Abstract
Biosorption, using cadmium-resistant bacterial isolates, is often regarded as a relatively inexpensive and efficient way of cleaning up wastes, sediments, or soils polluted with cadmium. Therefore, many efforts have been devoted to the isolation of cadmium-resistant isolates for the efficient management of cadmium remediation processes. However, isolation, identification and in situ screening of efficient cadmium-resistant isolates are primary challenges. To overcome these challanges, in this study, cadA, cadmium resistance coding gene, specific primers and DNA probes were used to identify and screen cadmium-resistant bacteria in the cadmium-polluted river waters through polymerase chain reaction (PCR) and fluorescein in situ hybridization (FISH). PCR amplification of the cadA amplicon coupled with 16S rRNA sequencing revealed various gram-positive and -negative bacterial isolates harboring cadA. Accordingly, a cadA-mediated DNA probe was prepared and used for in situ screening of cadmium-resistant isolates from water samples collected from cadmium-polluted river waters. The FISH analyses of cadA probe showed highly specific and efficient hybridization with cadA harboring isolates. The use of primers and DNA probes specific for cadA gene seems to be very helpful tools for the selection and screening of cadmium biosorbents with potential to be used in the remediation of cadmium-polluted sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
One of the most toxic pollutants in our environment is the heavy metal element cadmium that has been classified by the World Health Organisation (WHO) to be of serious health concern. Apart from the itai–itai disease which is a manifestation of cadmium toxicity in humans, kidney dysfunction, hepatic damage and hypertension are also other health implications of cadmium (Klaassen 2001). Cadmium metal, its alloys and compounds have been used in a variety of different industrial and consumer products, although most uses are now declining due to concerns about its toxicity. A major source of cadmium in the environment is the discharge of untreated industrial effluents from industries such as paint, plating, fertilizers, mining, textile dyeing, textile processing, automobile manufacturing and cadmium processing (Habib-Ur-Rahman et al. 2006). Thus the treatment or purification of cadmium-polluted water and effluents is one of the major areas of active research (Rao et al. 2010; Kheriji et al. 2015). According to WHO’s recommendation cadmium limit in drinking water is 5 μg L−1. Several conventional methods such as coagulation/flocculation (Amuda et al. 2006), cementation (Younesi et al. 2006), membrane separation (He et al. 2007), oxidation (Rangel-Mendez et al. 2000) reverse osmosis (Kumar et al. 2009), chemical precipitations (Ku and Jung 2001), ion exchange (Kocaoba 2007) and the use of activated charcoal (Nadeem et al. 2009) have been in use over the years for the removal of cadmium from wastewater. However, some of these technologies cannot be effective for complete removal of the cadmium or are too expensive. Another powerful technique for cadmium remediation is biosorption that utilizes various natural materials of biological origin, including bacteria, fungi, yeast, algae, etc. These biosorbents possess metal-sequestering properties and are highly helpful to decrease the concentration of heavy metal ions in solution (Wang and Chen 2006). Over conventional treatment methods, biosorption is advantageous with low cost, high efficiency of metal removal from dilute solution, no additional nutrient requirements, recycling of the biosorbent and possibility of cadmium recovery. The cadmium-resistant biological materials that have been investigated for cadmium uptake include fungi, bacteria, yeast, microalgae and others (Hrynkiewicz et al. 2015). Many microorganisms are able to immobilize toxic metals by a precipitation process through the formation of metal complexes, biosorption to an extracellular membrane ligand, or bioaccumulation by energy dependent transport system. This can result in the direct conversion of metal pollutants from a soluble mobile form to a sparingly soluble or less bioavailable form and facilitate the removal of metals from aqueous solutions. As in the cadmium resistance through efflux mechanism encoded by cadA gene is needed for effective cadmium biosorption. Resistant cells are expected to bind substantially more metals, which in turn is a prerequisite for enhanced bioprecipitation, intracellular accumulation, and development of an efficient process (Malik 2004).
Cadmium resistance in bacteria is mediated by several genetical systems (Naz et al. 2005) and the well-studied one is the cadmium efflux system encoded by cadmium-resistant gene cadA (Zhang et al. 2008). The homologs of cadA gene were reported to be widespread in gram-positive bacteria such as Staphylococcus aureus, Bacillus firmus, Listeria monocytogenes and Lactococcus lactis (Blencowe and Morby 2003), and in gram-negative bacteria Stenotrophomonas maltophilia (Pages et al. 2008) and Pseudomonas sp. (Lee et al. 2001). The cadA homologs are carried by plasmids in L. lactis and L. monocytogenes and on the chromosome in B. firmus. The cadA gene has also been found on the chromosome of the gram-negative species, S. maltophilia, upstream of an IS257 insertion sequence (Oger et al. 2003). The flanking insertion sequences and the unusual GC content of the locus lead to the conclusion of lateral transfer of cadmium-resistant genes from a gram-positive to this gram-negative bacteria (Alonso et al. 2000). This conclusion was further supported by the findings of Oger et al. (2003) who showed that exposure to a toxic concentration of dissolved cadmium enhances the expression of cadA gene in cadmium-resistant bacteria. Bacteria harboring cadA gene evidences the mechanism of cadmium biosorption to avoid re-entry of cadmium into the cell, once that the efflux of the metals is accomplished (Maynaud et al. 2014). Although the basis of cadmium resistance for most of the bacteria has not been well-characterized yet, the findings of lateral transfer of cadmium-resistant genes and presence of cadA homologs in several bacterial genome suggest that cadA plays a broader role in cadmium resistance and provides a good target for the selection of cadmium biosorbents with potentials to be used in the remediation strategies of cadmium-polluted sites. Therefore, in this study, cadA-specific primers and DNA probes were used to identify and screen cadmium biosorbents isolated from cadmium-polluted river waters through polymerase chain reaction (PCR) and fluorescein in situ hybridization (FISH), respectively.
Materials and Methods
Water samples were collected from 12 stations along the river Kızılırmak extending from 39°22′16.39″N, 33° 26′49.26″E, 890 m to 39°57′22.98″N, 33°25′04.35″E, 679 m of the city Kırıkkale, Turkey. For cadmium analysis, as indicated previously by Icgen and Yilmaz (2014), the samples were put in high density polyethylene containers previously washed in a solution of 10 % nitric acid in an ultrasonic bath for 15 min, followed by repeated rinsing with distillated water and finally rinsing with ultrapure water. Until collection containers were kept in sealed polyethylene bags. Water samples were stabilized with ultrapure nitric acid (0.5 % HNO3). For accurate determination of concentrations of cadmium in waters, a quantitative method was used, therefore a multi-standard calibration method was applied: Perkin Elmer 10 mg mL−1 of seventeen metals (ICP-MS Standard, Matrix: 5 % HNO3, Perkin Elmer Life and Analytical Sciences), a standard of 10 mg mL−1 metal solutions (metal standard 5 % HNO3 matrix, Perkin Elmer). All the determinations were done with inductively coupled plasma mass spectrometry (Perkin Elmer Elan DRC-ICP-MS) in triplicate and results were expressed as mean.
For microbial analysis, water samples were put into sterile screw capped bottles aseptically, kept in an icebox containing ice packs and taken immediately to the laboratory. A quantity of 1 mL of water from each of the collected samples was dissolved in 9 mL sterile distilled water and serial dilutions were made. Each dilution was plated on nutrient agar (NA) plates supplemented with 326 mM of cadmium containing Cd(NO3)2·4H2O were used. Stocks of Cd(NO3)2·4H2O were prepared in distilled water, sterilized by filter membrane (0.22 μm), and stored at 4°C. Plates were incubated at 30°C for 3 days and colonies differing in morphological characteristics were selected. After the growth of different microorganisms on the plate, each bacterial colony on the basis of its morphological characteristics was picked up and further purified by repeated streaking on nutrient agar plates and identified with 16S rRNA sequencing. Extraction of DNA from selected colonies was performed by using High Pure PCR Template Preparation Kit (Roche, Germany) following the manufacturer’s instructions. Bacterial 16S rRNA was amplified by using the universal bacterial 16S rRNA primers, 27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R 5′-GGTGTTTGATTGTTACGACTT-3′ (Lane et al. 1985). PCR was performed with a 50 μL reaction mixture containing 1 μL (10 ng) of DNA extract as a template, each primer at a concentration of 5 mM, 25 mM MgCl2 and dNTPs at a concentration of 2 mM, as well as 1.5 U of Taq polymerase and buffer used as recommended by the manufacturer (Fermentas, Germany). After the initial denaturation for 5 min at 94°C, there were 35 cycles consisting of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. PCR was carried out in a gene Piko Thermal Cycler (Thermo Scientific, USA). The obtained PCR products were purified, using the GeneJET™ PCR Purification Kit (Fermentas, Germany), according to the instructions of the manufacturer and sequenced. The PCR product was sequenced by 3730x1 DNA synthesizer (Applied Biosystems, USA). The two 16S rRNA sequences were aligned and compared with other 16S rRNA genes in the GenBank by using the NCBI basic local alignment search tools BLASTn program (Benson et al. 2002).
Water samples were put into sterile screw capped bottles aseptically, kept in an icebox containing ice packs, and taken immediately to the laboratory. Total DNA was extracted by using the method of Tan and Yiap (2009). DNA extractions were used for the amplification of cadA gene through PCR. The DNA of previously identified cadmium-resistant isolates Staphylococcus warneri and Delftia acidovorans was used as a positive control of the cadA gene. The cadA gene was amplified by using primers cad1 (CAAAYTGYGCRGGHAARTTYGA) and cad2 (AACTAATGCACAAGGACA) (Oger et al. 2001). PCR was performed as stated above. The purified PCR products were electrophoresed and separated on a 1.0 % agarose gel. The gel was visualized under UV after staining with ethidium bromide. The DNA extracted from cadA positive isolates were amplified by using the universal bacterial 16S rRNA primers as stated above. The 16S rRNA sequences were aligned and compared with other 16S rRNA genes in the GenBank by using the NCBI basic local alignment search tools BLASTn program (Benson et al. 2002). A distance matrix was generated using the Kimura two-parameter using MEGA version 5.2 (Shigematsu et al. 2003). The 16S rRNA gene sequences have been deposited to GenBank using BankIt submission tool and has been assigned with NCBI accession numbers.
The cadA-specific DNA probe was designed from the entire 3.5 kb BglII-XbaI fragment of cadA operon from pI258 by using Vector NTI Express Software (Life Technologies, USA). Obtained sequences were subsequently confirmed for their specificity to cadA gene in S. aureus by using BLAST. The designed 36 bp DNA fragment (5′-GCAGTATCCGTTCCAGCACCGCCCATTGCAATTCCA-3′) was labelled with fluorescein isothiocyanate (FITC) at the 5′ end (Alpha DNA, Montreal, Canada). Cadmium-resistant S. warneri and D. acidovorans isolates were used to calibrate the optimal hybridization stringency for the target genera to the cadA probe used. E. coli DH5α was used as negative control. 16S rRNA-targeted oligonucleotide probe sequences were selected from those deposited at probeBase (Loy et al. 2003). The following 16S rRNA-targeted oligonucleotide probes and hybridization conditions were used: (1) EUB338 (Daims et al. 1999), (2), EUB338 II (Wallner et al. 1993), (3) EUB338 III (Wallner et al. 1993), (4) NON338 (Schleifer et al. 1992) as a negative control and (5) cadA probe. All probes were commercially synthesized and labelled with FITC at the 5′ end (Alpha DNA, Montreal, Canada). 5 μL fixed samples were spotted on glass slides and dried at 45°C for 30 min and finally dehydrated sequentially in 50 %, 80 % and 96 % ethanol for 3 min each (Amann et al. 1990). All in situ hybridizations were performed according to the procedure described by Amann (1995). To determine the total bacteria present in the samples, each slide was counter-stained with 10 μL DAPI (4,6-diamidino-2-phenylindole dihydrochloride) solution (1 μg mL−1) for 15 min at room temperature, rinsed with deionized water and allowed to air-dry prior to microscopic observation. For in situ application, optimal stringency conditions were adjusted for each FITC-labelled probe performing whole cell hybridizations using target and nontarget organism(s). The hybridization stringency was gradually increased by the addition of formamide to the hybridization buffer in concentration steps of 5 % (v/v) according to Wagner et al. (1994). The sodium chloride concentration was adjusted according to the formamide concentration used in the hybridization buffer.
The cadA harboring postive control pure culture S. warneri and D. acidovorans cells were observed by a Leica DM 5000B fluorescence microscope equipped with A and I3 two filter sets: A was used for total microorganisms in samples stained by DAPI and filter set I3 for isolates hybridized with FITC-labelled cadA probe. Triplicate images were captured with CCD camera and saved in Leica QWin Plus software. The captured digital images were first processed using Microsoft Photoshop, in which the blur (or out-of-focus) areas were removed. The pixel areas of green region conferred by FITC-labelled cadA probe and blue region conferred by DAPI were counted separately for each image. Based on the quantification of pixel areas of images, cadA was determined as follows (Li et al. 2007);
% of cadA was calculated using average values of taken images for pixel areas of FITC probe and DAPI. Before the calculation, the pixel areas were subtracted by the areas of non-binding probe (NON338) to remove autofluorescence and background interference Daims et al. 1999). In this study, the total amount of cadA was assumed as the images conferred by oligonucleotide probe cadA and the total amount of positive control pure culture biomass was the images conferred by DAPI stained cells. After optimization of hybridization conditions for cadA probe with pure cultures of positive controls, the collected water samples were also screened for the cadA harboring isolates by using FISH.
All statistical analyses were performed using Origin Pro 8.5 software (OriginLab Corporation, Northampton, Massachusetts, USA). The significance of all parameters in the regression analyses presented has been verified (p < 0.05 significance level) by one-way analysis of variance (ANOVA) and Tukey test.
Results and Discussion
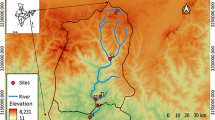
The Kızılırmak River has been affected by industrial and agricultural heavy metal pollution from the surrounding facilities and the domestic effluents from the city Kırıkkale, in Turkey. For this reason, water samples of the river from 12 different stations (Fig. 1; Table 1) collected starting from the June of 2011 till the June of 2012. Significant variations observed in the concentrations of cadmium in the river water were probably the result of industrial operations and the subsequent accumulation of pollutants. The annual loads of cadmium showed variations in between 0.005 and 0.977 mg L−1 (Table 2). Higher levels of cadmium in aerobic waters are usually associated with industrial pollution. For this reason, the samples collected from these stations were further used for the selective isolation of cadmium-resistant isolates. Waters from the vicinity of cadmium-bearing mineral deposits may have cadmium concentrations of ≥1000 μg L−1. Faroon et al. (2012) reports that the cadmium concentration of natural surface water and groundwater is usually <1 μg L−1. Water sources near cadmium-emitting industries, both with historic and current operations, have shown a marked elevation of cadmium in water bodies (Brumbaugh et al. 2005). Additional releases of cadmium to the environment occur from natural sources and from processes such as combustion of fossil fuel, incineration of municipal or industrial wastes, or land application of sewage sludge or fertilizer (Järup 2003).
During selective isolation and identification of cadmium-resistant isolates various colonies were collected from the selective medium. Based on the phylogenetic analysis of the collected colonies cadmium-resistant isolates of Staphylococcus aureus, Staphylococcus warneri, Micrococcus luteus, Delftia acidovorans, Pantoea agglomerans, Enterococcus faecalis, several Acinetobacter and Pseudomonas spp. were identified. Staphylococci are ubiquitous and widespread in the environment. S. aureus, a coagulase-positive species which produces a series of other enzymes and toxins, is the best known and has been frequently implicated in the etiology of a series of infections and intoxications in animals and humans (Cunha et al. 2004). S. warneri and other coagulase negative staphylococci have been recognized less frequently as significant human pathogens (Arslan et al. 2011). Micrococcus is routinely isolated from skin, but can also be found in soil, sediments, marine and fresh water (Dib et al. 2010). D. acidovorans is an aerobic bacterium from the genus of Comamonas previously classified within the genus Pseudomonas (Chou et al. 2007). Pseudomonads are ubiquitous group of environmental bacterial organisms. P. agglomerans is member of Enterobacteriaceae that inhabits plants, soil, water and such species includes bacteria reported as both commensal and pathogen of animals and humans (Cerit et al. 2015). Generally, E. faecalis is common in the feces of humans and birds and rare in the feces of other animals (Kuntz et al. 2004). The presence of enterococci in the water is indicative of fecal pollution. Acinetobacter spp. are a major concern because of their rapid development of resistance to a wide range of antimicrobials, ability to transform rapidly, surviving desiccation, and persistence in the environment for a very long time (Doughari et al. 2011).
Out of 28 water samples collected from the cadmium-polluted stations, only 15 isolates were found to harbor cadA gene (Fig. 2a, b). The rest of the isolates did not contain cadA gene after PCR amplification. For the identification of cadA containing amplicons, 16S rRNA sequencing was used. The distance matrix generated highlighted the distances among the sequences aligned (Data not shown). These distances were used for the phylogenetic analysis and the 16S rRNA gene sequences were deposited to GenBank using BankIt submission tool. Based on the phylogenetic analysis, the cadA harboring isolates with their respective NCBI accession numbers were identified as follows: two Staphylococcus spp. (S. aureus Al11 [KJ395360] and S. warneri Co11 [KJ395373]), two M. luteus Sr02, Sr11 (KJ395374 and KJ395375), one D. acidovorans Cd11 (KJ209817), P. agglomerans Sn11 (KJ395361), one E. faecalis Pb06 (KJ395380), 4 Acinetobacter spp. (A. johnsonii Sb01 [KJ395376], A. calcoaceticus Fe10 [KJ395366], two A. haemolyticus Mn12, Zn01 [KJ396367 and KJ395368]) and 4 Pseudomonas spp. (P. plecoglossicida Ag10 [KJ395363], P. corrugata SDS10-3 [KJ937676], two P. koreensis SDS4, SDS10-1 [KJ937669, KJ937674]).
The cadA gene harboring surface water isolates; E. coli DH5α (negative control) lane 1, M. luteus Sr01 lane 2, M. luteus S111 lane 3, D. acidovorans Cd11 lane 4, S. warneri Co11 lane 5, S. aureus Al11 lane 6, P. corrugata SDS10-3 lane 7, A. haemolyticus Mn12 lane 8, A. johnsonii Sb01 lane 9, A calcoaceticus Fe10 lane 10, E. faecalis Pb06 lane 11, A. haemolyticus Zn01 lane 12, P. plecoglossicida Ag10 lane 13, P. agglomerans Sn11 lane 14, P. koreensis SDS4 lane 15, P. koreensis SDS10-1 lane 16. M marker gene ruler 200–1500 bp (Top-Bio Prague, Czech Republic), arrows indicate cadA gene
The cadA gene provides resistance to cadmium by an energy dependent efflux mechanism. The cadA protein is a Cd2+/ATPase transporter, containing the eight transmembrane domains characteristic of P-class ATPases (Argüello et al. 2007). The cadA gene has been reported mostly in gram-positive bacteria (Lee et al. 2001; Oger et al. 2003). Lee et al. (2001) and Alonso et al. (2000) also showed the presence of cadA gene in gram-negative bacteria. The flanking IS257 sequences were explained as the reason for the presence of a cadA gene in S. maltophilia by Alonso et al. (2000). Our results also supported the presence of cadA gene in both gram-positive and -negative bacteria. In this study, we showed the presence of cadA gene in gram-positive (S. aureus, S. warneri, M. luteus and E. faecalis) and gram-negative (D. acidovorans, P. agglomerans, A. johnsonii, A. calcoaceticus, A. haemolyticus, P. plecoglossicida, P. corrugata, P. resinovorans and P. koreensis) surface water isolates. As stated Oger et al. (2003) cadA harboring bacteria seem to be members of different species or even genera and most have never previously been reported to be carriers of the cadA gene. The prokaryotic heavy metal-translocating ATPases detoxify the cell cytoplasm by effluxing the divalent ions of cadmium, (Nies 2003). The presence of ATPase genes on mobile genetic elements like plasmids and transposons in both Gram-positive bacteria and Gram-negative bacteria has been shown previously (Nucifora et al. 1989). Analysis of completed microbial genomes has indicated that horizontal gene transfer is an important factor contributing to the innovation of microbial genomes. Due to the horizontal transfer of resistance genes among bacteria, the cadA may be hosted by various bacteria in the environment for a long time and needs to be more accurately assessed.
The determination of bacterial community composition and their activities in nature is fundamental but has long been a challenge to scientists. FISH has provided information about absolute abundance, morphology, and cell size of bacteria with defined phylogenetic affiliations and been applied to the investigation of community composition in lakes, oceans, activated sludge, and drinking water (Wagner et al. 1994; Amann 1995; Kalmbach et al. 1997; Pernthaler et al. 1997). In the current study, cadA% was determined by calculating the pixel areas (pp2) of DAPI and FITC images of cadmium-resistant pure culture isolates by using FISH. Under the hybridization conditions of 46°C, 50 % formamide and 0.028 M NaCl, the designed cadA probe appeared to be highly specific to the cadA positive S. warneri and D. acidovorans isolates tested (Fig. 3). Our results indicated that cadA probe was highly efficient up to 90.7 % ± 0.98 % (p > 0.05) for the selection of cadA harboring pure culture isolates. Non-target bacteria only gave 3.50 % ± 0.69 % (p > 0.05) hybridization with cadA probes. Water samples collected from cadmium-polluted river water were also checked for the screening of cadA harboring isolates. The results showed that cadA probe was also successful for screening of cadA harboring isolates in surface waters.
FISH analyses to localize all bacteria; cadA probe applied to pure cultures of D. acidovorans (a 1 ), S. warneri as positive controls (b 1 ), E.coli DH5α as a negative control (c 1 ), water sample (d 1 ) and their corresponding total cell populations stained with DAPI (a, b, c, d), respectively. Bar 10 µm and applies to all photomicrographs
Bacterial biosorption is mainly used for the removal of pollutants from effluents contaminated with pollutants that are not biodegradable, like metals ions. Many researchers have studied biosorption performance of different microbial biosorbents to implement biosorption technologies for heavy metal removal from solutions. The extent of biosorption not only depends on the type of metal ions, but also on the bacterial genus. It is well known that many microbial biosorbents usually lack specificity in metal-binding, which may cause difficulties in the recovery and recycling of the desired metal ions. Therefore, the isolation, screening and harvesting of microbial biosorbents specific for heavy metals on a larger scale may be complicated. The use of tools like specific primers and DNA probes, on the other hand, are very helpful to select these microbial biosorbents with potential to be used in remediation strategies to solve these difficulties.
The consequences arising from cadmium overuse and subsequent disposal in waterways are of serious concern. Biosorption of cadmium seems to be an efficient and inexpensive method for successful removal of this heavy metal in surface waters providing that the biosorbent isolates are available. In this study, we showed that, the isolation, identification and screening of potential cadmium biosorbents in cadmium-polluted surface waters could be managed by using cadA-specific primers and DNA probes. PCR amplification with cadA-specific primers revealed the presence of cadA gene in both gram-positive (S. aureus Al11, S. warneri Co11, M. luteus Sr02, Sr11 and E. faecalis Pb06) and gram-negative (D. acidovorans Cd11, P. agglomerans Sn11, A. johnsonii Sb01, A. calcoaceticus Fe10, A. haemolyticus Mn12, Zn01, P. plecoglossicida Ag10, P. corrugata SDS10-3, and P. koreensis SDS4, SDS10-1) surface water isolates. The cadA-mediated DNA probe showed high efficiency for the selection of cadA harboring isolates in pure cultures and cadmium-polluted water samples. Use of cadA-specific primers and DNA probes seem to be highly efficient for the selection and screening of cadmium biosorbents with potentials to be used in remediation strategies. However, the relative importance of these biosorbents in cadmium remediation strategies of soil, sewage and WWTPs remains to be assessed.
References
Alonso A, Sanchez P, Martinez JL (2000) Stenotrophomonas maltophilia D457R contains a cluster of genes from gram-positive bacteria involved in antibiotic and heavy metal resistance. Antimicrob Agents Chemother 44:1778–1782
Amann RI (1995) In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Akkerman ADL, van Elsas JD, de Brujin FJ (eds) Molecular microbial ecology manual. Kluwer, Dordrecht, pp 1–15
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Amuda OS, Amoo IA, Ipinmoroti KO, Ajayi OO (2006) Coagulation/flocculation process in the removal of trace metals present in industrial wastewater. J Appl Sci Environ Manag 10:159–162
Argüello JM, Eren E, González-Guerrero M (2007) The structure and function of heavy metal transport P1B-ATPases. Biometals 20:233–248
Arslan F, Saltoglu N, Mete B, Mert A (2011) Recurrent Staphylococcus warnerii prosthetic valve endocarditis: a case report and review. Ann Clin Microbiol Antimicrob 10:14
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL (2002) GenBank. Nucl Acids Res 30:17–20
Blencowe DK, Morby AP (2003) Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev 27:291–311
Brumbaugh WG, Schmitt CJ, May TW (2005) Concentrations of cadmium, lead, and zinc in fish from mining-influenced waters of north eastern Oklahoma-sampling of blood, carcass, and liver for aquatic biomonitoring. Arch Environ Contam Toxicol 49:76–88
Cerit S, Yilmaz F, Icgen B (2015) Challenging tin toxicity by a novel strain isolated from fresh waters. Desalin Water Treat 53:3244–3252
Chou JH, Sheu SY, Lin KY, Chen WM, Arun AB, Young CC (2007) Comamonas odontotermitis sp. nov., isolated from the gut of the termite Odontotermes formosanus. Inter J Syst Evol Microbiol 57:887–891
Cunha MLRS, Sinzato YK, Silveira LV (2004) Comparison of methods for the identification of coagulase-negative staphylococci. Mem Inst Oswaldo Cruz 99:855–860
Daims H, Brühl A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Dib JR, Wagenknecht M, Hill RT, Farías ME, Meinhardt F (2010) First report of linear megaplasmids in the genus Micrococcus. Plasmid 63:40–45. doi:10.1016/j.plasmid.2009.10.001
Doughari HJ, Ndakidemi PA, Human IS, Benade S (2011) The ecology, biology and pathogenesis of Acinetobacter spp.: an overview. Microbes Environ 26:101–112
Faroon O, Ashizawa A, Wright S et al (2012) Toxicological profile for cadmium. Agency for Toxic Substances and Disease Registry (US), Atlanta
Habib-Ur-Rahman MS, Imtiaz A, Sher S, Hameedullah H (2006) Sorption studies of nickel ions onto sawdust of Dalbergia sissoo. J Chin Chem Soc 53:1045–1052
He D, Gu S, Ming Ma M (2007) Simultaneous removal and recovery of cadmium (II) and CN− from simulated electroplating rinse wastewater by a strip dispersion hybrid liquid membrane (SDHLM) containing double carrier. J Membr Sci 305:36–47
Hrynkiewicz K, Złoch M, Kowalkowski T, Baum C, Niedojadło K, Buszewski B (2015) Strain-specific bioaccumulation and intracellular distribution of Cd2+ in bacteria isolated from the rhizosphere, ectomycorrhizae, and fruitbodies of ectomycorrhizal fungi. Environ Sci Pollut Res Int 22:3055–3067
Icgen B, Yilmaz F (2014) Co-occurrence of antibiotic and heavy metal resistance in Kızılırmak river isolates. Bull Environ Contam Toxicol 93:735–743
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Kalmbach S, Manz W, Szewzyk U (1997) Isolation of new bacterial species from drinking water biofilms and proof of their in situ dominance with highly specific 16S rRNA probes. Appl Environ Microbiol 63:4164–4170
Kheriji J, Tabassi D, Hamrouni B (2015) Removal of Cd(II) ions from aqueous solution and industrial effluent using reverse osmosis and nanofiltration membranes. Water Sci Technol 72:1206–1216
Klaassen CD (2001) Toxic effects of metals. In: Klaassen CD (ed) Casarett and Doull’s toxicology: the basic science of poisons, 6th edn. McGaw-Hill, New York, pp 811–869
Kocaoba S (2007) Comparison of Amberlite IR 120 and dolamite’s performance for the removal of heavy metals. J Hazard Mater 147:488–496
Ku Y, Jung IL (2001) Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35:135–142
Kumar V, Kumar M, Jha MK, Jeong J, Jae-chun Lee JC (2009) Solvent extraction of cadmium from sulfate solution with di-(2-ethylhexyl)phosphoric acid diluted in kerosene. Hydrometallurgy 96:230–234
Kuntz RL, Hartel PG, Rodgers K, Segars WI (2004) Presence of Enterococcus faecalis in broiler litter and wild bird feces for bacterial source tracking. Water Res 38:3351–3557
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci 82:6955–6959
Lee SW, Glickmann E, Cooksey DA (2001) Chromosomal locus for cadmium resistance in Pseudomonas putida consisting of a cadmium-transporting ATPase and a MerR family response regulator. Appl Environ Microbiol 67:1437–1444
Li B, Irvin S, Baker B (2007) The variation of nitrifying bacterial population sizes in a sequencing batch reactor (SBR) treating low/mid/high concentrated wastewater. Environ Sci Eng 6:651–663
Loy A, Horn M, Wagner M (2003) ProbeBase: an online resource for rRNA-targeted oligonucleotide probes. Nucleic Acids Res 31:514–516
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278
Maynaud G, Brunel B, Yashiro E, Mergeay M, Cleyet-Marel JC, Le Quéré A (2014) CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a P(IB-2)-type ATPase involved in cadmium and zinc resistance. Res Microbiol 165:175–189
Nadeem M, Shabbir M, Abdullah MA, Shah SS, McKay G (2009) Sorption of cadmium from aqueous solution by surfactant modified carbon adsorbents. Chem Eng J 148:365–370
Naz N, Young HK, Ahmed N, Gadd GM (2005) Cadmium accumulation and DNA homology with metal resistance genes in sulfate-reducing bacteria. Appl Environ Microbiol 71:4610–4618
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339
Nucifora G, Chu L, Misra TK, Silver S (1989) Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proc Natl Acad Sci USA 86:3544–3548
Oger C, Berthe T, Quillet L, Barray S, Chioleau JF, Petit F (2001) Estimation of the abundance of the cadmium resistance gene cadA in microbial communities in polluted estuary. Res Microbiol 152:671–678
Oger C, Mahillon J, Petit F (2003) Distribution and diversity of a cadmium resistance (cadA) determinant and occurrence of IS257 insertion sequences in Staphylococcal bacteria isolated from a contaminated estuary (Seine, France). FEMS Microbiol Rev 43:173–183
Pages D, Rose J, Conrod S, Cuine S, Carrier P, Heulin T, Achouak W, Ward N (2008) Heavy metal tolerance in Stenotrophomonas maltophilia. PLoS ONE. doi:10.1371/journal.pone.0001539
Pernthaler J, Alfreider A, Posch T, Andreatta S, Psenner R (1997) In situ classification and image cytometry of pelagic bacteria from a high mountain lake (Gossenköllesee, Austria). Appl Environ Microbiol 63:4778–4783
Rangel-Mendez JR, Tai MH, Streat M (2000) Removal of cadmium using electrochemically oxidized activated carbon. Process Saf Environ Protect 78:143–148
Rao KS, Mohapatra M, Venkateswarlu P (2010) Review on cadmium removal from aqueous solutions. Int J Eng Sci Technol 2:81–103
Schleifer KH, Amann RI, Ludwig W, Rothemund C, Springer N, Dorn S (1992) Nucleic acid probes for the identification and in situ detection of Pseudomonads. In: Galli E, Silver S, Withold B (eds) Pseudomonas: molecular and biology biotechnology. Washington American Society of Microbiology, Washington, DC, pp 127–134
Shigematsu T, Yumihara K, Ueda Y, Numaguchi M, Morimura S, Kida K (2003) Delftia tsuruhatensis sp. nov., a terephthalate-assimilating bacterium isolated from activated sludge. Int J Syst Evol Microbiol 53:1479–1483
Tan SC, Yiap BC (2009) DNA, RNA and protein extraction: the past and the present. J Biomedicine Biotech. doi:10.1155/2009/574398
Wagner M, Erhart R, Manz W, Amann RI, Lemmer H, Wedi D et al (1994) Development of an rRNA-targeted oligonucleotide probe specific for the genus Acinetobacter and its application for in situ monitoring win activated sludge. Appl Environ Microbiol 60:792–800
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136–143
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451
Younesi SR, Alimadadi H, Alamdari EK, Marashi SPH (2006) Kinetic mechanisms of cementation of cadmium ions by zinc powder from sulphate solutions. Hydrometallurgy 84:155–164
Zhang Y, Zhang H, Li X, Su Z, Zhang C (2008) The cadA gene in cadmium-resistant bacteria from cadmium-polluted soil in the Zhangshi area of Northeast China. Curr Microbiol 56:236–239
Acknowledgments
This work was supported through a Research Fund Project (BAP-03-11-2014-003) by Middle East Technical University, Ankara-Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Icgen, B., Yilmaz, F. Use of cadA-Specific Primers and DNA Probes as Tools to Select Cadmium Biosorbents with Potential in Remediation Strategies. Bull Environ Contam Toxicol 96, 685–693 (2016). https://doi.org/10.1007/s00128-016-1767-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1767-x