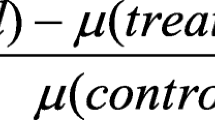Abstract
Sensitivity of four tropical cyanobacteria viz. Coelosphaerium sp., Synechococcus sp., Oscillatoria sp. and Chroococcus sp. to environmentally relevant concentrations of Cr6+, Cd2+ and Zn2+was assessed based on fluorescence change as a proxy for growth reduction. At 24 h exposure, the growth reduction inthe cyanobacteria followed the order: Zn2+ < Cr6+ ≤ Cd2+. Of the four cyanobacteria, Synechococcus was the most sensitive for Cr6+, where as Chroococcus was the most sensitive for Cd2+and Zn2+. Sensitivity was gradually decreased by 96 h implying the acquisition of tolerance by cyanobacteria to heavy metal ions with prolonged exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Heavy metals are continuously released into the biosphere by natural and anthropogenic sources. Chromium, cadmium and zinc are such heavy metals causing pollution of aquatic ecosystems. Chromium is a transition metal existing in oxidation states varying from −2 to +6, and 0, +3 and +6 being the most common oxidation states (Wong and Trevors 1988). Chromium is used in alloys including stainless steel and in chemical industrial processes like leather tanning, pigments and dye, pulp and paper, automobile and electroplating (McGrath and Smith 1990). Being the most abundant, Cr (III) and Cr (VI) attract more attention in terms of their toxicity to aquatic biota. Cadmium usually has an oxidation state of +2. Cadmium occurs as a minor component in most zinc ores and therefore is a byproduct of zinc production. It was used for a long time as a pigment and for corrosion resistant plating on steel. Zinc acts as an essential trace element in development, growth and differentiation of all living systems. Zinc occurs exclusively as the Zn2+ divalent cation and due to its completely filled d orbitals; Zn2+ cannot undergo redox changes under biological conditions (Nies 1999). However, Zn2+ can be toxic to biological systems at high concentrations. The release of these heavy metals into aquatic ecosystems from industrial waste and other sources has been a serious environmental issue because heavy metals are non-degradable tending to accumulate in the water bodies and cause toxicity to aquatic organisms as well as terrestrial organisms via bioaccumulation through food chains.
Cyanobacteria which are prokaryotic photosynthetic microorganisms, found in almost every terrestrial and aquatic habitats (Waterbury 2006). Cyanobacteria are regarded as bioindicators for environmental monitoring and assessment as they are sensitive to a number of pollutants (Campanella et al. 2000). Recently, use of cyanobacteria as biomonitoring agents for the detection of toxicity of Cr6+, Cd2+, and Zn2+ in aquatic environments has been exploited due to their high sensitivity and reproducibility. The most preferred biological process to assess the growth reducing effect of Cr6+, Cd2+, Zn2+ on microalgae is the photosynthetic activity, estimated by chlorophyll a fluorescence of photosystem II (PSII) (Altamirano et al. 2004). During the photosynthesis process a small portion of energy absorbed as sunlight is emitted as fluorescence (Wong et al. 2013). The amount of this fluorescence emission can be changed due to the inhibition of growth in the presence of contaminants like Cr6+, Cd2+, and Zn2+ in the environment or their direct effect on PSII. Therefore fluorescence changes in these organisms can be used as an indicator for the monitoring heavy metals in the aquatic systems (Buonasera et al. 2011). Information on sensitivity of tropical cyanobacteria to environmentally realistic levels of heavy metal ions is meager in the scientific literature. The objective of the present study was to assess the effect of environmentally realistic concentrations of Cr6+, Cd2+, and Zn2+ on the growth of four cyanobacteria isolated from different tropical freshwater environments based on fluorescence change patterns.
Materials and Methods
Four cyanobacteria viz. Coelosphaerium sp., Synechococcus sp., Oscillatoria sp. and Chroococcus sp. used in this study have been isolated from the water samples collected from different freshwater sites in the Gampaha district, Sri Lanka. Coelosphaerium sp. and Synechococcus sp. were isolated from two domestic freshwater ponds whereas sources of Oscillatoria sp. and Chroococcus sp. were from Kelani River and a shallow well respectively. For cyanobacteria isolation, flasks containing BG11 medium were inoculated with respective water samples and incubated at 25 ± 2°C on a bench top orbital shaker (GFL® 3005) at 100 rpm, under continuous illumination (200 µE m−2 s−1 PPFD). After green color cyanobacterial growth was observed, the organisms were isolated and purified into single axenic cultures using the isolation streak method on the BG11 agar medium. Cyanobacteria were identified to generic level according to Bellinger (1992) based on observable morphological characters under the light microscope (Olympus CX21FS1). Axenic cultures of cyanobacterial isolates were maintained in BG11 medium and incubated at 25 ± 2°C in flasks on the bench top orbital shaker (GFL® 3005), under continuous illumination. Cultures were periodically transferred to fresh media. Chromium, cadmium, and zinc content of water samples where the cyanobacteria were isolated, were determined using graphite furnace Atomic Absorption Spectrometer (Analytik Jena novAA® 400P) following the procedure described by APHA (1999). For toxicity assessments, stock solutions of Cr6+, Cd2+ and Zn2+ were prepared in de-ionized water using K2Cr2O7 (≥99 % purity, NORMAPUR, Belgium), Cd(NO3)2·4H2O (≥99 % purity, Sigma-Aldrich, USA), and Zn(NO3)2·6H2O (≥99 % purity, Sigma-Aldrich, USA) respectively and working solutions for Cr6+, Cd2+ (0.8, 0.4, 0.2, 0.1, 0.05, 0.025, and 0.0125 mg/L) and for Zn2+ (16.0, 8.0, 4.0, 2.0, 1.0, 0.5, and 0.25 mg/L) were prepared by appropriate dilutions. The metal levels in the working solutions were analytically verified by atomic absorption spectrometry [Analytik Jena model: novAA 400P atomic absorption spectrometer with a graphite furnace and auto sampler or flame mode (Acetylene/air) where appropriate] following the standard analytical procedures (APHA 1999). Limit of quantification (LOQ) for each analyte was calculated as the metal concentration that correspond to the sum of the mean and ten times the standard deviation of 10 independent measurement of the blank medium (nitric acid). The LOQ for Cr, Cd and Zn were 0.014, 0.001 and 0.1 mg/L respectively. Each sample was analyzed in duplicates. The measured concentrations of metals in the working solutions were for Cr 0.89, 0.42, 0.21, 0.08, 0.044, 0.023 and <0.014 (LOQ) mg/L; for Cd 0.76, 0.41, 0.21, 0.11, 0.045, 0.022 and 0.013 mg/L; for Zn 14, 7, 3.5, 2, 0.75, 0.45, and 0.19 mg/L. In general measured concentrations of the metal did not show much deviations from the nominal concentrations. Metal solutions were sterilized by autoclaving. All glassware was acid washed before use to avoid binding of metal to the glass surface. Sensitivity of the cyanobacteria to Cr6+, Cd2+, and Zn2+ wastested individually under BG11 growth medium using seven nominal concentrations of each metal ion (final concentration for Cr6+, Cd2+:1–66 µg/L and for Zn2+ 20–1330 µg/L).The control media contained only the growth medium (240 μL). The protocol of ‘Algal microplate toxicity test suitable for heavy metals’ (Peterson et al. 2005) was followed in the toxicity assessments. Bioassays were conducted in quadruplicates in 96-well microplates (Sterilin®, flat bottom, sterile, with lid). The cyanobacteria were incubated on an orbital shaker (GFL® 3005) at 100 rpm under continuous illumination using cool white fluorescent lamps (200 µE m−2 s−1 PPFD). Growth patterns of the cyanobacteria were measured as chlorophyll a fluorescence (using 440/40 nm excitation filter and 680/30 nm emission filter) at 24 h intervals from the time of initial inoculation up to 96 h using the BioTek Synergy™ HT Microplate Reader using Gen5 software (OECD 2011). Relative growth of cyanobacteria based on fluorescence was calculated as a percentage in relation to the untreated control for each tested concentration of each metal ion. Effective Concentrations for growth reduction (ECx) x = 50, 20 and 10 (estimated metal ion concentration where the organisms show the relevant % reduction in fluorescence compared to the control) were estimated by Probit analysis (Finney 1971), using MINITAB 15 Statistical Software™. In addition, no observed effect concentration (NOEC) and lowest observed effect concentration (LOEC) for each metal ion were estimated based on Analysis of Variance test followed by Dunnett’spost-hoc test. p < 0.05 was considered as statistically significant.
Results and Discussion
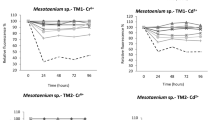
Four cyanobacteria (Coelosphaerium sp., Synechococcus sp., Oscillatoria sp. and Chroococcus sp.) isolated from tropical freshwaters were tested for their sensitivity to environmentally relevant concentrations of three heavy metal ions, Cr6+, Cd2+ and Zn2+ was assessed based on fluorescence change as a proxy for growth reduction. The concentration of Cr was 27 µg/L in the water from which Coelosphaerium sp was isolated whereas the waters from which the other three cyanobacteria were isolated contained only 2–3 µg/L of chromium. The water from which Oscillatoria sp. was isolated had 20 µg/L cadmium in comparison to the other water samples (below 3 µg/L). Zinc levels were relatively low (52 µg/L) in the water from which Coelosphaerium sp. was isolated compared to the waters from which the other cyanobacteria were collected (110–138 µg/L). The range of metal ion concentrations used in this study also covers the metal levels in the natural habitat of the four cyanobacteria. The metal ion concentration–growth response relationships (as % relative fluorescence) for the four cyanobacteria at 24 h exposure are presented in Fig. 1. In all cyanobacteria, relative fluorescence (%) decreased with increasing metal ion concentration in the growth medium at 24 h of exposure. Estimated NOEC and LOEC of the three metal ions for growth reduction of the cyanobacteria at 24 h are presented in Table 1. Of the four cyanobacteria, NOEC and LOEC of Cr6+ are 16 and 33 µg/L for Synechococcus sp., whereas for the other three cyanobacteria corresponding concentrations are 33 and 66 µg/L respectively. The toxicity thresholds estimated by hypothesis testing indicate that Synechococcus sp. is the most sensitive cyanobacteria for Cr6+. Estimated NOEC (16 µg/L) and LOEC (33 µg/L) for Cd2+ are two-fold greater in Coelosphaerium sp. and Synechococcus sp. in comparison to those (NOEC: 8 µg/L and LOEC: 16 µg/L) in Oscillatoria sp. and Chroococcus sp. For Zn2+, estimated NOEC and LOEC for growth reduction of Chroococcus sp. are 166 and 330 µg/L respectively whereas corresponding values for the other three cyanobacteria are two-fold greater (NOEC: 330 and LOEC: 660 µg/L) indicating greater sensitivity of Chroococcus sp. to Zn2+.
Table 2 shows the estimated 24 h effective concentrations (EC50, EC20 and EC10) of metal ions Cr6+, Cd2+ and Zn2+, for the growth reduction of cyanobacteria based on Probit analysis. Of the four cyanobacteria, Synechococcus sp. showed the lowest EC50 for all three metals (Cr6+, Cd2+ and Zn2+). However the differences in EC50s among the four cyanobacteria are not statistically significant as the respective 95 % confidence limits overlap. Moreover except for effects of Cr6+on Synechococcus sp., the concentration-response curves did not exceed 50 % decrease in relative fluorescence levels (Fig. 1) and the EC50 estimates are based on extrapolations of the measured effects at the tested concentrations. Hence, estimated EC50s have to be interpreted with caution.
When considering the 24 h EC20s for Cr6+ (Table 2), Synechococcus sp. exhibited the lowest EC20 value (p < 0.05) indicating the highest sensitivity. Chroococcus sp. showed the lowest EC20 for Cd2+ and Zn2+ butthe differences among four cyanobacteria are not statistically significant. Toxicity threshold estimates for the three metal ions for the cyanobacteria tested in this study based on two methods viz. LOEC based on hypothesis testing and EC10 based on probit analysis correspond well for Zn2+ except for Chroococcus sp. For Cr6+, EC10 estimates are much lower than the LOEC estimates but correspond well with the NOEC estimates for cyanobacteria except in Synechococcus sp. where the LOEC agrees with the EC10 estimate. For Cd2+, EC10 estimates for Coelosphaerium sp. and Synechococcus sp. correspond well with the LOEC values but LOEC estimates for Oscillatoria sp. and Chroococcus sp. are much lower than the respective EC10 estimates.
The growth response of the four cyanobacterial isolates (as relative fluorescence %) decreased with increasing metal ion concentration for 24 h and the growth reduction followed the increasing order, Zn2+ < Cr6+ ≤ Cd2+. Cadmium shows the highest toxicity to the tested cyanobacteria at elevated concentrations. It has been reported earlier that in cyanobacteria, Cd causes severe inhibition of growth, photosynthesis (Zhou et al. 2008) and nitrogen fixation (Singh et al. 2014). Reduction of growth at elevated chromium concentrations may be due to the toxicity of hexavalent chromium to cyanobacteria. Chromium can interfere with the uptake of some essential elements such as Fe and S due to its structural similarity. Once enters the cell, chromium stress can also result in alterations of photosynthetic pigments such as chlorophyll (Pereira et al. 2013). Chromium can also produce reactive oxygen species that cause oxidative damage to cells and cellular mechanisms (Hameed and Hasnein 2014). Hence, changes of chlorophyll fluorescence as observed in this study with Cr6+ and Cd2+ exposure may be due to inhibition of physiological processes in the cells. Although being an essential metal, zinc is not as toxic as chromium or cadmium, at high concentrations zinc also shows toxic effects to the tested cyanobacteria. In a study by Loez et al. (1995), it was shown that even at low concentrations, Zn was deleterious to the algae Euglenophyceae, Cyanophyceae and Xanthophyceae.
After 24 h, it was observed that sensitivity of the cyanobacteria to the Cr6+, Cd2+ and Zn2+ was decreased and growth response of cyanobacterial cultures gradually reached closer to the control levels by the end of 96 h exposure (Fig. 1S: Supplementary figure). This may be due to the development of metal tolerance mechanisms in the cyanobacterial cells with the increase in exposure time. Prokaryotic cells like cyanobacteria employ ATP-consuming efflux of heavy metals or enzymatic change of speciation to achieve detoxification (Nies 1999). Most cyanobacteria have one or more endogenous plasmids responsible for many cellular functions including heavy metal resistance (Lee et al. 2013). Metallothionein (MT) and efflux ATPases also assist in zinc homeostasis and cadmium tolerance (Chauvat and Chauvat 2015). For example Synechococcus PCC7942 have Zn- and Cd- binding MT encoded by smtA gene and various Oscillatoria strains also have genes for both MT and efflux pump (Blindauer 2011).
In conclusion, sensitivity of four cyanobacteria viz. Coelosphaerium sp., Synechococcus sp., Oscillatoria sp. and Chroococcus sp. isolated from tropical freshwaters to environmentally relevant concentrations of Cr6+, Cd2+& Zn2+ based on fluorescence changing patterns followed the increasing order of toxicity, Zn2+ < Cr6+ ≤ Cd2+. Synechococcus sp. was the most sensitive isolate for Cr6+, exhibiting the lowest NOEC/LOEC as well as the lowest 24 h-EC20 of 38 μg/L. With respect to Cd2+ and Zn2+, statistically significant differences were not found in 24 h-EC20s among the four cyanobacteria but estimated 24 h LOECs/NOECs of Cd2+ for growth retardation are lower in Oscillatoria sp and Chroococcus sp. than those of Coelosphaerium sp. and Synechococcus sp. Based on NOEC/LOEC estimates Chroococcus sp. was the most sensitive cyanobacteria for Zn2+. By 96 h exposure, sensitivity to the Cr6+, Cd2+ and Zn2+ was decreased implying the acquisition of tolerance by cyanobacteria to the tested metal ions. Of the four tropical cyanobacteria isolates, Synechococcus sp. is the most sensitive cyanobacteria for Cr6+, and Chroococcus sp seems to be the most sensitive isolate for Cd2+ and Zn2+. The results imply that sensitivity of cyanobacteria to specific heavy metal ions can vary depending on the cyanobacterial strain and generalizations cannot be made as to the most sensitive cyanobacteria for all heavy metal ions. Hence the most sensitive consortium of organisms needs to be selected for monitoring and assessments of heavy metal contamination in tropical aquatic environments.
References
Altamirano M, García-Villada L, Agrelo M, Sánchez-Martín L, Martín-Otero L, Flores-Moya A, Rico M, López-Rodas V, Costas E (2004) A novel approach to improve specificity of algal biosensors using wild-type and resistant mutants: an application to detect TNT. Biosens Bioelectron 19:1319–1323
APHA (1999) Standard methods for the examination of water and wastewater. American Public Health Association, 20th edn. http://www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdf. Accessed 20 Jan 2014
Bellinger EG (1992) A key to common algae, 4th edn. The Institution of Water and Environmental Management, London
Blindauer CA (2011) Bacterial metallothioneins: past, present, and questions for the future. J Biol Inorg Chemo 16(7):1011–1024
Buonasera K, Lambreva M, Rea G, Touloupakis E, Giardi MT (2011) Technological applications of chlorophyll a fluorescence for the assessment of environmental pollutants. Anal Bioanal Chem 401:1139–1151
Campanella L, Cubadda F, Sammartino MP, Saoncella A (2000) An algal biosensor for the monitoring of water toxicity in estuarine environments. Water Res 35(1):69–76
Chauvat CC, Chauvat F (2015) Responses to oxidative and heavy metal stresses in cyanobacteria: recent advances. Int J Mol Sci 16(1):871–886
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Hameed A, Hasnein S (2014) Role of cyanobacterial strains on Triticumae stivum growth under chromium stress in laboratory. Sci Int (Lahore) 26(4):1737–1742
Lee LH, Garrett R, Slusarczyk A, Perez J, Patel J, Chu TC (2013) Bioinformatic analyses of chromium tolerant genes in cyanobacteria and identification of chromium tolerant operon in Synechococcussp. IU 625. Growth 5(11):1–7
Loez CR, Topalián ML, Salibián A (1995) Effects of zinc on the structure and growth dynamics of a natural freshwater phytoplankton assemblage reared in the laboratory. Environ Pollut 88:275–281
McGrath SP, Smith S (1990) Chromium and nickel. In: Alloway BJ (ed) Heavy metals in soils. Wiley, New York, pp 125–150
Nies DH (1999) Microbial heavy metal resistance. Appl Microbiol Biotechnol 51:730–750
OECD TG 201 (2011) Organization for economic co-operation and development (OECD) guidelines for the testing of chemicals, section 2: effects on biotic systems test no. 201: freshwater alga and cyanobacteria, growth inhibition test OECD. OECD Publishing, Paris
Pereira M, Bartholomew MC, Sánchez-Fortún S (2013) Biosorption and bioaccumulation of chromium trivalent in Cr(III)-tolerant microalgae: a mechanism for chromium resistance. Chemosphere 93:1057–1063
Peterson HG, Nyholm H, Ruecker N (2005) Algal microplate toxicity test suitable for heavy metals. In: Blaise C, Ferard J (eds) Small scale freshwater toxicity investigations. Springer, Netherlands, pp 243–270
Singh S, Shrivastava AK, Singh VK (2014) Arsenic and cadmium are inhibitors of cyanobacterial dinitrogenase reductase (nifH1) gene. Funct Integr Genomics. doi:10.1007/s10142-014-0375-2
Waterbury JB (2006) The cyanobacteria-isolation, purification and identification. In: The prokaryotes Springer US, 4:1053–1073
Wong PTS, Trevors JT (1988) Chromium toxicity to algae and bacteria. In: Nriagu JO, Nieboe E (eds) Chromium in the natural and human environments. Wiley, New York, pp 335–348
Wong LS, Lee YH, Surif S (2013) Performance of a cyanobacteria whole cell-based fluorescence biosensor for heavy metal and pesticide detection. Sensors 13:6394–6404
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150
Acknowledgments
Financial support by the National Research Council (NRC) Sri Lanka (Grant No. 12-092). Atomic absorption spectrometer purchased from the Equipment grant (RG/2012/EQ/12) of the National Science Foundation of Sri Lanka was used for metal analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Munagamage, T., Rathnayake, I.V.N., Pathiratne, A. et al. Sensitivity of Four Cyanobacterial Isolates from Tropical Freshwaters to Environmentally Realistic Concentrations of Cr6+, Cd2+ and Zn2+ . Bull Environ Contam Toxicol 96, 816–821 (2016). https://doi.org/10.1007/s00128-016-1809-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1809-4