. Then, the mixture was extracted by ultrasonication for 4 h. The solution was passed through 0.45 μm filter membrane and diluted with distilled water till 2 L to obtain a final extract concentration of 15 g L−1. The extracts were stored in the dark at 4 °C before experimentation.
Assessment of the antialgal activities of GBE
Experimental cultures were conducted in 250 mL glass flasks. M. flos-aquae (1.63 × 106 cells mL−1) cultures were treated with additions of the extract at a concentration gradient of 0.00, 0.75, 1.50, 3.00, 6.00, and 12.00 g L−1. All experiments were carried out in triplicate and conducted for 96 h. The experimental conditions were the same as mentioned in the “Algal culture and preparation of GBE” section. A volume of 0.10 mL samples of M. flos-aquae were taken every 24 h from the flasks and counted immediately in a phytoplankton counter frame (CC-F, Beijing Purity Instrument Co., Ltd., China) with an optical microscope (Nikon, Y-TV55, Japan).
Photosynthetic fluorescence parameters of M. flos-aquae were measured every 24 h by a pulse amplitude-modulated (PAM) fluorescence monitoring system (Phyto-PAM, Walz, Effeltrich, Germany) under dark adaptation for 5 min (Lin et al. 2015; Zhao et al. 2015). The details of the method refer to Schreiber (1998) with minor modifications, and data analysis was performed in PhytoWin v2.13.
RNA extraction, reverse transcription, and real-time PCR analysis
The culture of M. flos-aquae in an amount of 150 mL was centrifuged at 8000 rpm for 5 min at 4 °C to collect algal cells and stored at −80 °C for total RNA isolation. Total RNA was extracted using a Cell/Bacteria RNA Extraction Kit (Tiange, China), treated with RNase-free DNase (Tiange, China), and then reverse transcribed into first-strand cDNA using a FastKing RT Kit (with gDNase) (Tiange, China) according to the manufacturer’s directions. Quantitative real-time PCR (RT-qPCR) was applied to determine the transcriptional level of three genes (psbD, rbcL, nblA) in M. flos-aquae treated by the extraction of G. biloba for 24 h, 48 h, and 96 h. The samples were performed in an FTC-3000 real-time PCR system (Funglyn Biotech, Canada) with a SYBR Green RT-PCR Kit (Tiangen, China) in a final 20 μL volume. The 16S rRNA gene of M. flos-aquae served as the reference gene, and primers used for RT-qPCR were designed (Table 1). The amplification reaction was performed under the following conditions: after heating at 95 °C for 2 min, amplification was programmed for 40 cycles of 15 s at 95 °C, 30 s at 55 °C, and 15 s at 72 °C. Gene expression data were evaluated using the Ct value, and the reference gene was used to normalize the expression levels of target genes (Shao et al. 2009). The experiment was carried out in triplicate, and the average was reported. The relative gene transcription was calculated using the 2-ΔΔCt method (Wu et al. 2018), where ΔΔCt was calculated using the following equation.
Ecological safety experiment
Zebrafish (Danio rerio) and submerged plants (Vallisneria natans, Elodea nuttallii, and Myriophyllum verticillatum) were selected as the nontarget test organisms and exposed to different concentrations of GBE (0.00, 3.00, 6.00, and 12.00 g L−1). Fifteen zebrafish (the average weight of the fish was 0.18 ± 0.02 g) and three selected submerged plants (the average fresh weight of the plant was 6.00 ± 0.50 g) were placed into each aquarium (capacity of 25 L). Triplicate treatments were cultivated under the same conditions. The total number of dead zebrafish was recorded every 24 h for the mortality rate, and the effect of GBE on submerged plants was measured by analyzing photosynthetic fluorescence parameters at 96 h. The method refers to Roháček (2010) with minor modifications. Three selected plants were adapted to the dark for 5 min, and the quantum yields of PSII were obtained by the steady state of slow kinetics mode under the condition of fluorescence and P700 mode (Zhao et al. 2015) in which the actinic light was 300 μmol m−2 s−1.
Data statistic and analysis
When algal growth was inhibited, the IC50 at the 95% confidence interval with an upper confidence limit and a lower confidence limit was calculated using probit analysis. The data are presented as the mean ± SD of triplicates, which were normally distributed according to the Shapiro-Wilk test, and homogeneity of variances was tested by Levene’s test. One-way ANOVA followed by least significant difference (LSD) was applied to test the difference between the control and treatment groups at a significance level of P < 0.05. Statistical analysis was performed using SPSS 24 (IBM SPSS Software, Chicago, USA), and figures were generated using Origin 8.0 (Origin Lab, USA). The inhibitory effect was estimated by the inhibition rate, which is defined by the following equation: IR (%) =[1-(N0/N)] ×100%, where N0 and N are the cell numbers in the treatment and control cultures, respectively.
Results
Effect of GBE on growth of M. flos-aquae
When no extracts were added into the system, M. flos-aquae density increased from around 1.63 × 106 cells mL−1 to 4.08 × 106 cells mL−1, indicating a rapid growth and reproduction (Fig. 1). However, as can be seen from Fig. 1, the growth and reproduction of M. flos-aquae steadily decreased in the systems when GBE were added. At GBE concentration level greater than 0.75 g L−1, a rapid decline in cell density was found. And the inhibitory increased along with increasing GBE dosages. For example, the inhibition rates of 3.00 g L−1 and 6.00 g L−1 reached 75.51% and 83.47% at 96 h, respectively. Based on the collected experiment data, the IC50,96h value of GBE was found to be approximately 0.79 g L−1. These data suggested that GBE have a strong inhibitory effect on M. flos-aquae.
Effect of GBE on photosynthetic activity of M. flos-aquae
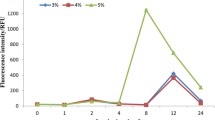
It has been reported that PAM fluorometry can effectively measure PSII activity in algae for better identification of physiological mechanisms (Wang et al., 2016b). Phyto-PAM was used to determine the Fv/Fm, Fq’/Fm’, and rETRmax of photosynthesis. After 24 h of exposure, no marked difference in Fv/Fm was observed between control and treatments with GBE concentration lower than or equal to 3.00 g L−1 (Fig. 2) (P > 0.05). However, for 96 h data, a significant decline in Fv/Fm was observed for the treatments of 1.50 g L−1 and 3.00 g L−1.This result can be attributed to an initial delay in the GBE effects on the photosynthetic system of M. flos-aquae. Effects of GBE on the parameter of Fq’/Fm’ in M. flos-aquae were investigated and depicted in Fig. 3. After 48 h of exposure, a significant reduction in Fq’/Fm’ was denoted at systems with GBE concentration greater than 0.75 g L−1 (P < 0.05). The inhibition rate of 1.50 g L−1 and 3.00 g L−1reached 21.54% and 75.36%, at 48 h, respectively. The value of Fq’/Fm’ also reached almost zero at 6.00 g L−1 and 12.00 g L−1 and maintained till the end of experiment. Similar to the results of Fq’/Fm’, rETRmax values significantly reduced at systems with GBE concentration greater than 0.75 g L−1 after 48 h exposing (P < 0.05). Furthermore, only after 24-h exposure, the value of Fq’/Fm’ decreased to nearly zero in systems with GBE at 6.00 g L−1 and 12.00 g L−1. However, compared with the control, GBE concentration at 0.75 g L−1 had no significant effect on rETRmax during the investigation (Fig. 4). The trend in change of Fv/Fm, Fq’/Fm’, and rETRmax appears to be consistent with the results of cell density. These results suggested that GBE caused substantial damage to photosynthesis in M. flos-aquae, thereby inhibiting cellular growth.
Effect of GBE on gene expression
This study investigated the effects of GBE on several photosynthesis genes that may be responsive to stress. From Fig. 5 a and b, expression of psbD and rbcL was found to be downregulated significantly with the treatment of GBE (P < 0.05). The relative expression levels of psbD were 15.47%, 25.03%, and 17.27% of the control at 24 h, 48 h, and 96 h, respectively. And this result was similar to the change of rbcL expression, which decreased by 87.64%, 90.07%, and 76.26% at 24 h, 48 h, and 96 h, respectively. For nblA expression, no significant change was observed at shorter exposure (24 h) but decreased by 21.12% and 38.18% after 48 h and 96 h exposure (Fig. 5c). The downregulation of photosynthesis genes is a marker of the photoinhibition. The result further proved that GBE reduced the efficiency of photosynthesis in M. flos-aquae.
The normalized expression of psbD (a), rbcL (b), and nblA (c) in M. flos-aquae under G. biloba extract at 3 g L−1. Open bars represent control treatments, and gray bars represent treatments. Asterisks (*) and double asterisks (**) indicate significant (P < 0.05) and extremely significant (P < 0.01) differences between groups, respectively
Effect of GBE on zebrafish and submerged plants
Table 2 showed the effect of GBE on zebrafish and three submerged plants after 96-h exposure. The Fv/Fm values of V. natans, E. nuttallii, and M. verticillatum were between 0.60 and 0.75 in control and treated systems. No insignificant differences were found between the treated groups and the control (P > 0.05). In addition, there were no deaths of zebrafish in any group after 96 h of exposure. These results indicated that the effective algal inhibitory concentration of GBE (3.00–12.00 g L−1) exerted no biotoxicity to zebrafish and submerged plants. Thus, employing GBE as a strategy in controlling algae bloom in aquatic environments would virtually have no adverse effect on other aquatic organisms in the ecosystem.
Discussion
For the past few years, many researches have focused on the algicidal activity of terrestrial plants, including eucalyptus (Zhao et al. 2019), Spartina alterniflora (Xu et al. 2020) and Ailanthus altissima (Meng et al. 2015). For example, the cell multiplication of Microcystis was reported to be markedly inhibited by the extract of Cinnamomum camphora fallen leaves and fresh leaves, with inhibition rates of 43% and 67% after 24 h with 15 g L−1, respectively (Chen et al. 2018; Yakefu et al. 2018). In our studies, the results confirmed that the number of cells of M. flos-aquae was efficiently inhibited by GBE, with a maximum inhibition rate higher than 83% (6.00 g L−1, 96 h). The present study also indicated that the inhibitory effect on M. flos-aquae occurred when exposed to GBE at lower concentrations (< 1 g L−1). However, it is unreasonable to conclude that GBE had more inhibition efficiency to M. flos-aquae than others because of the different extract process and bioassay protocols. In addition, Zhang et al. (2014) demonstrated that ginkgolic acids, which are extracted from G. biloba exocarp, inhibited M. aeruginosa growth effectively with IC 50,3d and IC 50,7d of 3.26 and 2.03 mg L−1, respectively, which is pretty lower than our result, which IC50,96h value was 0.79 g L−1. The huge gap means that perhaps many biologically derived substances in crude plant extract are ineffective to Microcystis. Therefore, it is meaningful to further research finding effective components for the sake of improving inhibition efficiency.
Photosynthetic parameters, such as Fv/Fm, Fq’/Fm’, and rETRmax, are often used as significant indexes to indicate the photoacclimation state of phytoplankton photosynthesis, which is associated with biomass and decreased earlier, meaning that phytoplankton photosynthesis changes more rapidly than cell density (Zhu et al. 2010). Fv/Fm usually is used to estimate the maximum quantum yield of PSII photochemistry (Baker 2008). When electron transfer between electron acceptors and donors in PSII was inhibited, Fv/Fm and the accumulation of carbohydrates decreased, which may slow algal cell growth (Leu et al., 2002 Chen et al. 2018). Fq’/Fm’ is a measure of PSII photosynthetic efficiency when the cells are exposed to ambient light, and the value represents a balance between the amount of light energy being funneled into the PSII reaction centers and the flow of electrons away from PSII (Mackey et al. 2013). As one of the parameters of rapid light curves, rETRmax is a measure of the capacity of the photosystems to utilize the absorbed light energy (Belshe et al. 2007). The depressions of Fq’/Fm’ and rETRmax demonstrated that electron transport was limited and that the efficiency of light capture and utilization declined (Kalaji et al. 2016, Terada et al. 2016). For example, some biologically derived substances such as pyrogallic acid and naphthoquinones interrupt the electron transport, resulting in the reduction in the effective quantum yield and photosynthesis damage (Wu et al. 2013; Hou et al. 2019). Some plant extracts, such as Dracontomelon duperreanum leaf litter extract (Wang et al. 2018) and eucalyptus extract (Zhao et al. 2019), were observed to affect the photosynthetic system and impair the PSII reaction center. In this study, the value of Fv/Fm, Fq’/Fm’, and rETRmax showed similar responses. With the increasing exposure time and doses of GBE, the photosynthetic parameters decreased roughly. Compared with temporary impairment at low concentrations, irreversible damage to photosynthesis was observed only if the dose was above a critical value (Wang et al., 2016a). Our results are generally consistent with these findings. As shown in Figs. 2–4, although the photosynthesis parameters of M. flos-aquae were significantly inhibited at low concentrations (1.50 g L−1and 3.00 g L−1), a full damage was observed at high concentrations (6.00 g L−1 and 12.00 g L−1) with photosynthetic parameters down almost to zero, which indicated the irreversible dysfunction of the PSII reaction center of M. flos-aquae at high extract concentrations.
Analysis of targeting gene sites at the molecular level is also a useful tool for elucidating toxic mechanisms (Qian et al. 2010). Interfering with the intracellular electron transfer rate and reducing the expression of the core proteins were the pathways of plant polyphenols affecting photosynthetic activity (Shao et al. 2009). The results of the present research showed that under GBE exposure, the expression of psbD, rbcL, and nblA was all downregulated, which is in agreement with photosynthetic parameters results. psbD is a gene encoding the D2 core protein subunits of PSII, which can form the reaction center of PSII (Lu et al. 2014; Zhang et al. 2014). The downregulated psbD under GBE may indicate that there is not enough D2 core protein to transfer electrons and replace damaged proteins. This result is in accordance with the phenomenon of M. aeruginosa exposure to ampicillin, atrazine, and cadmium chloride by Qian et al. (2012), who assumed that electron transport-related proteins were not sufficient to transport electrons and led to a decrease in Fv/Fm, Fq’/Fm’, and rETRmax. rbcL encodes the large subunit of Rubisco, a key protein involved in the carbon assimilation process whose downregulation would cause the accumulation of excess electrons and induce oxidative stress, meaning reactive oxygen species (ROS) production (Zhang et al. 2013). Similar phenomena were shown when M. aeruginosa was exposed to neo-przewaquinone A, extracted from Salvia miltiorrhiza Bung (Zhang et al. 2013) and sodium chloride (Chen et al. 2015). Accordingly, the downregulation of psbD and rbcL expression demonstrated that GBE can block electron transport and affect PSII function and carbon assimilation, which are consistent with the results of photosynthetic parameters. The nblA gene encodes the phycobilisome degradation protein, whose upregulation means that phycobiliproteins degrade rapidly, leading to photoinhibition. The degradation of numerous phycobiliproteins results in a decline in the ability to capture and absorb light, thereby inhibiting photosynthetic activity (Lu et al. 2014). The relative copies of nblA were significantly different from the copies of the control group at 96 h, which attest to the decrease in the number of phycobilisome degradation protein, indicating that the ability of algae cells to capture and absorb light increased and the photosynthetic activity was restored to some degree. In addition, many studies have shown that some algaecides exhibit temporary and reversible effects on photosynthesis at low concentrations (Guo et al. 2015; Wang et al. 2018). In this study, the relative copies of nblA decreased gradually; however, photosynthetic parameters still showed a downward trend. One possible reason for this result is that the light repair rate is lower than the damage rate of D2 protein. At the same time, the translation elongation of the D2 protein could be inhibited by singlet oxygen molecules (Nishiyama et al. 2006), which may lead to oxidative damage. To the best of our knowledge, the equilibrium state of intracellular oxidation plays a necessary role in algae growth. The formation of ROS is enhanced by the leakage of electrons from the photosynthetic electron transport chain to oxygen (Zhou et al. 2014). Zhang et al. (2014) demonstrated that the inhibition of photosynthesis would cause an imbalance or even destruction in the antioxidant system by disrupting the electron transport. However, in this study, further research is needed to verify how the GBE caused oxidative damage in M. flos-aquae and the impacts between photosynthesis and ROS levels.
It is important to consider the environmental risks posed by introducing plant extracts into waterbodies. In this study, acute zebrafish and three submerged plants toxicity tests were carried out to ascertain the GBE toxicity, which gained better empirical insights on the validity of using GBE in aquatic ecosystem. As the results showed in Table 2, we did not note a remarkable toxicity of GBE toward nontarget organisms at concentration less than 12.00 g L−1, indicating the safety of GBE in aquatic ecosystems. Several studies also have shown that extracts from plants do not pose a threat to ecosystems (Shao et al. 2018; Wu et al. 2018). Thus, employing GBE as a strategy in controlling algae would virtually have no adverse effect on other aquatic organisms in aquatic ecosystem. Taken together, the GBE could replace other chemical approaches providing an environmentally safe way to control algal blooms.
Conclusions
This work showed that GBE is a promising algaecide that can be used to control harmful algal blooms. The growth of M. flos-aquae was effectively inhibited after the addition of GBE. The results from photosynthetic fluorescence and the expression of photosynthesis genes support the opinion that photosynthesis is one of the potential mechanisms for the inhibitory effect of GBE. The reaction center of PSII and the electron transfer process are important targets for GBE damage. In addition, GBE has been confirmed to be safe and environmentally friendly in aquatic ecosystems. In further researches, more experiments are needed to analyze the biologically derived substances in GBE to improve the inhibition efficiency.
Data availability
The related data generated during this study are included in this published article and are available from the corresponding author on reasonable request.
References
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Belshe EF, Durako MJ, Blum JE (2007) Photosynthetic rapid light curves (RLC) of Thalassia testudinum exhibit diurnal variation. J Exp Mar Biol Ecol 342:253–268. https://doi.org/10.1016/j.jembe.2006.10.056
Brooks BW, Lazorchak JM, Howard MD, Johnson MV, Morton SL, Pwekins DAK, Reavie ED, Scott GI, Smith SA, Steevens JA (2016) Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ Toxicol Chem 35(1):6–13. https://doi.org/10.1002/etc.3220
Bukowska A, Kalinski T, Koper M, Kostrzewska-Szlakowska I, Kwiatowski J, Mazur-Marzec H, Jasser I (2017) Predicting blooms of toxic cyanobacteria in eutrophic lakes with diverse cyanobacterial communities. Sci Rep 7(1):8342. https://doi.org/10.1038/s41598-017-08701-8
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijn P, Fochtman P, Gourmelon A, Hübler N, Kleensang A, Knöbel M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E et al (2014) OCED validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul Toxicol Pharmacol 69(3):496–511. https://doi.org/10.1016/j.yrtph.2014.05.018
Chen GN, Pan LS, Sun Z, Xiong JH, Zhu HX, Wang SF, Song HN, Lin HF, Chen YL, Liang JX (2020) Removal of nitrogen and phosphorus from black-odor water by different submerged plants. J Biobased Mater Bio 14(4):524–530. https://doi.org/10.1166/jbmb.2020.1972
Chen L, Mao FJ, Kirumba GC, Jiang C, Manefield M, He YL (2015) Changes in metabolites, antioxidant system, and gene expression in Microcystis aeruginosa under sodium chloride stress. Ecotox Environ Safe 122:126–135. https://doi.org/10.1016/j.ecoenv.2015.07.011
Chen SL, Zheng TF, Ye CL, Huannixi WL, Yakefu Z, Meng YY, Peng X, Tian ZF, Wang JH, Ma YD, Yang YY, Ma ZQ, Zuo ZJ (2018) Algicidal properties of extracts from Cinnamomum camphora fresh leaves and their main compounds. Ecotox Environ Safe 15(163):594–603. https://doi.org/10.1016/j.ecoenv.2018.07.115
Crafton EA, Glowczewski J, Ott DW, Cutright TJ (2018) In situ field trial to evaluate the efficacy of Cutrine Ultra to manage a cyanobacteria population in a drinking water source. Environ Sci Water Res Technol 4:863–871. https://doi.org/10.1039/c8ew00124c
Guo PY, Liu Y, Liu C (2015) Effects of chitosan, gallic acid, and algicide on the physiological and biochemical properties of Microcystis flos-aquae. Environ Sci Pollut Res 22:13514–13521. https://doi.org/10.1007/s11356-015-4500-0
Hedgpeth BM, Redman AD, Alyea RA, Letinski DJ, Connelly MJ, Butler JD, Zhou HP, Lampi MA (2019) Analysis of sublethal toxicity in developing zebrafish embryos exposed to a range of petroleum substances. Environ Toxicol Chemi 38(6):1302–1312. https://doi.org/10.1002/etc.4428
Hou XY, Huang J, Tang JH, Wang N, Zhang L, Gu L, Sun YF, Yang Z, Huang Y (2019) Allelopathic inhibition of juglone (5-hydroxy-1, 4-naphthoquinone) on the growth and physiological performance in Microcystis aeruginosa. J Environ Manage 232:382–386. https://doi.org/10.1016/j.jenvman.2018.11.105
Hua Q, Liu YG, Yan ZL, Zeng GM, Liu SB, Wang WJ, Tan XF, Deng JQ, Tang X, Wang QP (2018) Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotox Environ Safe 148:953–959. https://doi.org/10.1016/j.ecoenv.2017.11.049
Huang HM, Xiao X, Ghadouani A, Wu JP, Nie ZY, Peng C, Xu XH, Shi JY (2015) Effects of natural flavonoids on photosynthetic activity and cell integrity in Microcystis aeruginosa. Toxins 7:66–80. https://doi.org/10.3390/toxins7010066
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Lukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102. https://doi.org/10.1007/s11738-016-2113-y
Kato-Noguchi H, Takeshita S (2013) Contribution of a phytotoxic compound to the allelopathy of Ginkgo biloba. Plant Signal Behav 8:e26999. https://doi.org/10.4161/psb.26999
Leu E, Krieger-Liszkay A, Goussias C, Gross EM (2002) Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II. Plant Physiol 130(4):2011–2018. https://doi.org/10.1104/pp.011593
Li HA, Ai HN, Kang L, Sun XF, He Q (2016) Simultaneous Microcystis algicidal and microcystin degrading capability by a single Acinetobacter bacterial strain. Environ Sci Technol 50(21):11903–11911. https://doi.org/10.1021/acs.est.6b03986
Lin L, Feng C, Li QY, Wu M, Zhao LY (2015) Effects of electrolysis by low-amperage electric current on the chlorophyll fluorescence characteristics of Microcystis aeruginosa. Environ Sci Pollut Res 22:14932–14939. https://doi.org/10.1007/s11356-015-4708-z
Lu YP, Wang J, Yang Y, Shi LM, Kong FX (2014) Changes in the physiology and gene expression of Microcystis aeruginosa under EGCG stress. Chemosphere 117:164–169. https://doi.org/10.1016/j.chemosphere.2014.06.040
Lürling M, Mackay E, Reitzel K, Spears BM (2016) Editorial – a critical perspective on geo-engineering for eutrophication management in lakes. Water Res 97:1–10. https://doi.org/10.1016/j.watres.2016.03.035
Mackey KRM, Paytan A, Caldeira K, Grossman AR, Moran D, McIlvin M, Saito M (2013) Effect of temperature on photosynthesis and growth in marine Synechococcus spp. Plant Physiol 163:815–829. https://doi.org/10.1104/pp.113.221937
Mahadevan S, Park Y (2008) Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci 74:14–19. https://doi.org/10.1111/j.1750-3841.2007.00597.x
Meng P, Pei H, Hu W (2015) Allelopathic effects of Ailanthus altissima extracts on Microcystis aeruginosa growth, physiological changes and microcystins release. Chemosphere 141:219–226. https://doi.org/10.1016/j.chemosphere.2015.07.057
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757:742–749. https://doi.org/10.1016/j.bbabio.2006.05.013
Patino R, Rashel RH, Rubio A, Longing S (2018) Growth-suppressing and algicidal properties of an extract from Arundo donax, an invasive riparian plant, against prymnesium parvum, an invasive harmful alga. Harmful Algae 71(JAN.):1–9. https://doi.org/10.1016/j.hal.2017.11.005
Qian HF, Li J, Pan XJ, Chen J, Zhou DM, Chen ZG, Zhang L, Fu ZW (2012) Analyses of gene expression and physiological changes in Microcystis aeruginosa reveal the phytotoxicities of three environmental pollutants. Ecotoxicology 3:847–859. https://doi.org/10.1007/s10646-011-0845-4
Qian HF, Yu SQ, Sun ZQ, Xie XC, Liu WP, Fu ZW (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99(3):405–412. https://doi.org/10.1016/j.aquatox.2010.05.018
Qian YP, Li XT, Tian RN (2019) Effects of aqueous extracts from the rhizome of Pontederia cordata on the growth and interspecific competition of two algal species. Ecotoxicol Environ Safe 168:401–407. https://doi.org/10.1016/j.ecoenv.2018.10.086
Rajasekhar P, Fan LH, Nguyen T, Roddick FA (2012) A review of the use of sonication to control cyanobacterial blooms. Water Res 46(14):4319–4329. https://doi.org/10.1016/j.watres.2012.05.054
Roháček K (2010) Method for resolution and quantification of components of the non-photochemical quenching (qN). Photosynth Res 105(2):101–113. https://doi.org/10.1007/s11120-010-9564-6
Sati P, Dhyani P, Bhatt ID, Pandey A (2019) Ginkgo biloba flavonoid glycosides in antimicrobial perspective with reference to extraction method. J Tradit Complem Med 9(1): 15-23. https://doi.org/10.1016/j.jtcme.2017.10.003
Schreiber U (1998) Chlorophyll fluorescence: new instruments for special applications. Photosynthesis: mechanisms and effects:4253–4258. https://doi.org/10.1007/978-94-011-3953-3_984
Shao JH, Liu DM, Gong DX, Zeng QR, Yan ZY, Gu JD (2013) Inhibitory effects of sanguinarine against the cyanobacterium Microcystis aeruginosa NIES-843 and possible mechanisms of action. Aquat Toxicol 142-143:257–263. https://doi.org/10.1016/j.aquatox.2013.08.019
Shao JH, Wu ZX, Yu GL, Peng X, Li RH (2009) Allelopathic mechanism of pyrogallol to Microcystis aeruginosa pcc7806 (cyanobacteria): from views of gene expression and antioxidant system. Chemosphere 75(7):924–928. https://doi.org/10.1016/j.chemosphere.2009.01.021
Shao JH, Yu GL, Wang ZJ, Wu ZX, Peng X, Li RH (2010) Towards clarification of the inhibitory mechanism of wheat bran leachate on Microcystis aeruginosa NIES-843 (cyanobacteria): physiological responses. Ecotoxicology 19:1634–1641. https://doi.org/10.1007/s10646-010-0549-1
Shao L, Li JY, Zhang YJ, Song YY, Yu KF, He PM, Shen AL (2018) Herbicidal effects of Chinese herbal medicine Coptis chinensis Franch extract on duckweed Spirodela polyrhiza (L.) Schleid.). Ecological Engineering 115:9–14. https://doi.org/10.1016/j.ecoleng.2018.02.002
Shi YX, Shen AL, Tan M, He PM, Shao L (2020) The effect of plant extracts on growth and photosynthetic fluorescence characteristics of Microcystis flos-aquae. Water Sci Technol 82(6):1102–1110. https://doi.org/10.2166/wst.2020.312
Sinha AK, Eggleton MA, Lochmann RT (2018) An environmentally friendly approach for mitigating cyanobacterial bloom and their toxins in hypereutrophic ponds: potentiality of a newly developed granular hydrogen peroxide-based compound. Sci Total Environ 637-638:524–537. https://doi.org/10.1016/j.scitotenv.2018.05.023
Song CY, Horiuchi T, Oba S (2002) Effects of dried fine pieces of herb plants on growth of large crabgrass (Digitaria adscendens Henr.). J Weed Sci 47(3):153–160. https://doi.org/10.3719/weed.47.153
Sun RG, Fan L (2019) Purification of eutrophic water by five aqua-cultured plants in lake Hongfeng, Guiyang, China. Wuhan Univ J Nat Sci 24(1):37–44. https://doi.org/10.1007/s11859-019-1366-x
Tazart Z, Douma M, Tebaa L, Loudiki M (2018) Use of macrophytes allelopathy in the biocontrol of harmful Microcystis aeruginosa blooms. Water Sci Technol 19(1):245–253. https://doi.org/10.2166/ws.2018.072
Terada R, Vo TD, Nishihara GN, Matsumoto K, Kokubu S, Watanabe Y, Kawaguchi S (2016) The effect of photosynthetically active radiation and temperature on the photosynthesis of two Vietname sespecies of Sargassum, S. Mcclurei and S. oligocystum, based on the field and laboratory measurements. Phycol Res 64(4):230–240. https://doi.org/10.1111/pre.12143
Visser PM, Ibelings BW, Bormans M, Huisman J (2016) Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol 50(3):423–441. https://doi.org/10.1007/s10452-015-9537-0
Wang SB, Wang YN, Ma XX, Xu ZR (2016a) Effects of garlic and diallyl trisulfide on the growth, photosynthesis, and alkaline phosphatase activity of the toxic cyanobacterium Microcystis aeruginosa. Environ. Sci. Pollut. Res. 23:5712–5720. https://doi.org/10.1007/s11356-015-5809-4
Wang XX, Jiang CC, Szeto YT, Li HK, Yam KL, Wang XJ (2016b) Effects of Dracontomelon duperreanum defoliation extract on Microcystis aeruginosa: physiological and morphological aspects. Environ Sci Pollut Res 23:731–8740 https://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs11356-016-6119-1
Wang XX, Szeto YT, Jiang CC, Wang XJ, Tao Y, Tu JG, Chen J (2018) Effects of Dracontomelon duperreanum leaf litter on the growth and photosynthesis of Microcystis aeruginosa. Bull Environ Contam Toxicol 100:690–694. https://doi.org/10.1007/s00128-018-2289-5
Wang YC, Li ZK, Zhou L, Feng LL, Fan NW, Shen J (2013) Effects of macrophyte-associated nitrogen cycling bacteria on denitrification in the sediments of the eutrophic Gonghu Bay, Taihu Lake. Hydrobiologia 700(1):329–341. https://doi.org/10.1007/s10750-012-1241-7
Wu X, Wu H, Wang SJ, Wang YM, Zhang RF, Hu XB, Ye JY (2018) Effect of propionamide on the growth of Microcystis flos-aquae colonies and the underlying physiological mechanisms. Sci Total Environ 630:526–535. https://doi.org/10.1016/j.scitotenv.2018.02.217
Wu Z, Shi J, Yang S (2013) The effect of pyrogallic acid on growth, oxidative stress, and gene expression in Cylindrospermopsis raciborskii (Cyanobacteria). Ecotoxicol 22:271–278. https://doi.org/10.1007/s10646-012-1023-z
Xu CC, Huang ST, Huang YZ, Effiong K, Yu SM, Hu J, Xiao X (2020) New insights into the harmful algae inhibition by Spartina alterniflora: cellular physiology and metabolism of extracellular secretion. Sci Total Environ 714:1–12. https://doi.org/10.1016/j.scitotenv.2020.136737
Yakefu Z, Huannixi W, Ye C, Zheng T, Chen S, Peng X, Tian ZF, Wang JH, Yang YY, Ma ZQ, Zuo ZJ (2018) Inhibitory effects of extracts from Cinnamomum camphora fallen leaves on algae. Water Sci Technol 77(11):2545–2554. https://doi.org/10.2166/wst.2018.199
Yi YL, Lei Y, Yin YB, Zhang HY, Wang GX (2012) The antialgal activity of 40 medicinal plants against Microcystis aeruginosa. J Appl Phycol 24(4):847–856. https://doi.org/10.1007/s10811-011-9703-2
Yuan R, Li Y, Li JH, Ji SH, Wang S, Kong FL (2020) The allelopathic effects of aqueous extracts from Spartina alterniflora on controlling the Microcystis aeruginosa blooms. Sci Total Environ 712:136332. https://doi.org/10.1016/j.scitotenv.2019.136332
Zhang C, Ling F, Yi YL, Zhang HY, Wang GX (2014) Algicidal activity and potential mechanisms of ginkgolic acids isolated from Ginkgo biloba exocarp on Microcystis aeruginosa. J Appl Phycol 26:323–332. https://doi.org/10.1007/s10811-013-0057-9
Zhang C, Yi YL, Hao K, Liu GL, Wang GX (2013) Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa—towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere 93:997–1004. https://doi.org/10.1016/j.chemosphere.2013.05.068
Zhang TT, Zheng CY, Hu W, Xu WW, Wang HF (2010) The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J Appl Phycol 22(1):71–77. https://doi.org/10.1007/s10811-009-9429-6
Zhao W, Zheng Z, Zhang JL, Roger SF, Luo XZ (2019) Allelopathically inhibitory effects of eucalyptus extracts on the growth of Microcystis aeruginosa. Chemosphere 225:424–433. https://doi.org/10.1016/j.chemosphere.2019.03.070
Zhao Y, Liu WS, Li Q, Yang Q, Chai WB, Zeng MJ, Li RH, Peng YY (2015) Multiparameter-based bioassay of 2-(4-chlorophenyl)-4-(4-methoxyphenyl) quinazoline, a newly-synthesized quinazoline derivative, toward Microcystis aeruginosa HAB5100 (Cyanobacteria). Bull Environ Contam Toxicol 943:376–381. https://doi.org/10.1007/s00128-015-1459-y
Zheng W, Li XM, Zhang L, Zhang YZ, Lu XP, Tian JK (2015) Improved metabolites of pharmaceutical ingredient grade Ginkgo biloba and the correlated proteomics analysis. Proteomics 15(11):1868–1883. https://doi.org/10.1002/pmic.201400258
Zhou Q, Han SQ, Yan SH, Guo JY, Song W, Liu GF (2014) Impacts of Eichhornia crassipes (Mart.) Solms stress on the physiological characteristics, microcystin production and release of Microcystis aeruginosa. Biochem Syst Ecol 55:148–155. https://doi.org/10.1016/j.bse.2014.03.008
Zhou W, Chai H, Lin PH, Lumsden AB, Yao QZ, Chen CY (2004) Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovascular Drug Reviews 22(4):309–319. https://doi.org/10.1111/j.1527-3466.2004.tb00148.x
Zhu JY, Liu BY, Wang J, Gao YN, Wu ZB (2010) Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat Toxicol 98(2):196–203. https://doi.org/10.1016/j.aquatox.2010.02.011
Funding
This work was supported by the Shanghai Science and Technology innovation action plan (19DZ1204500), the National Natural Science Foundation of China (31502172), and the Major Projects of Water Pollution Control and Management of China (2017ZX07205003).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yuxin Shi, Liu Shao, and Peimin He planned and constructed the experimental setup. Material preparation, data collection, and analysis were performed by Yuxin Shi. The first draft of the manuscript was written by Yuxin Shi and Liu Shao. Anglu Shen and Peimin He reviewed and edited the manuscript together. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Vitor Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ginkgo biloba extracts was found to be an efficient botanical algaecide.
• The expression of some key photosynthesis related genes was influenced by GBE
• Photosynthetic damage is one of the main toxic mechanisms of GBE on M. flos-aquae.
• GBE (< 12.00 g L−1) had no significant potential risk to aquatic ecological safety.
Rights and permissions
About this article
Cite this article
Shi, Y., Shen, A., Shao, L. et al. Effects of Ginkgo biloba extract on growth, photosynthesis, and photosynthesis-related gene expression in Microcystis flos-aquae. Environ Sci Pollut Res 29, 87446–87455 (2022). https://doi.org/10.1007/s11356-022-21663-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21663-3