Abstract
Adult zebrafish pairs were exposed to sub-lethal concentrations of BaCl2 for 21 days, and the effects on reproduction, sex steroid hormones, and transcription of the genes belonging to the hypothalamic–pituitary–gonad (HPG) axis were investigated. The adverse effects on performances of F1 generation were further examined with or without subsequent exposure to BaCl2. Egg production was significantly decreased, and parental exposure to BaCl2 resulted in lesser rates of hatching. In males, exposure to BaCl2 resulted in greater concentrations of E2 along with greater mRNA expression of cyp19a. The results demonstrated that BaCl2 could modulate gene transcriptions and hormone production of the HPG axis in a sex-dependent way, which could cause adverse effects on reproduction and the development of offspring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Barium is known one of the most abundant elements of the Earth’s crust, and has been used in massive amount worldwide. In cosmetics and personal care products, barium sulfate is allowed to be used in the formulation of eye makeup, lipsticks, and skin products (Cosmetic Ingredient Review 2014). In Korea, face painting is a popular activity among children in various festival, however many of face paints currently on the market contained high level of barium (maximum 4325 ppm) which is greater than regulated safety limit (Korea Consumer Agency 2013). Continuous use of barium compounds in consumer products could led to release into the water environment, hence their potential impact on aquatic organisms is an important concern.
Various studies have reported that barium could cause numerous toxic effects. However, information on toxicity in freshwater organisms is mostly limited to acute lethal effects. For barium chloride (BaCl2), currently available chronic toxicity information only includes 21 days median effective concentration for immobilization (20.4 mg/L) and reproduction (13.4 mg/L) of Daphnia magna, and 30 days median lethal concentration (89.3 mg/L) of Orconectes limosus (Hazardous Substances Data Bank (HSDB) 2014).
In fish, reproductive system is primarily controlled by the hypothalamic–pituitary–gonadal axis (HPG) axis which is responsible for regulating gonadotropin and sex hormone dynamics (Nagahama and Yamashita 2008; Sofikitis et al. 2008). Environmental contaminants that disrupt at any point in this axis can adversely affect the function of endocrine system and subsequently affect reproduction. It has been previously shown that several environmental contaminants could disrupt production of steroid hormones or gene transcriptions in HPG axis, subsequently leading to decreased egg production and reduced hatching rates (Ji et al. 2013; Liu et al. 2013; Ma et al. 2012). Until now, the effects of barium compounds on reproduction, hormone production, and gene transcription of the HPG axis are not well understood.
To fill this knowledge gap, we investigated the endocrine disrupting effects of BaCl2 on HPG axis and reproduction of zebrafish. The developmental effects on F1 generation were further examined with or without exposure to BaCl2.
Materials and Methods
Barium chloride (CAS number 10361-37-2) was purchased from Sigma-Aldrich (St. Louise, MO, USA). To measure actual concentrations of the exposure media, water samples were collected from each tank for 3 times during the 21 days exposure period. To determine the content of barium, exposure media were analyzed by ICP-AES (Perkin Elmer Optima 4300 DV, Waltham, MA, USA) with the following conditions: RF power 1300 W, flow rate 1.5 mL/min, plasma gas flow 15 mL/min, auxiliary gas flow 0.2 mL/min, nebulizer gas flow 0.8 mL/min, and wavelength 233.57 nm. The limit of detection (LOD) and limit of quantification (LOQ) were calculated at a signal-to-noise ratio of 3 and 10, respectively. The LOD for barium was 0.0025 mg/L, and the LOQ was 0.0083 mg/L. The acceptance criteria for the quality control was below 10 % coefficient of variation, and were considered acceptable.
Adult zebrafish (Danio rerio; AB strain) were obtained from a commercial fishery (Green Fish, Seoul, Korea). The fish were acclimated in tanks containing dechlorinated tap water for 3 weeks at 28 ± 1°C under a photoperiod 16:8 h light/dark in Molecular and Environmental Toxicology Laboratory at Yongin University (Yongin, Korea). The fish were fed with commercially available mosquito larvae (Green Fish, Seoul, Korea) twice daily ad libitum, and the remaining food and feces were removed 30 min after the feeding.
In the F0 exposure study, 10 mating pairs per treatment group were exposed to control, 0.1, 1, or 10 mg/L BaCl2 for 21 days, following OECD test guideline 229 (OECD 2009). Definitive test concentrations for each compound were determined via preliminary range-finding tests. Two replicates with 5 mating pairs were used for each control or treatment, and fish were placed in a spawning aquarium (30 L tank with 25 L exposure medium). Exposure medium was renewed daily, by carefully decanting 50 % of old medium and adding freshly prepared test medium. Fish were fed with mosquito larvae twice daily. Dead fish were removed as soon as detected. The number of eggs spawned was counted and recorded daily. Exposure medium was routinely monitored for pH, conductivity, temperature, and dissolved oxygen. On conclusion of the exposure, all surviving fish were euthanized using 2-phenoxyethanol (Sigma-Aldrich, St. Louis, MO, USA). Gonad and total weight were measured for each fish, and gonadosomatic index (GSI; 100 × gonad weight/body weight) were calculated. For measurement of sex steroid hormones and gene transcription, 4 male and 4 female fish were randomly sampled from each treatment.
After exposure for 21 days, the tail of each zebrafish was transected. Blood per each fish was collected from caudal vein in a glass capillary tube, and centrifuged at 2000×g for 5 min. Plasma sample (1 μL) was dissolved in 120 μL ELISA buffer, and used to quantify E2 (Cat no. 582251) and T (Cat no. 582701) by use of ELISA kit (Cayman Chemical Company), following the manufacturer’s instructions.
Brain and gonad were collected from each fish after exposure for 21 days and reserved in 250 μL RNAlater reagent (QIAGEN, Korea Ltd., Seoul, Korea) at −20°C until analysis. Effects on steroidogenic transcription were evaluated as described elsewhere (Ji et al. 2013) with minor modification. Briefly, Transcriptions of 22 genes as well as two housekeeping gene including β-actin and 18s ribosomal RNA (18S rRNA) were measured. Total RNA was isolated from the sample by use of the RNeasy mini kit (QIAGEN). One micrograms of total RNA for each sample were used for reverse transcription by use of the iScript™ cDNA Synthesis Kit (BIORAD, Hercules, CA, USA). The LightCycler 480 System (Roche Diagnostics GmbH, Mannheim, Germany) was used to perform quantitative real time polymerase chain reaction (PCR). For quantification of PCR results, the threshold cycle (Ct) values for each gene of interest were normalized to that of reference genes using the delta delta Ct method. Normalized values were used to calculate the degree of up-regulation or down-regulation expressed as a fold difference compared to normalized control values.
On days 7, 8, and 13 of fish exposure, fertilized eggs were collected from each tank. Among them, forty-eight eggs were randomly selected per each tank, i.e., 96 eggs per each treatment group, and separately placed in 96-well plate (Corning Life Sciences, CA, USA) containing 250 μL exposure water (the same concentration) or clean water (control) for 6 days under static conditions. Hatching rate and time to hatch were also determined.
Statistical analyses were performed using SPSS® version 18.0 K, for Windows® (SPSS, Chicago, IL, USA). The value p less than 0.05 were considered statistically significant. Differences between control and exposure groups were investigated using Kruskal–Wallis test or one-way analysis of variance (ANOVA) with Dunnett’s test. Before conducting ANOVA, normality of distributions and homogeneity of variances were confirmed. To evaluate dose–response relationship, the linear regression analysis was conducted.
Results and Discussion
The present study demonstrates that BaCl2 has the potential to induce endocrine disruption in reproductive system by changing sex hormone homeostasis and accompanying genes along the HPG axis. The average measured concentrations for the nominal concentrations of 0.1 mg/L BaCl2 (0.06 mg Ba/L), 1 mg/L BaCl2 (0.66 mg Ba/L), and 10 mg/L BaCl2 (6.60 mg Ba/L) were 0.08, 0.42, and 1.66 mg Ba/L, respectively. Measured levels at the lowest concentration were within 20 % of difference from the nominal concentration. At higher concentrations, however, the measured concentrations were relatively lower than the nominal concentrations. The average measured concentrations were mainly used for presentation of the results throughout the present study.
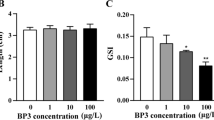
Dramatic decrease in egg production along with significant decrease in GSI of male zebrafish observed at ≥0.08 mg Ba/L (i.e., ≥0.1 mg/L BaCl2 for nominal concentration) (Fig. 1a, b) suggest that BaCl2 has the potential to inhibit the normal growth of gonad and further affect fertility. Gonadosomatic index, an index of gonad size relative to fish size, has been used as a biomarker to examine possible adverse effects of endocrine disrupting chemicals (Hutchinson et al. 2006). Alterations of GSI value have been reported in several fish species exposed to xenoestrogens such as E2 (Bjerselius et al. 2001) and 4-nonylphenol (Kang et al. 2003). Decreases in GSI may indicate alteration in the numbers and sizes of Sertoli cells and germ cells (Miles-Richardson et al. 1999; Kinnberg et al. 2000).
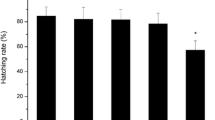
Important indicators for zebrafish embryogenesis, i.e., hatchability and time to hatch, were affected by parental exposure to BaCl2. Significant decrease in hatchability in eggs exposed to 1.66 mg Ba/L (10 mg/L BaCl2 for nominal concentration) continuously throughout the hatching period (Fig. 1c). In addition, parental exposure to BaCl2 fastened hatching period even when the eggs were transferred to clean water (Fig. 1d). The present observations clearly show that parental exposure to barium compounds could influence the development of the next generation embryo without the exposure.
Exposure to BaCl2 significantly modulated concentrations of sex hormones and transcripts of genes involved in HPG axis, and these effects were sex dependent. Sex steroid hormones (E2 and T) and their balance play important roles in sex differentiation, determination and maturation of teleost (Devlin and Nagahama 2002; Fenske and Segner 2004). Therefore, altered sex hormone levels observed in the present study may explain reproduction failure. In male fish exposed to BaCl2, significant increase of E2 at 1.66 mg Ba/L was accompanied by up-regulation of cyp19a gene, while no significant difference was observed in concentrations of T (Figs. 2a, b, 3b). These results indicate that changes in E2 hormones were regulated by cyp19 (aromatase) enzyme, which catalyzes the conversion of androgen to estrogen. In female fish, significant decrease of E2 was accompanied by down-regulation of cyp19a, which was the opposite direction to those observed in male fish (Figs. 2a, 3d). In addition, concentrations of T were significantly decreased following exposure to 1.66 mg Ba/L (Fig. 2b). This result was accompanied by the down-regulation of 17βhsd gene (Fig. 3d), which has an important role for basal biosynthesis of T. The reason for the sex-dependent alterations of the steroid hormones and steroidogenic gene transcriptions was not determined in the present study, and warrants further investigations.
Profiles of gene expression in zebrafish exposed to BaCl2. Observations in a male zebrafish brain, b male zebrafish gonad, c female zebrafish brain, and d female zebrafish gonad. The results are shown as mean ± SD, gene expression is expressed as fold change relative to control. Asterisk (*) indicates significant difference between exposure groups and the control group (p < 0.05)
The transcriptions of the genes involved in steroidogenic pathways were affected by exposure to BaCl2 (Fig. 3). In males, expressions of gnrh2, gnrhr1, fshβ, cyp19b, er2β, and ar mRNAs in brain, and fshr and cyp19a mRNAs in testis were significantly up-regulated by the exposure to BaCl2 (Fig. 3a, b). Expression of gnrhr2 mRNA in male zebrafish exposed to 1.66 mg Ba/L was significantly lesser compared to those in the control male fish (Fig. 3a). In females, expressions of gnrhr1 and gnrhr2 mRNAs in brain and cyp19a mRNA in ovary were significantly down-regulated (Fig. 3c, d). Expressions of 3βhsd and cyp17 mRNAs in ovary from female fish exposed to BaCl2 were significantly up-regulated in a dose–response manner (Fig. 3d). Gonadotropin hormones play important roles in regulation of spermatogenesis in male fish, by binding to receptors such as follicle-stimulating hormone receptor (fshr) and luteinizing hormone receptor (lhr). Up-regulation of gnrh2, gnrhr1, and fshβ genes in the male brain observed in the present study suggests possible change in spermatogenesis and subsequent reproductive dysfunction. The greater abundances of transcription level of fshr gene in the gonads from male fish might be a response to greater fshβ gene transcription of the pituitary. These results correspond well with the fact that exposure to BaCl2 could indirectly affect gonadotropin hormones.
Our results clearly show that chronic exposure to BaCl2 could affect reproduction, sex hormone production and the transcription of genes in HPG axis in fish, although the detailed mechanisms of sex dependent responses remain unknown. Further study on the crossover interactions with other endocrine systems at environmentally relevant concentration will provide more holistic understanding of the observed endocrine disruption by barium exposure.
References
Bjerselius R, Lundstedt-Enkel K, Olsén H, Mayer I, Dimberg K (2001) Male goldfish reproductive behaviour and physiology are severely affected by exogenous exposure to 17β-estradiol. Aquat Toxicol 53:139–152
Cosmetic Ingredient Review (2014) Safety assessment of barium sulfate as used in cosmetics
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Fenske M, Segner H (2004) Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat Toxicol 67:105–126
Hazardous Substances Data Bank (HSDB) (2014) http://toxnet.nlm.nih.gov/. Accessed 16 Jun 2014
Hutchinson TH, Ankley GI, Segner H, Tyler CR (2006) Screening and testing for endocrine disruption in fish-biomarkers as “signposts”, not “traffic lights”, in risk assessment. Environ Health Perspect 114:106–114
Ji K, Liu X, Lee S, Kang S, Kho Y, Giesy JP, Choi K (2013) Effects of non-steroidal anti-inflammatory drugs on hormones and genes of the hypothalamic–pituitary–gonad axis, and reproduction of zebrafish. J Hazard Mater 254–255:242–251
Kang IJ, Yokata H, Oshima Y, Tsuruda Y, Hano T, Maeda M, Imada N, Tadokoro H, Honjo T (2003) Effects of 4-nonylphenol on reproduction of Japanese medaka, Oryzias latipes. Environ Toxicol Chem 22:2438–2445
Kinnberg K, Korsgaard B, Bjerregaard P, Jespersen AS (2000) Effects of nonylphenol and 17β-estradiol on vitellogenin synthesis and testis morphology in male platyfish Xiphophorus maculates. J Exp Biol 203:171–181
Korea Consumer Agency (2013) Children’s face paint found to be toxic. http://www.kca.go.kr/brd/m_32/view.do?seq=1428&multi_itm_seq=8. Accessed 02 Jun 2015
Liu X, Ji K, Jo A, Moon HB, Choi K (2013) Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol 134–135:104–111
Ma Y, Han J, Guo Y, Lam PKS, Wu RSS, Giesy JP, Zhang X, Zhou B (2012) Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol. Aquat Toxicol 106–107:173–181
Miles-Richardson SR, Pierens SL, Nichols KM, Kramer VJ, Snyder SA, Render JA, Fitzgerald SD, Giesy JP (1999) Effects of waterborne exposure to 4-nonylphenol and nonylphenol ethoxylate on secondary sex characteristics and gonads of fathead minnows (Pimephales promelas). Environ Res 80:S122–S137
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50:S195–S219
OECD (2009) OECD guideline for testing of chemicals, test no. 229: fish short term reproduction assay. Paris, France
Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N (2008) Hormonal regulation of spermatogenesis and spermiogenesis. Steroid Biochem Mol Biol 109:323–330
Acknowledgments
This study was supported by National Research Foundation of Korea (NRF; Project No. 2013R1A1A1061684).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, B., Ha, N., Jung, J. et al. Effects of Barium Chloride Exposure on Hormones and Genes of the Hypothalamic–Pituitary–Gonad Axis, and Reproduction of Zebrafish (Danio rerio). Bull Environ Contam Toxicol 96, 341–346 (2016). https://doi.org/10.1007/s00128-016-1731-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1731-9