Abstract
The present study evaluated the impact of cesium (133Cs) at four concentrations (0, 0.001, 0.01, and 0.1 mg L−1) on growth, concentrations of chlorophyll and carotenoid pigments, and oxidative stress responses in the charophyte, Nitella pseudoflabellata, over 30 days. Oxidative stress was quantified by measuring anti-oxidant enzyme activities and H2O2 content. When compared with the control, significantly elevated activity levels of the anti-oxidative enzymes ascorbic peroxidase, catalase and guaiacol peroxidase were observed at 0.1 mg L−1 (all p < 0.05), even though the H2O2 level was not significantly elevated. Carotenoid and chlorophyll a and b pigment levels were significantly reduced (all p < 0.05) at Cs exposures of 0.01 and 0.1 mg L−1. Photosynthetic efficiency (i.e., Fv/Fm) was significantly reduced (p < 0.05) at Cs concentrations ≥0.001 mg L−1. Significant reduction (p < 0.05) of plant growth (i.e., shoot length) was also observed after 1 week of exposure at Cs concentrations ≥0.001 mg L−1. Our results suggested that Cs exposure reduced plant growth and affected plant functioning via activating the defense mechanism against oxidative stress in Nitella.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Aquatic systems are challenged by an array of fluctuations in abiotic stress vectors. In particular, anthropogenic inputs, including heavy metals and other toxic substances, have been prominent in the last few decades and have exceeded the tolerance limits for certain species in aquatic systems (Nagajyoti et al. 2010). Stable Cs (133Cs) is an alkali metal that originates from an aluminosilicate mineral called pollucite (White and Broadley 2000). The major anthropogenic sources of 133Cs are mining of pollucite ores and the production and use of Cs compounds in electronic and energy production (especially coal-burning power plants) (ATSDR 2004). The Cs concentrations found in freshwater and marine ecosystems range from 1 × 10−5 to 12 × 10−3, and 5 × 10−4 to 2 × 10−3 mg L−1, respectively (Komarov and Bennett 1983), whereas soils have been reported to contain 0–26 mg kg−1 133Cs (Cook et al. 2007). Although the naturally occurring Cs levels are harmless, Cs accumulation over longer time periods can be toxic to plants (Bystrzejewska-Piotrowska et al. 2005; Hampton et al. 2004). Therefore, 133Cs accumulation may pose a risk to aquatic plants in areas with anthropogenic inputs in the long run.
Aquatic flora provide a wide spectrum of ecological functions and play a crucial role in maintaining the integrity of aquatic ecosystems. Their functions include the provisioning of habitats, refuge and food for fish and other invertebrates, primary production, retention of substances, and contributions to biogeochemical cycles (Bennett et al. 2005; Bornette and Puijalon 2011; Folkard 2011; Nepf 2012). Algae are plant-like aquatic organisms containing chlorophyll for photosynthesis, while their bodies are not differentiated into true leaves, stems or roots. The macro-algae of the taxon Charophyta, which are commonly known as stoneworts/brittleworts, are a group of non-vascular hydrophytes commonly found in many regions, with potential for phytoremediation (García 1994; Gomes and Asaeda 2009; Siong and Asaeda 2009).
The effects of Cs exposure on growth, metabolism and genetics have been reported for some terrestrial plants (Hampton et al. 2004; Kanter et al. 2010; Zhu and Smolders 2000). However, there is little information regarding the impact of Cs exposure and Cs accumulation on aquatic flora, including charophytes. Furthermore, Cs-induced stress responses in charophytes are largely unknown, and the relationship between plant stress and the Cs accumulation in aquatic plants remains unclear. In this study, we used the charophyte, Nitella pseudoflabellata, as a model species to evaluate our hypothesis that Cs exposure would cause oxidative stress and negatively impact growth of the algae.
Materials and Methods
Nitella pseudoflabellata plants were obtained from a laboratory maintained culture tank which was previously collected from nearby paddy fields. A glass beaker (1 L) with a layer (~2 cm) of commercial river (90 % <1 mm) sand purchased from the local market (DOIT, Saitama, Japan) with 1 % Hoagland’s nutrient solution (800 mL) was used as an experimental unit. Cs solutions (CsCl, purity was 99 %) and Hoagland’s nutrient medium were prepared from pure analytical-grade chemicals (Wako chemicals, Osaka, Japan) and distilled water. The total nitrogen (TN) and total phosphorus (TP) contents of the medium were 2.1 and 0.3 mg L−1, respectively. Each treatment, with three replicates (n = 3), was randomly allocated into 12 (4 × 3) glass beakers in a complete randomized design. Six similar size apical tips of N. pseudoflabellata [initial length (IL) ~2–3 cm] were planted in each beaker.
The measured initial Cs concentrations of the 0.001, 0.01 and 0.1 mg L−1 exposures were 0.005, 0.010 and 0.142 mg L−1, respectively. The lowest test concentration approximated the upper limit of concentrations reported for polluted waters in the literature. Light intensity was maintained at ~100 µmol m−2 s−1 using florescent lamps with a photoperiod of 12 h light and 12 h dark. The average temperature of the glass beakers was maintained at 24 ± 1°C throughout the experimental period (30 days).
The shoot length of Nitella was measured once per week. At the end of the experiment, the final shoot length (FL), shoot elongation rate (SER, SER = (FL − IL)/time)), Cs content, pigment concentration (chlorophyll-a, chlorophyll-b, and carotenoids) and the stress responses of plants were compared. Plant stress was assayed by measuring the chlorophyll fluorescence, H2O2 concentration and antioxidant enzyme activities. To characterize the antioxidant enzyme activities, ascorbic peroxidase (APX), catalase (CAT) and guaicol peroxidase (POD) activities were assayed. The chlorophyll fluorescence was determined using the chlorophyll fluorescence imaging technique (FC 1000-H; Photon Systems Instruments, Drasov, Czech Republic), and the maximum quantum efficiency of photo-system II photochemistry (Fv/Fm) was calculated (DeEll and Toivonen 2003). It should be noted that there were some attached algae grown in the microcosm. The algae were carefully removed with the aid of forceps before analysis.
The pigments (chlorophyll and carotenoids) were extracted by keeping fresh N. pseudoflabellata (~5 mg) overnight in N,N-dimethylformamide. After extraction, the absorbance was measured spectrophotometrically (Shimadzu UV mini 1210, Kyoto, Japan) at the wave lengths of 663.8, 646.8 and 480 nm. The pigment contents were calculated according to Wellburn (1994). For the stress assay, plant materials (~100 mg fresh weight (FW)) were ground to extract hormone and antioxidants using an ice-cold phosphate buffer (50 mM, pH = 6.0) which contained polyvinylpyrrolidone (PVP). After extraction, extracts were centrifuged at 3000 rpm for 20 min at 4°C. The supernatant was separated and stored at −80°C until analysis. The H2O2 content was determined according to Jana and Choudhuri (1982). Briefly, 750 µL of extract was mixed with 2.5 mL of 0.1 % titanium sulfate in 20 % H2SO4 (v/v). The mixture was centrifuged at 5000×g for 15 min at room temperature, and the intensity of the resulting yellow color was measured at 410 nm. The H2O2 concentration was estimated using a standard curve and the H2O2 content is presented as µmol g−1 FW.
Catalase activity was assayed following Aebi (1984). Briefly, the reaction mixture contained 100 µL of 10 mM H2O2, 2.00 mL of 100 mM potassium phosphate buffer (pH = 7.0) and 500 µL of extract. The decrease in absorbance at 240 nm was recorded for 0.5 min. The CAT activity was calculated using the extinction coefficient of 40 mM−1 cm−1. APX activity was determined according to Nakano and Asada (1981). The reaction mixture contained 100 µL of extract, 200 µL of 0.5 mM ascorbic acid in 50 mM potassium phosphate buffer (pH = 7.0) and 2.00 mL of 50 mM potassium phosphate buffer (pH = 7.0). The reaction was started after adding 60 µL of 1 mM H2O2. The decrease in absorbance at 290 nm was recorded every 15 s. The APX activity was calculated using the extinction coefficient of 2.8 mM−1 cm−1. Guaiacol peroxidase activity was measured based on guaiacol oxidation according to MacAdam et al. (1992), The reaction mixture contained 3.0 mL of 50 mM potassium phosphate buffer (pH = 6), 40 µL of 30 mM H2O2 and 50 µL of 0.2 M guaiacol. The reaction was initiated by adding 100 µL of enzyme extract, and the absorbance was measured immediately and then every 15 s for 3 min. The rate of absorbance change was calculated, and the POD activity was determined using the extinction coefficient of 26.6 mM−1 cm−1. All enzyme activities are presented as nkat g−1 FW (‘nkat’ designates nanokatal, where one katal is the amount of enzyme that converts one mole of substrate per second) (Dybkaer 2001).
For metal analysis (Cs and K), the remaining plants at the end of the experiment were dried at 65°C in an oven (Eyela NDO-700, Tokyo, Japan) until a constant weight was achieved. A 20 mg dry sample was digested with 60 % HNO3 for 1 h at 125°C. After cooling, 30 % H2O2 was added and the mixture was further digested until it finished bubbling (Plank 1992). The extraction was adjusted to 10 mL using milli-Q water and used to measure K. Cs speciation (i.e., organically bound, inorganically bound and exchangeable) were determined using dry samples following the method described by Siong and Asaeda (2009). Briefly, dried sample was mixed with 10 mL of 1 M MgCl2 for 30 min to obtain the exchangeable fraction. The residue after former extraction was extracted using 10 mL of 1 M NaOAc for 5 h and the carbonate-bound fraction was obtained. The residue in the NaOAc was digested using a mixture of HNO3 and H2O2 to extract the organic-bound fraction. The solution was evaporated to approximately 5 mL and then diluted using distilled water to a final volume of 25 mL. The content of each Cs species was summed to determine the total Cs content. Air/acetylene flame atomic absorption spectrophotometry (Shimadzu AA-6300, Kyoto, Japan) was used for metal analyses according to standard methods (APHA 1998).
Quality assurance and control (QA/QC) procedures were carried out for Cs estimation from water and plant samples. Method detection limit (MLD) of Cs estimation by atomic absorption spectrophotometry was calculated from seven replicate analyses with 99 % confidence level. The MLD of Cs estimation was 0.0175 mg L−1. During Cs analysis by flame atomization, the matrix effects were negligible for both water samples and plant extract. The standard addition technique was used with atomic absorption spectrophotometry. The concentrations obtained for the standard reference material were always within the 95 % CI of certified values. Recalibration of Cs standards was performed after every 10 determinations.
All of the data were analyzed and figures were created by using R (R Development Core Team 2010). Data were presented as the mean ± standard deviation (SD) (n = 3). The homogeneity of variance test and Levine’s check for equality of variances were performed on the datasets prior to the statistical analysis to verify the assumptions of normal distribution and homogeneity of variances. Data recorded at the end of the experiment were subjected to a one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test to evaluate the mean difference at the 0.05 significance level. Pearson’s correlation analyses were conducted to determine the relationships between concentrations of cesium in media and growth, and biochemical parameters.
Results and Discussion
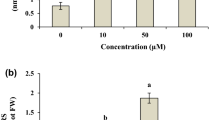
Plants grew and were alive until the end of the experiment (30 days) in all of the treatments, while the shoot lengths increased with increasing exposure duration irrespective of the treatments (Fig. 1). However, Cs exposure significantly affected the plant growth (F = 7.80, p = 0.01). The longest shoots were observed in the control, followed by the 0.001, 0.01 and 0.1 mg L−1 Cs treatments. The final lengths (length at harvest, Fig. 1) of the control plants were statistically similar to the shoot length of the 0.001 mg L−1 treatment. The shoot elongation rate (SER) of N. pseudoflabellata varied significantly among the treatments (F = 13.5, p < 0.01), with SERs in the control, 0.001, 0.01 and 0.1 mg L−1 Cs treatments of 2.2 ± 0.1, 2.1 ± 0.1, 1.9 ± 0.0 and 1.7 ± 0.1 mm day−1, respectively. The observed trend in SER was in close agreement with the trend observed for the final length (FL). The FL of the control plants was approximately 1.5-fold longer than that of the plants exposed to the highest Cs concentration (0.1 mg L−1) (Fig. 1). The trends in growth reduction for charophytes after exposure to chromium (0.8 mg L−1), cadmium (0.025–0.15 mg L−1) and zinc (0.15–1 mg L−1) for 35 days (Gomes and Asaeda 2009; Hawa Bibi et al. 2010; Siong and Asaeda 2009) were similar to the results in this study. Furthermore, the total length inhibition in Spiroplasma floricola was reported after exposure to ~1.13 mg L−1 Cs (Chang 1986). A similar growth reduction was also reported for Arabidopsis thaliana (Hampton et al. 2004). Even though the accumulation of excess metals in plants inhibits growth, some toxic metals appear to promote growth at very low concentrations. For example, Strauss (1980) observed a better growth rate in Chara fragilis and Chara vulgaris after growing in a medium that contained minute contents of Cs (0.007–0.003 mg L−1).
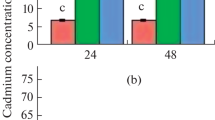
The Cs treated plants of this study (0.001–0.1 mg L−1) contained significantly higher concentrations of Cs (Fig. 2a) when compared to the control plants (F = 6.20, p = 0.05). In addition, there was a positive correlation between Cs accumulation and the exposure concentration (r = 0.81, p = 0.02). There was a decreasing trend in the K content in plants with increasing Cs concentration (Fig. 2b). The Cs+ ion shows similar properties as K+ and thus both ions compete with K+ binding sites; therefore, K starvation might occur in stressed plants (Hampton et al. 2004; Isaure et al. 2006). We observed a decreasing trend in the K content in plants with increasing Cs concentration (Fig. 2a). But the former trend was not statistically significant. The Cs accumulation might reduce the K+ uptake. As K is one of the major nutrients in plants, this may impact cellular metabolism, leading to reduced growth in Nitella.
According to the speciation analysis (Fig. 2b), the organically bound Cs fraction (Cs-ORG/Total Cs) of Nitella was significantly different among treatments (F = 6.68, p = 0.04). Further, this fraction was positively correlated to the total Cs content of the plant (R = 0.94, p < 0.01). Similar concentrations of exchangeable Cs (EX) were observed in the control, 0.001 and 0.01 mg L−1, while the plants in 0.1 mg L−1 had significantly higher concentrations of EX Cs (F = 28.2, p < 0.01). The organically bound Cs (Org) was assumed to gradually accumulate in plants with the Cs exposure (Fig. 2b), and this could be considered as a sign of the bioaccumulation of Cs. This fraction in Cs-treated plants was 60–80 µg g−1 DW, nearly three-fold higher than that of the control plants (~20 µg g−1 DW). However, the behavior of inorganically bound (IB) accumulation deviated from the former observations, as we observed an increasing trend followed by a decline at higher Cs exposure.
Photosynthesis plays a prime role in plant functioning, and thus maintenance of appropriate chlorophyll levels in plant cells is essential for plant functioning. Chlorophyll levels decreased in Nitella in response to Cs exposure (Fig. 3). Therefore, the reduced plant growth could also be associated with the reduced chlorophyll content in stressed Nitella. Cesium exposure significantly affected chlorophyll-a (F = 5.50, p = 0.02) and chlorophyll-b (F = 4.76, p = 0.04) concentrations (Fig. 3). The negative impact of Cs exposure on photosynthesis was further explained by the correlations with shoot length observed for chlorophyll-a (r = 0.62, p = 0.046) and chlorophyll-b (r = 0.55, p = 0.076). The reduction in chlorophyll and carotenoids in the Cs treated plants may be explained by the degradation of some enzymes, which were essential in pigment biosynthesis (Shalygo et al. 1997). Similar to the present study, concentration-dependent reduction of chlorophyll was observed in barley leaves after exposure to CsCl for 8 h (Shalygo et al. 1997).
The observed changes in pigments (caroten.: Carotenoids, Chl. a: chlorophyll-a, Chl. b: chlorophyll-b), chlorophyll fluorescence (Fv/Fm) and stress responses (H2O2 content, APX and POD activity). Different letters in each bar indicate treatments were significantly different based on ANOVA followed by Tukey’s post hoc test (p < 0.05)
Generally, stress-free plants exhibit the optimum value (0.83) of the maximum quantum yield of PSII [i.e., the efficiency of PSII (Fv/Fm)] for most plant species, whereas this optimum Fv/Fm ratio decreases when plants are stressed, indicating the phenomenon of photo-inhibition (Atapaththu and Asaeda 2015; Maxwell and Johnson 2000). The observed Fv/Fm ratios were significantly different among the treatments (F = 12.04, p < 0.01). The highest ratio was observed for the control plants (0.80 ± 0.01), which was close to the optimum value. Similar to the present study, lower Fv/Fm ratios have been reported for charophytes (Gomes and Asaeda 2009), algae (Lu et al. 2000) and aquatic macrophytes (Valderrama et al. 2013) in response to metal toxicity. Therefore, the observed reduction in Fv/Fm ratio suggests that photoinhibition occurred in Cs-treated Nitella.
When compared with the control group, the observed activities of CAT, POD and APX indicated the activation of oxidative defense mechanisms responding to Cs exposure (Fig. 3). The stress factors (i.e., response of antioxidant enzymes, efficiency of PS-II) are highly correlated to plant growth and photosynthesis. Plants produce different forms of reactive oxygen species in stress conditions, especially in chloroplasts, mitochondria, peroxisomes etc. Therefore, the metal induced oxidative stress might damage either the chlorophyll structure or chlorophyll membranes (Dinakar et al. 2012). This possibility would appear to be supported by the significant negative correlations in our study between chlorophyll-a concentration and APX and POD activities (Table 1). Further, chlorophyll fluorescence (Fv/Fm ratio) and SER were negatively correlated with CAT, POD and APX (Table 1). Therefore, the Cs-induced stress was considered to have impacted the photosynthetic mechanism of Nitella.
The APX activity of plants was significantly different among the treatments (F = 49.10, p = 0.00), and the activity of this enzyme increased responding to exposure concentration (Fig. 3). Further, the former relationship was clearly explained by the positive correlation between APX activity and the Cs content of plants (r = 0.79, p = 0.02). The APX activity at the 0.1 mg L−1 Cs treatment was approximately two-fold higher than that of the control group. Similarly, the CAT activity of plants was significantly different (F = 5.7, p = 0.02) among the treatments (Fig. 3), with the CAT activity of plants exposed to 0.1 mg L−1 Cs being approximately two-fold higher than that of the control plants (Fig. 3). The POD activity also varied significantly among the treatments (F = 6.35, p = 0.02), where elevated levels of POD activity were exhibited in plants exposed to 0.1 and 0.01 mg L−1 treatments. However, the POD activity of the plants exposed to the lowest Cs concentration (0.001 mg L−1) was not statistically different either from the control or other treatments.
Cs is known to be a potentially toxic mineral element that is released into the environment and taken up by plants (Qi et al. 2008). Due to the large hydrated ion radius of Cs, the free mobile single electron can react with water and oxygen to form reactive oxygen species (Sahr et al. 2005), leading to the activation of the anti-oxidative defense system in plants. The increased activity of antioxidant enzyme activities (POD, CAT and APX) indicated the activation of defense mechanisms against the Cs-induced oxidative stress in Nitella. Similarly, the Cs application resulted in the induction of peroxidases, catalases, and an increased amount of metabolites, such as glutathione, in other plants (Ghosh et al. 1993). In the present study, the H2O2 content was not significantly different among the four treatments (F = 2.65, p = 0.12).
Cs exposure negatively impacted the chlorophyll content and significantly reduced the growth of Nitella. The antioxidant activities (POD, CAT and APX) changed in an exposure dependent manner where elevated levels of activities were detected in plants exposed to the highest Cs (0.1 mg L−1) concentration. In summary, the Cs content of the stressed plants was significantly higher than that of the control plants. Cs (133Cs) induced oxidative stress and negatively affected photosynthetic function and growth in Nitella. However, the present study merely studied the effects of stable Cs on charophytes. Even though the ability of radioactive Cs (137Cs) elimination was reported for the charophyte; Chara braunii (Fukuda et al. 2014), the impacts of radioactive Cs on function and stress physiology of charophytes remain unclear, and further studies are recommended.
References
Aebi H (1984) Catalase in vitro. In: Lester P (ed) Methods in enzymology. Academic Press, Massachusetts, pp 121–126
APHA (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington
Atapaththu K, Asaeda T (2015) Growth and stress responses of Nuttall’s waterweed Elodea nuttallii (Planch) St. John to water movements. Hydrobiologia 747:217–233
ATSDR (2004) Toxicolgical profile for cesium, US Department of Health and Human Services. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=578&tid=107. Accessed 26 Nov 2015
Bennett ER, Moore MT, Cooper CM, Smith S, Shields FD, Drouillard KG, Schulz R (2005) Vegetated agricultural drainage ditches for the mitigation of pyrethroid-associated runoff. Environ Toxicol Chem 24:2121–2127
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14
Bystrzejewska-Piotrowska G, Drożdż A, Stęborowski R (2005) Resistance of heather plants (Calluna vulgaris L.) to cesium toxicity. Nukleonika 50:31–35
Chang CJ (1986) Inorganic salts and the growth of spiroplasmas. Can J Microbiol 32:861–866
Cook LL, Inouye RS, McGonigle TP, White GJ (2007) The distribution of stable cesium in soils and plants of the eastern Snake River Plain in southern Idaho. J Arid Environ 69:40–64
DeEll JR, Toivonen PMA (2003) Practical applications of chlorophyll fluorescence in plant biology. Springer, London
Dinakar C, Djilianov D, Bartels D (2012) Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Plant Sci 182:29–41
Dybkaer R (2001) Unit “katal” for catalytic activity. Pure Appl Chem 73:927–931
Folkard AM (2011) Vegetated flows in their environmental context: a review. Proc Inst Civ Eng Eng Comput Mech 164:3–24
Fukuda S, Iwamoto K, Atsumi M, Yokoyama A, Nakayama T, Ishida K, Inouye I, Shiraiwa Y (2014) Global searches for microalgae and aquatic plants that can eliminate radioactive cesium, iodine and strontium from the radio-polluted aquatic environment: a bioremediation strategy. J Plant Res 127:79–89
García A (1994) Charophyta: their use in paleolimnology. J Paleolimnol 10:43–52
Ghosh A, Sharma A, Talukder G (1993) Effects of cesium on cellular systems. Biol Trace Elem Res 38:165–203
Gomes PIA, Asaeda T (2009) Phycoremediation of chromium (VI) by Nitella and impact of calcium encrustation. J Hazard Mater 166:1332–1338
Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136:3824–3837
Hawa Bibi M, Asaeda T, Azim E (2010) Effects of Cd, Cr, and Zn on growth and metal accumulation in an aquatic macrophyte, Nitella graciliformis. J Chem Ecol 26:49–56
Isaure MP, Fraysse A, Devès G, Le Lay P, Fayard B, Susini J, Bourguignon J, Ortega R (2006) Micro-chemical imaging of cesium distribution in Arabidopsis thaliana plant and its interaction with potassium and essential trace elements. Biochimie 88:1583–1590
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Bot 12:345–354
Kanter U, Hauser A, Michalke B, Dräxl S, Schäffner AR (2010) Caesium and strontium accumulation in shoots of Arabidopsis thaliana: genetic and physiological aspects. J Exp Bot 61:3995–4009
Komarov E, Bennett BG (1983) Selected radionucleotides. World Health Organization, Geneva
Lu CM, Chau CW, Zhang JH (2000) Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium, S. platensis—assessment by chlorophyll fluorescence analysis. Chemosphere 41:191–196
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99:872–878
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nepf HM (2012) Hydrodynamics of vegetated channels. J Hydraul Res 50:262–279
Plank CO (ed) (1992) Plant analysis reference procedures for the southern region of the United States. The University of Georgia, Athens
Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP (2008) The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J Exp Bot 59:595–607
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 01 July 2011
Sahr T, Voigt G, Paretzke HG, Schramel P, Ernst D (2005) Caesium-affected gene expression in Arabidopsis thaliana. New Phytol 165:747–754
Shalygo NV, Averina NG, Grimm B, Mock H (1997) Influence of cesium on tetrapyrrole biosynthesis in etiolated and greening barley leaves. Physiol Plant 99:160–168
Siong K, Asaeda T (2009) Calcite encrustation in macro-algae Chara and its implication to the formation of carbonate-bound cadmium. J Hazard Mater 167:1237–1241
Strauss R (1980) Chara fragilis and Chara vulgaris were cultivated in a natural medium containing rubidium and cesium as chloride. Hydrobiologia 71:87–93
Valderrama A, Tapia J, Peñailillo P, Carvajal DE (2013) Water phytoremediation of cadmium and copper using Azolla filiculoides Lam. in a hydroponic system. Water Environ J 27:293–300
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
White PJ, Broadley MR (2000) Mechanisms of caesium uptake by plants. New Phytol 147:241–256
Zhu Y, Smolders E (2000) Plant uptake of radiocaesium: a review of mechanisms, regulation and application. J Exp Bot 51:1635–1645
Acknowledgments
This research was supported financially by grants from the Ministry of Education, Culture, Sports, Science and Technology (Research Grant-in-Aid), Japan; River Foundation and the Japanese Society for the Promotion of Science (Wakate-kenkyu B: 25820221). The assistance of others at the Ecological Engineering Laboratory, Saitama University, during laboratory analyses is gratefully acknowledged. Two anonymous reviewers are acknowledged for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atapaththu, K.S.S., Rashid, M.H. & Asaeda, T. Growth and Oxidative Stress of Brittlewort (Nitella pseudoflabellata) in Response to Cesium Exposure. Bull Environ Contam Toxicol 96, 347–353 (2016). https://doi.org/10.1007/s00128-016-1736-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1736-4