Abstract
Bisphenol F (BPF) has been frequently detected in various environmental compartments, and previous studies found that BPF exhibits similar estrogenic and anti-androgenic effects on the mammalian endocrine system to those of bisphenol A (BPA). However, the potential disrupting effects of BPF on aquatic organisms and the underling disrupting mechanisms have not been investigated. In this study, the potential disrupting mechanisms of BPF on the hypothalamic–pituitary–gonadal (HPG) axis and liver were probed by employing the OECD 21-day short-term fecundity assay in zebrafish. The results show that BPF exposure (1 mg/L) impaired the reproductive function of zebrafish, as exemplified by alterations to testicular and ovarian histology of the treated zebrafish. Homogenate testosterone (T) levels in male zebrafish decreased in a concentration-dependent manner, and 17β-estradiol (E2) levels increased significantly when fish were exposed to 0.1 and 1 mg/L BPF. The real-time polymerase chain reaction was performed to examine gene expression in the HPG axis and liver. Hepatic vitellogenin expression was significantly upregulated in males, suggesting that BPF possesses estrogenic activity. The disturbed hormone balance was enhanced by the significant changes in gene expression along the HPG axis. These alterations suggest that BPF leads to adverse effects on the endocrine system of teleost fish, and that these effects were more prominent in males than in females.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is an important raw material in the production of polycarbonate and epoxy plastics, as well as many consumer products, including baby bottles, food packaging, dental sealants, and thermal receipts. The results of recent in vivo and in vitro testing indicate that BPA is a weak estrogen that elicits multiple adverse effects on the animal endocrine system (Chen et al. 2016; Rochester 2013). BPA has been banned in certain products by several countries (European Commission 2011; FDA 2008). These stringent regulations have led to the development of alternatives, such as more heated-stable bisphenol analogs, e.g., bisphenol F (4, 4′-dihydroxydiphenylmethane, BPF). Notably, the molecular structures of these replacements are similar to BPA. Thus, whether these BPA replacements could also disturb the endocrine system has become an increasing concern.
BPF is used in several consumer products, such as lacquers, varnishes, liners, adhesives, plastics, water pipes, dental sealants, and food packaging (Rochester and Bolden 2015). As a result, BPF has already been detected in products and biota: e.g., foodstuffs (Liao and Kannan 2013; Zoller et al. 2015), household waste paper (Pivnenko et al. 2015), and human urine samples (Zhou et al. 2014; Yang et al. 2014b). BPF is the second-most abundant analog in a US survey of various food items (Chen et al. 2016). It is also detectable in various environmental compartments, such as indoor dust (Liao et al. 2012a), river and seawater (Yamazaki et al. 2015), sediments (Liao et al. 2012b), and municipal sewage sludge (Lee et al. 2015; Song et al. 2014b). BPF is frequently detected as the second-most abundant analogue in environmental samples after BPA. It is also the most abundant bisphenol analog in surface water from sites in Japan, Korea, and China, contributing >70% of the total concentration on average (Chen et al. 2016). As BPF has a marked structural resemblance to BPA, it may lead to similar adverse effects on the endocrine system as BPA. However, limited information is available on the hazards of BPF, until now. More importantly, the major focus of previous BPF studies was limited to test disruption of the mammalian endocrine system (Rochester and Bolden 2015). Considering that the aquatic environment is a major sink for BPF, it is of vital importance to evaluate the potential disrupting effects of BPF on aquatic organisms and to clarify the underling disrupting mechanisms.
Small freshwater fish, such as zebrafish (Danio rerio), are often appropriate models for investigating biological effects of endocrine-disrupting chemicals (EDCs) (Ankley and Villeneuve 2006; Segner 2009). Genes and sex steroid hormones in the hypothalamic–pituitary–gonadal (HPG) axis and liver may be affected by EDCs. Disruption at any point in this axis may adversely affect function of the endocrine system.
Albeit some recent studies investigated the endocrine disrupting potency of bisphenol substitutes in aquatic species notably in the zebrafish using both in vitro and in vivo models (Ji et al. 2013; Le Fol et al. 2017; Yang et al. 2017), the consequences of the estrogenic activity of BPF need to be further investigated by studying functional genes in the HPG axis. Therefore, the purpose of this study was to investigate the potential disrupting mechanisms of BPF on steroid hormone signaling pathways and functionally relevant genes along the HPG axis and liver of zebrafish. The effects of BPF on reproduction of adult fish were also investigated. In addition, the effects of BPF on embryo–larval development of the F1 generation were studied.
Materials and methods
Chemicals and instrumental analysis
BPF (CAS No. 620-92-8) was purchased from J&K Scientific Ltd. (Shanghai, China) and dissolved in dimethyl sulfoxide to form a stock solution (104 mg/L). All solvents used in this study were of analytical grade and purchased from Merck (Darmstadt, Germany). All chemicals had purity ≥98%. The water was deionized and purified by a Milli-Q plus system (Millipore, Billerica, MA, USA).
As half the water in each tank was renewed every 2 days, BPF concentrations in the exposure solution were determined only at the beginning of exposure and after 48 h of exposure (before renewing the exposure solution). The samples were analyzed by the following methods: water samples were filtered to remove particulates and then injected into Oasis HLB cartridges (Waters, Milford, MA, USA), which were conditioned with 5 mL methanol and 5 mL ultrapure water. Then, the filtrates were passed through the cartridges at a flow rate of about 5 mL/min. The extracts were eluted with 10 mL methanol/ultrapure water (99/1, v/v) after drying the solvent for 30 min under a gentle stream of N2. The residue was concentrated to 1 mL methanol for high performance liquid chromatography-tandem mass spectrometry (LC-Agilent 160 Technologies 1290 Infinity, MS-AB SCIEX QTRAP4500, Palo Alto, CA, USA) analysis. Details are shown in supporting information (Tables S1 and S2). The correlation coefficient (R 2) of the calibration curve was 0.998, and relative recovery rate was 86.3%, with a relative standard deviation of 9.2%. The BPF detection limit was 0.15 μg/L.
Fish maintenance and chemical exposure
Four-month-old male and female zebrafish (Danio rerio, AB strain) were acclimated in recirculating aquaria at 28 ± 0.5 °C for 2 weeks. The fish were maintained on a 16/8 h light/dark cycle and were fed twice daily with fresh Artemia sp. (nauplii). Six male and six female fish were selected randomly from the acclimatized fish and were placed in 10-L glass aquaria containing control, 0.001, 0.01, 0.1, and 1 mg/L BPF for 21 days following OECD guidelines 229 (OECD 2009a) and 230 (OECD 2009b). The range of exposure concentrations was based on acute toxicity testing and environmental concentration information (Chen et al. 2016; Yamazaki et al. 2015). The exposed and control groups received 0.01% (v/v) DMSO, which has been demonstrated not to affect reproduction in fish (Han et al. 2013). Each treatment group was comprised of two replicate aquaria. Half of the water in each tank was renewed every 2 days. During the exposure period, eggs were collected 1 h post-fertilization and cleaned with fresh water. The fertilized eggs were incubated in aerated tap water at 28 °C until 6 days post-fertilization (dpf).
Sampling
All fish were humanely sacrificed on ice after the 21 days of exposure. Body length and weight were recorded, and the condition factor (k = (weight (g) × 100)/(length (cm)3)) was calculated for each individual fish. Samples of the liver, gonad, and brain were collected and weighed. The hepatosomatic index (HSI, (liver weight (mg)/total weight (mg)) × 100)), gonadosomatic index (GSI, (gonad weight (mg)/total weight (mg)) × 100)), and brain somatic index (BSI, (brain weight (mg)/total weight (mg)) × 100)) were calculated for all fish. The liver and gonad were divided into two groups. In the first groups, the liver and gonad from three male and three female fish in each treatment group were fixed in 4% (w/v) paraformaldehyde. The fixed samples were stored overnight at 4 °C for histological analysis. In the second group, the liver, brain (including hypothalamus and pituitary), and gonad were preserved in RNA stabilization solution (Life Technology, Carlsbad, CA, USA) for later RNA extraction and gene expression analysis. Gene expression was measured in quadruplicate and repeated four times. The homogenates were prepared from the combined anterior and posterior parts of three males or three females in each treatment group at a rate that was 4× the tissue weight of ice-cold homogenization buffer (50 mM Tris–HCl, pH 7.4, and 1% protease inhibitor cocktail) and stored at −80 °C for later hormone measurements. Homogenates of the same gender from each exposure group were pooled as one replicate, measured in quadruplicate, and repeated four times.
Histological analysis
Samples fixed in paraformaldehyde were dehydrated in ethanol, cleared in xylene, embedded in paraffin, and cut into 5-μm sections for the histological analysis. The sections were stained with hematoxylin-eosin (HE) and observed under a microscope (Leica, Zena, Germany).
Hormone measurements
The homogenate was centrifuged at 12,000×g for 20 min at 4 °C. The supernatant was collected to detect levels of 17β-estradiol (E2) and testosterone (T) using specific enzyme-linked immunosorbent assays (ELISAs). The commercial kits for E2 (ml025762), and T (ml025781) were purchased from Shanghai Enzyme-linked Biotechnology (Shanghai, China), and all of the manufacturer’s instructions were followed.
RNA extraction and gene expression analysis
The expression of various genes with the functional processes of the HPG axis and liver were measured. The primer sequences for the genes measured are listed in Table S3. Total RNA was extracted from brains, gonads, and livers using trizol reagent according to the manufacturer’s protocol (Life Technologies), and purified RNA samples were stored at −80 °C until analysis. Total RNA (2 μg) was used to synthesize first-strand complementary DNA (cDNA) using the HIFI-MMLV First-Strand cDNA Synthesis Kit (Cwbio, Beijing, China). The cDNA samples were stored at −20 °C until further analysis.
The real-time polymerase chain reaction (PCR) analysis was performed on a CFX96 Real-Time PCR System (Bio-Rad, Singapore, Thailand) in 96-well PCR plates. The PCR reaction mixture for one reaction contained 10 μL of SYBR Green Master Mix (Bio-Rad), 2 μL of sense/antisense gene-specific primers (Genscript, Nanjing, China), and 8 μL of cDNA that was diluted in RNase-free water (Genray Biotech, Shanghai, China). The PCR reaction mix was denatured at 95 °C for 30 s before the first PCR cycle. The thermal cycle profile was as follows: denaturation at 95 °C for 5 s and annealing and extension at 60 °C for 5 s for a total of 40 PCR cycles. After the amplification reactions were completed, melting curves were generated to ensure amplification of a single product. The threshold cycle (Ct) was determined for each reaction to quantify the PCR results. Ct values for each gene of interest were normalized to the endogenous control gene β-actin using the 2−ΔΔCt method (Ji et al. 2013).
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistic 19 (IBM Corp., Armonk, NY, USA). All data are expressed as mean ± standard error. Homogeneity of variances was analyzed by Levene’s test. The Kolmogorov–Smirnov test was conducted to test the normality of the data. If necessary, data were log-transformed to approximate normality. Statistical differences were evaluated by one-way analysis of variance followed by Tukey’s post-hoc test. A P value <0.05 was considered significant.
Results
Chemical analysis
The actual BPF concentrations at the beginning of exposure were 0.00074, 0.0076, 0.081, and 0.79 mg/L in the 0.001, 0.01, 0.1, and 1 mg/L exposure groups, respectively (Table 1). After 48 h of exposure, the concentrations were 0.00069, 0.0068, 0.077, and 0.71 mg/L, respectively, with a decreasing trend but not significantly altered (Table 1). Concentrations in the control were below the limit of detection. Subsequent analyses of biological effects were based on the nominal concentrations for simplicity.
Mortality and growth
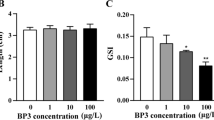
No mortalities were observed in any treatment during the exposure period. No significant differences in body weight or length of male or female zebrafish were observed in the BPF-treated groups. The effects of BPF exposure on k, BSI, HSI, and GSI of adult zebrafish are presented in Table 2. No evident differences in k or BSI were observed between the control and exposure groups. However, the HSI value of the 1 mg/L exposure group was significantly higher than that of the control group at the end of the exposure in male zebrafish, whereas GSI decreased significantly in males and females after exposure to 1 mg/L BPF.
Reproductive performance and F1 generation effects
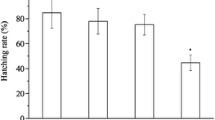
Egg production, hatching rate, and survival rate changed significantly in the 1 mg/L exposure group compared with those in control fish (Fig. 1). In addition, exposing parental fish to BPF resulted in malformed embryos and larvae (Fig. 2 and Table S4). As shown in Table S4, malformation rates, such as pericardial edema, tail malformation, and trunk curvature, in the 0.1 and 1 mg/L exposure groups, were significantly higher than those in the control group after 6 days, even when the eggs were transferred to clean water. These results clearly indicate that fecundity of parental fish and survival rates of their offspring decreased significantly after exposure to 1 mg/L BPF. The embryos that did not hatch within 6 dpf were dead.
Histological examination
Fertility can be influenced by alterations in oocyte competence and sperm quality or release (Daouk et al. 2011). Therefore, the morphology of the testis and ovary in adult male and female zebrafish exposed to 0.1 and 1 mg/L BPF was analyzed. Under normal conditions, sperm differentiation consists of four stages, namely, spermatogonia, early and late spermatocytes, spermatids, and spermatozoa. Similarly, follicles consist of four stages, such as pre-vitellogenic stages (I and II), vitellogenic III, and post-vitellogenic IV. Cysts in control testis contained all spermatogenic stages and were arranged in tubules, and the lumina were filled with spermatozoa (Fig. 3a). BPF caused a decrease in the number of spermatogonia and spermatocytes but increased the number of spermatids compared with those of control fish. An enlargement of the interstitial space was also observed in the exposed male fish (Fig. 3b). There were four clear follicular stages in the control female zebrafish (Fig. 4a); the 1 mg/L exposed ovaries showed a higher proportion of pre-vitellogenic stage I oocytes but lower proportions of pre-vitellogenic stage II, vitellogenic III, and post-vitellogenic IV oocytes. However, one ovary contained all stages of oocytes; the remaining two ovaries had a reduced number of post-vitellogenic oocytes. One even contained large numbers of pre-vitellogenic stages with nearly the absence of vitellogenic and post-vitellogenic stages in the 1 mg/L BPF exposed groups (Fig. 4b). No intersex individuals were found in any treatment.
Histology of the ovaries from adult female zebrafish from the control fish and 1 mg/L BPF exposed fish. a Ovary from a control female (HE stain, 20×). b 1 mg/L BPF exposed zebrafish (HE stain, 10×). c 1 mg/L BPF exposed zebrafish (HE stain, 20×). d 1 mg/L BPF exposed zebrafish (HE stain, 40×). The ovaries were categorized into pre-vitellogenic stages (I and II), vitellogenic (III), and post-vitellogenic (IV)
Hormone concentrations in the homogenate
Significant differences in the concentrations of steroid hormones were detected among the control and different zebrafish treatments. Exposing male fish to BPF resulted in a significant decrease in T concentration in the homogenate (75.98, 70.59, and 65.71% in the 0.001, 0.1, and 1 mg/L groups, respectively, compared with the control group) (Fig. 5a). In contrast, E2 concentration increased significantly by 26.51 and 28.4% in the 0.1 and 1 mg/L groups, respectively, compared with the control group (Fig. 5b). As a result, the T/E2 ratio in all exposure groups of male fish decreased significantly (Fig. 5c). A significant decrease in the T level was detected in the 1 mg/L BPF exposed female fish (Fig. 5a) whereas E2 increased significantly (Fig. 5b). The T/E2 ratio in female zebrafish was also significantly influenced by 1 mg/L BPF (Fig. 5c).
BPF-induced gene expression
The abundance of target gene transcription in males and females was generally affected after exposure to BPF (Fig. 6). However, the gene expression profiles and the magnitude of the effects differed between the sexes. In the livers, BPF exposure (≥0.01 mg/L) significantly increased vtg1 expression in a concentration-dependent manner in males, but no significant change in vtg1 expression was observed in females (Fig. 6a).
Gene expression of the HPG axis in zebrafish exposed to 0, 0.001, 0.01, 0.1, and 1 mg/L of BPF for 21 days. The results are shown as the mean ± SEM and expressed as fold change relative to the corresponding control. Asterisk indicates significant difference from the control (p < 0.05). a Vtg gene expression in male and female liver. b Male brain. c Female brain. d Male gonad. e Female gonad
In the brains, gene expression of the androgen receptor (AR), cyp19a1b (aromatase B), estrogen receptor (ER)2β, ERα, follicle-stimulating hormone (FSH)β, luteinizing hormone (LH)β, gonadotropin-releasing hormone (GnRH)2, GnRH3, gonadotropin-releasing hormone receptor (GnRHR)1, and GnRHR2 was examined. cyp19a1b, FSHβ, LHβ, GnRH2, GnRH3, GnRHR1, and GnRHR2 were significantly upregulated in a concentration-dependent manner in male zebrafish exposed to BPF (Fig. 6b). Exposure to all concentrations of BPF caused upregulation of cyp19a1b and FSHβ (Fig. 6b). AR and ER2β were not significantly affected by any of the treatments, whereas ERα increased significantly (Fig. 6b). cyp19a1b gene expression was significantly upregulated in the 0.1 mg/L-treated group of females (Fig. 6c). ERα gene expression was significantly upregulated in the 0.1 and 1 mg/L groups, whereas FSHβ gene expression was significantly downregulated (Fig. 6c).
In the gonads, the expression of 3β-hydroxysteroid dehydrogenase (3β-HSD), 17βHSD, cyp11a, cyp17, cyp19a, follicle-stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHR), and steroidogenic acute regulatory protein (StAR) was examined. In testis, expression of cyp17, 17βHSD, and StAR in the 1 mg/L exposed group was significantly downregulated, whereas cyp11a gene expression was significantly upregulated (Fig. 6d). Exposure to 0.1 and 1 mg/L BPF caused a significant upregulation of cyp19a, FSHR, and LHR (Fig. 6d). Significant downregulation of 17β-HSD and StAR gene expression was observed in the ovaries in the 0.1 and 1 mg/L exposed groups, whereas FSHR gene expression was upregulated (Fig. 6e). Exposure to 1 mg/L BPF caused significant upregulation of cyp11a expression and downregulated expression of LHR (Fig. 6e).
Discussion
The present study demonstrates that exposure to BPF impaired reproductive functions in zebrafish, caused a reduction in the number of eggs as well as hatching and survival rates, and increased malformation in the F1 generation. The histological examination revealed that exposure to BPF damaged the testis and ovary of treated zebrafish. The steroid hormone levels as well as gene expression in the HPG axis and liver were also significantly altered in male fish. These alterations suggest that BPF may lead to adverse effects on the endocrine system of teleost fish. Similar estrogenic activities of BPF were also observed from mammalian tests (Stroheker et al. 2003; Yamasaki et al. 2002, 2004).
In this study, zebrafish reproduction was disrupted by BPF. Previous studies have demonstrated that exposure to EDCs affects reproduction in teleost fish (Ji et al. 2013; Nash et al. 2004; Shi et al. 2015; Sohoni et al. 2001; Song et al. 2014a; Uren Webster et al. 2014). The survival rate of zebrafish larvae was significantly lower when the parents were exposed to 125 μg/L BPAF compared with the control group at 6 dpf (Shi et al. 2015). These bisphenols also produce a series of sublethal effects during development of zebrafish embryos, such as cardiac edema, delayed hatching, and malformations. These abnormalities were also found in the F1 generation in the current study. As the offspring were maintained in clean water, we conclude that the mortality and malformations were direct results of BPF exposure to the parental fish. Furthermore, Uren Webster et al. (2014) reported that exposure to roundup and glyphosate during gametogenesis increases early-stage zebrafish embryo mortality and premature hatching. In the present study, mature spermatids were the most frequent testicular stage and other stages were quite low in frequency. As a result, the balance between proliferating, differentiating, and maturing spermatogenic stages, as well as mature sperm, was disturbed. Similar effects have been reported previously in zebrafish treated with E2 (Christianson-Heiska et al. 2008) and guppies treated with octylphenol and E2 (Kinnberg et al. 2003). However, exposure to the BPA analog BPAF induced a significant reduction in spermatids (Yang et al. 2014a). The discrepancies in the gonadal effects in different studies may be due to structural differences in the compounds. BPF caused a depletion of late-stage oocytes, indicating that follicular growth and ovarian maturation were inhibited, which agrees with previous studies on other bisphenol analogs. BPA exposure resulted in severe deterioration of the ovary, e.g., increased number of atretic oocytes; structurally distorted and less developed oocytes were also observed, but the exposure concentrations were two to four times higher than those used in our study (Yön and Akbulut 2014). Yang et al. (2014a) also observed similar results when fish were exposed to 1 mg/L BPAF. In the present study, the reduced GSI value in males and females was due to a decrease in the number of viable oocytes and spermatogonia. A lower GSI value accompanies inhibited egg production in fish exposed to estrogenic compounds (Van den Belt et al. 2001; Ji et al. 2013).
The observed histological alterations can be explained by the negative feedback exerted by estrogens on gonadotropin (Viganò et al. 2010). Gonadal growth and maintenance are known to be under endocrine control. Steroid hormones exert both positive and negative feedback control on the synthesis and release of gonadotropins. Hence, the steroid hormones of exposed fish were determined. The results of our study showed that BPF exposure significantly decreased T production but enhanced E2 production in male and female zebrafish. However, the adverse effects of BPF on hormone levels in males were more serious than those in females. The mechanism of BPF to induce E2 may be attributed to the estrogenic properties of BPF reported by in vivo and in vitro studies. Goldinger et al. (2015) evaluated the steroidogenic effect of BPF and found that BPF increases E2 concentration but decreases free T level in the H295R steroidogenesis assay. Furthermore, the T/E2 ratio was significantly reduced. Previous studies have shown that exposure to bisphenols, e.g., BPA, BPS, and BPAF, disturbs the T and E2 balance (Mandich et al. 2007; Ji et al. 2013; Shi et al. 2015). The T/E2 ratio is a sensitive biomarker of abnormal steroid hormone secretion in fish. Disturbing the balance between T and E2 can affect reproduction, sexual development, gametogenesis, and sex determination (Shang et al. 2006.). Hence, the disrupted steroid hormone balance can explain the induced egg production and histological alterations in the gonads of zebrafish exposed to BPF. Steroid hormone synthesis and balance are generally regulated and controlled by genes in the HPG axis. In addition, the T/E2 ratio has been proposed to be an indicator of aromatase activity, the enzyme that transforms T to E2 (Liu et al. 2009). Hence, abundance of gene transcripts in the HPG axis and liver were quantified in both male and female zebrafish exposed to different concentrations of BPF.
Significant upregulation of vtg1 gene expression was observed in the livers of male fish in 0.01, 0.1, and 1 mg/L BPF exposure groups. However, no significant difference was observed in females. VTG is a biomarker for estrogenic endocrine disruption in males and is usually produced in response to stimulation by estrogenic chemicals. Hence, the increase in vtg1 gene expression coincided with the increased E2 level after exposure to BPF. In a recent study, 7-day exposure of adult male zebrafish to 0.02 mg/L of BPF resulted in plasmatic vitellogenin induction (Le Fol et al. 2017), which was consistent with our study to some extent. Previous studies have demonstrated that exposure to some bisphenols induces upregulation of vtg gene expression in male fish. Exposure to BPA (10, 200, or 400 μg/L) for 180 days increases the expression of vtg genes in F1 male zebrafish in a concentration-dependent manner (Keiter et al. 2012). Exposure to 1 mg/L BPAF for 28 days also significantly increased vtg gene expression in male zebrafish (Yang et al. 2014a). Moreover, life-long exposure to 25 and 125 μg/L BPAF induced an increase in VTG levels (Shi et al. 2015). Plasma VTG level increased significantly in BPS-treated zebrafish embryos but the male and female responses differed. BPS ≥10 μg/L caused a significant increase in plasma VTG level in females, whereas VTG level in males only increased significantly in response to 100 μg/L BPS (Naderi et al. 2014). As VTG is synthesized by the liver, the increased HSI value of exposed male fish may have been caused by the excessive expression of vtg1.
GnRH is the primary hormone regulating the synthesis and release of FSH and LH (Filby et al. 2008). Any alteration in the balance of GnRH and the GnRHR could lead to interruptions in the sex hormone balance (Liu et al. 2013). In the present study, GnRH2, GnRH3, GnRHR1, and GnRHR2 were significantly upregulated in the brains of male fish in the BPF-treated groups. This result suggests that GnRH concentration can be modulated by BPF, which could subsequently affect the production of gonadotropic hormones. Here, expression of FSHβ and LHβ in males was indeed upregulated. Previous studies have shown that exposure to other BPS and BPAF significantly increases GnRH, FSHβ, and LHβ in male zebrafish (Ji et al. 2013; Yang et al., 2015a). Upregulation of these genes in response to BPS corresponds well with the changes in gonadotropic hormones. The increased expression of these genes in response to BPAF and BPF was consistent with the observed changes in steroid hormones. In fish, the gonadotropins FSH and LH are secreted from the pituitary and act by binding to gonadal receptors, such as FSHR and LHR, to regulate steroidogenesis and gametogenesis in the gonads (Kwok et al. 2005; Andersson et al. 2009). The alterations in gonadotropins may be an important reason for the histological response of testes exposed to BPF. Ovarian follicular growth is primarily controlled by FSH, whereas oocyte maturation is primarily controlled by LH (Clelland and Peng 2009). In this study, significant downregulation of FSHβ gene expression in females after exposure to 1 mg/L BPF suggested possible delays in oogenesis and maturation. This finding was also revealed in the histological examination of ovaries exposed to BPF. ERα expression was significantly upregulated in BPF-exposed brains of males and females, whereas there were no changes in ER2β gene expression. ERα plays a more important role in autoregulation due to the increased E2 levels induced by BPF. One study demonstrated that BPF activates both human estrogen receptors (hERα and hERβ) but is more active in the hERα assay (Molinamolina et al. 2013). Furthermore, Joel et al. (2016) reported that BPF physically binds zebrafish estrogen receptor alpha (zfERα) both in in vitro ligand competition assays and in a functional cyp19a1b-luciferase reporter gene assay of glial cells. In contrast, no significant luciferase activity was found in cell transfected with the ERβ1 or ERβ2 subtype receptors upon stimulation with BPF. However, Le Fol et al. (2017) showed that BPF was slightly more potent towards zfERβs than zfERα by using a set of in vitro reporter gene assays based on stable expression of subtypes of zebrafish ER coupled to estrogen response element-driven luciferase in a zebrafish liver cell line and pointed out the variations between studies may be caused by different cell contexts related to the tissue and/or species of origin. No changes were observed in either ERα or ERβ gene expression in BPS- or BPAF-exposed zebrafish (Ji et al. 2013; Shi et al. 2015), suggesting that ERs are differentially regulated by BPA analogs. Yamaguchi et al. (2015) reported that bisphenol analogs, such as BPC and BPAF, interact with medaka (Oryzias latipes) and common carp (Cyprinus carpio) ERα with different interaction energies during an in silico docking simulation analysis and revealed that the differences in interaction energies and key amino acid residues between medaka and carp ERα may be involved in the species-specific differences of the interactions with BPA, BPAF, and BPC.
The expression of cyp11a in male zebrafish suggested an enhanced capacity for steroidogenesis. The downregulated expression of cyp17 and 17β-HSD in males, which converts progesterone to testosterone, directly caused the decline of T in males exposed to BPF. Aromatase (Cyp19) is the key enzyme in estrogen biosynthesis from testosterone. Our study demonstrated that cyp19a expression in males was significantly upregulated after exposure to BPF. Downregulation of the cyp17 and 17β-HSD genes and upregulation of the cyp19a gene are good evidence for decreased T and increased E2 levels and the disrupted steroid hormone balance.
In summary, our study demonstrated that exposure to 1 mg/L BPF impaired reproductive functions in zebrafish, by reducing the number of viable eggs, reducing hatching and survival rates, and increasing the malformation rate in F1 generation embryos and larvae. The histological examination revealed that exposure to BPF damaged the testis and ovary of treated zebrafish. T and E2 levels, as well as gene expression in the HPG axis and liver, were also significantly altered in male fish exposed to BPF. These alterations suggest that BPF adversely affects the endocrine system of teleost fish, and the effects were more prominent in males than in females. BPF has been frequently detected in environmental samples. Future investigations on long-term exposure of environmentally relevant concentrations of BPF, as well as effects on fish reproduction through disruption of the HPG axis in a systematic manner are necessary to predict the risk of BPF.
References
Andersson E, Nijenhuis W, Male R, Swanson P, Bogerd J, Taranger GL, Schulz RW (2009) Pharmacological characterization, localization and quantification of expression of gonadotropin receptors in Atlantic salmon (Salmo salar L.) ovaries. Gen Comp Endocrinol 163(3):329–339
Ankley GT, Villeneuve DL (2006) The fathead minnow in aquatic toxicology: past, present and future. Aquat Toxicol 78(1):91–102
Joel CN, Colette V, Elisabeth P, Charlier TD, Olivier K, Pascal C (2016) Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front Neurosci 10:112
Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol 50(11):5438–5453
Clelland E, Peng C (2009) Endocrine/paracrine control of zebrafish ovarian development. Mol Cell Endocrinol 312(1):42–52
Christianson-Heiska IL, Haavisto T, Paranko J, Bergelin E, Isomaa B (2008) Effects of the wood extractives dehydroabietic acid and betulinol on reproductive physiology of zebrafish (Danio rerio)—a two-generation study. Aquat Toxicol 86(3):388–396
Daouk T, Larcher T, Roupsard F, Lyphout L, Rigaud C, Ledevin M, Loizeau V, Cousin X (2011) Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat Toxicol 105(3):270–278
European Commission, 2011. Bisphenol a: EU ban on baby bottles to enter into force tomorrow
Filby AL, van Aerle R, Duitman J, Tyler CR (2008) The kisspeptin/gonadotropin-releasing hormone pathway and molecular signaling of puberty in fish. Biol Reprod 78(2):278–289
FDA (2008) Draft assessment of Bisphenol A for use in food contact applications. Food and Drug Administration, U.S.
Goldinger DM, Demierre AL, Zoller O, Rupp H, Reinhard H, Magnin R, Becker TW, Bourqui-Pittet M (2015) Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Reg Toxicol Pharmacol 71(3):453–462
Han XB, Yuen KW, Wu RS (2013) Polybrominated diphenyl ethers affect thereproduction and development, and alter the sex ratio of zebrafish (Daniorerio). Environ Pollut 182:120–126
Ji K, Hong S, Kho Y, Choi K (2013) Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol 47(15):8793–8800
Keiter S, Baumann L, Färber H, Holbech H, Skutlarek D, Engwall M et al (2012) Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol A (BPA) in zebrafish (Danio rerio). Aquat Toxicol 118–119:116–129
Kinnberg, K., Korsgaard, B., Bjerregaard, P. 2003. Effects of octylphenol and 17 beta-estradiol on the gonads of guppies (poecilia reticulata) exposed as adults via the water or as embryos via the mother. Comp Biochem Physiol Part C Toxicol Pharmacol 134(1), 45–55
Kwok HF, So WK, Wang Y, Ge W (2005) Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors—evidence for their distinct functions in follicle development. Biol Reprod 72(6):1370–1381
Lee S, Liao C, Song GJ, Ra K, Kannan K, Moon HB (2015) Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere 119:1000–1006
Le Fol V, Aïtaïssa S, Sonavane M, Porcher JM, Balaguer P, Cravedi JP, Zalko D, Brion F (2017) In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf 142:150–156
Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K (2012a) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46(16):9138–9145
Liao C, Liu F, Moon HB, Yamashita N, Yun S, Kannan K (2012b) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Scie Technol 46(21):11558–11565
Liao C, Kannan K (2013) Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 61(19):4655–4662
Liu C, Yu L, Deng J, Lam PK, Wu RS, Zhou B (2009) Waterborne exposure to fluorotelomer alcohol 6: 2 FTOH alters plasma sex hormone and gene transcription in the hypothalamic–pituitary–gonadal (HPG) axis of zebrafish. Aquat Toxicol 93(2):131–137
Liu X, Ji K, Jo A, Moon HB, Choi K (2013) Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol 134:104–111
Mandich A, Bottero S, Benfenati E, Cevasco A, Erratico C, Maggioni S, Massari A, Pedemonte F, Vigano L (2007) In vivo exposure of carp to graded concentrations of bisphenol A. Gen Comp Endocrinol 153(1):15–24
Molinamolina JM, Amaya E, Grimaldi M, Sáenz JM, Real M, Fernández MF, Balaguer P, Olea N (2013) In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–136
Naderi M, Wong MY, Gholami F (2014) Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol 148(2):195–203
Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, Allner PS, Tyler CR (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect:1725–1733
Organization for Economic Co-operation and Development (2009a) Fish short term reproduction assay. OECD guideline 229. Paris, France
Organization for Economic Co-operation and Development (2009b) 21-day Fish Assay. OECD guideline 230. Paris, France
Pivnenko K, Pedersen GA, Eriksson E, Astrup TF (2015) Bisphenol a and its structural analogues in household waste paper. Waste Manag 44:39–47
Rochester JR (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155
Rochester JR, Bolden AL (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650
Segner H (2009) Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol C 149(2):187–195
Shang EHH, Yu RMK, Wu RSS (2006) Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio). Environ Sci Technol 40:3118–3122
Shi J, Jiao Z, Zheng S, Li M, Zhang J, Feng Y, Yin J, Shao B (2015) Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 128:252–257
Sohoni PCRT, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Sumpter JP (2001) Reproductive effects of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas). Environ Sci Technol 35(14):2917–2925
Song M, Liang D, Liang Y, Chen M, Wang F, Wang H, Jiang G (2014a) Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 112:275–281
Song S, Song M, Zeng L, Wang T, Liu R, Ruan T, Jiang G (2014b) Occurrence and profiles of bisphenol analogues in municipal sewage sludge in china. Environ Pollut 186(1):14–19
Stroheker T, Chagnon MC, Pinnert MF, Berges R, Canivenc-Lavier MC (2003) Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol 17:421–432
Uren Webster TM, Laing LV, Florance H, Santos EM (2014) Effects of glyphosate and its formulation, roundup, on reproduction in zebrafish (Danio rerio). Environ Sci Technol 48(2):1271–1279
Van den Belt K, Verheyen R, Witters H (2001) Reproductive effects of ethynylestradiol and 4t-octylphenol on the zebrafish (Danio rerio). Arch Environ Contam Toxicol 41(4):458–467
Viganò L, Benfenati E, Bottero S, Cevasco A, Monteverde M, Mandich A (2010) Endocrine modulation, inhibition of ovarian development and hepatic alterations in rainbow trout exposed to polluted river water. Environ Pollut 158(12):3675–3683
Yamaguchi A, Ishibashi H, Arizono K, Tominaga N (2015) In vivo and in silico analyses of estrogenic potential of bisphenol analogs in medaka (oryzias latipes) and common carp (cyprinus carpio). Ecotoxicol Environ Saf 120:198–205
Yamasaki K, Noda S, Imatanaka N, Yakabe Y (2004) Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett 146:111–120
Yamasaki K, Takeyoshi Y, Yakabe Y, Sawaki M, Imatanaka N, Takatsuki M (2002) Comparison of gene reporter assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology 170:21–30
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PK, Moon HB, Jeong Y, Kannan P, Achyuthan H, Munuswamy N, Kannan K (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572
Yang Q, Yang X, Liu J, Ren W, Chen Y, Shen S (2017) Exposure to bisphenol B disrupts steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Water Air Soil Pollut 228(3):112
Yang X, Liu Y, Li J, Chen M, Peng D, Liang Y, Zhang J, Jiang G (2014b) Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ Toxicol 31(3):285–294
Yang Y, Guan J, Yin J, Shao B, Li H (2014a) Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 112:481–486
Yön ND, Akbulut C (2014) Histological changes in zebrafish (Danio rerio) ovaries following administration of bisphenol A. Pakistan J Zool 46(4):1153–1159
Zhou X, Kramer JP, Calafat AM, Ye X (2014) Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol a, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analytic Technologi Biomedical Life Sci 944:152–156
Zoller O, Brüschweiler BJ, Magnin R, Reinhard H, Rhyn P, Rupp H, Zeltnenr S, Felleisen R (2015) Natural occurrence of bisphenol F in mustard. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33(1):137–146
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (General Program) (No. BK20151100), National Natural Science Foundation of China (No. 21507038), and Graduate Research and Innovation Projects of Jiangsu Province (No. KYLX15_0813).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Cinta Porte
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Yang, X., Liu, J. et al. Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Environ Sci Pollut Res 24, 21311–21322 (2017). https://doi.org/10.1007/s11356-017-9773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9773-z