Abstract
This study focused on the analysis of organochlorine pesticides and PCBs in tissue samples of the clam, Ruditapes decussatus, in the Oualidia lagoon. Tissue assays were conducted during February to December 2005 and sediment (October 2005) was also tested. 13 organochlorine compounds and eight PCBs congeners were investigated, is HCB, γ-HCH, chlordane, cis-chlordane and trans-nonachlor, DDT and its metabolites DDD, DDE, heptachlor, its epoxide, mirex and PCBs (PCB28 + 50, PCB52, PCB101, PCB 118, PCB138, PCB153, PCB180). Analysis of these compounds was performed using a gas chromatography capillary column and an electron capture detector. Organochlorine contamination of clams and sediments in the lagoon did not exceed tolerable thresholds according to European standards. The levels of tPCB, tDDT and tOCP in clams are high at 49.4, 22.2, and 7.1 ng g−1 dw respectively. Concentrations of trans nonachlor and mirex are low compared to other chlorinated pesticides. PCB28 + 50, PCB52 and PCB101 show typical values in sediment, at 18.5, 10.8 and 17.8 ng g−1 dw respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Oualidia lagoon is an important water body on the west coast of Morocco; it has high amenity value and supports a commercial shellfishery. It is nevertheless exposed to various threats from pollution, including those caused by organochlorine pesticides and polychlorinated biphenyls (PCBs). Indeed, due to their high chemical stability and low biodegradability, contamination by these compounds can significantly alter physicochemical parameters for the marine biological components and the associated habitats within this lagoon. Their toxic effects can degrade filter and suspension feeding organisms and contamination can accumulate through the food chain (Custer et al. 2010a, b; Kelly et al. 2008). Investigations into the sampling station of Oualidia lagoon were partly motivated by the importance of the Oualidia wetland as a biological reserve of international importance according to Ramsar criteria. Economic interests are also important since the oyster and clam shell fisheries are exploited and have high commercial value. Since 1950, the lagoon has become the main traditional oyster center in Morocco. However, land near by the lagoon has been developed for intensive crop farming with heavy use of insecticides, pesticides and fungicides for weed control and for fighting animal pests (mosquitoes, slugs and snails). Sources of contamination by persistent organic compounds include the agricultural areas as well as local pharmaceutical and industrial activities (Kamara et al. 2005, 2008; Jayed et al. 2010). The problems are chronic and intensifying around the lagoon so that accumulation of many of these compounds was suspected in the lagoon sediments and in the marine organisms.
Located between two hills, the Oualidia lagoon is influenced by many small point water sources including seepage effluent from septic tanks. Socio-economic activities in the area are based on intensive agriculture, livestock, fishing, salt mining, tourism and oyster farming. This last activity exploits approximately one-sixth of the lagoon area. The production of oyster farming reached 300 tons/year in 2013. Local residents also exploit mussels on the rocks and reef flats (Perna perna and Mytilus galloprovincialis). Additionally collecting clams (Ruditapes decussatus) is an important cottage industry on the lagoon marshes (Kamara et al. 2008). Shellfish production in the Oualida lagoon area has been monitored since 2009.
Organochlorine pesticides are in widespread use in Morocco and elsewhere in the world. These compounds are typically very persistent in the environment, and are well known to accumulate in sediments, plants and animals (Naso et al. 2005; Jayed 2011; Jayed et al. 2010). Organochlorines produce a wide-range of acute and chronic health effects, including cancer, neurological damage, and birth defects. Many organochlorines are suspected endocrine disruptors.
To help to rehabilitate and safeguard the lagoon, a monitoring network for coastal health was initiated by the Institute of Fisheries at Casablanca. Integrated actions in the Oualidia area include a multidisciplinary study of the watershed and adjacent areas. The various activities revolve around routine health monitoring of oyster farming in oyster parks and other lagoon fishery resources. This study on the bioaccumulation of chlorinated pesticides and PCBs was undertaken as part of Oualidia area action plan and aims to assess the current state of contamination by organochlorine pesticides (OCPs) and PCBs and so contributes to ensuring biosecurity of the rich natural lagoonal shellfish resource. The study also undertakes to determine the extent of contamination by sampling and assaying samples of the mollusk R. decussatus and of sediment collected over a period of 1 year.
Materials and Methods
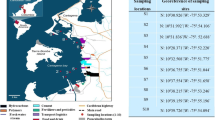
One representative station where R. decussatus was common was used for monthly sampling during 10 months from February to December 2005 (Fig. 1). During this period the levels of organochlorine pesticides and PCBs bioaccumulated in the tissues of the bivalve and/or trapped in fine sediment particles were monitored. The Oualidia ‘oyster park’ 7′ (32°44′50″N–09°01′52″W) sampling station (Fig. 1) was selected because of the abundance of clams and the muddy sandy substrate (Fig. 1). This bivalve was chosen as a sentinel species, due to its high commercial value, its presence in the lagoon due to its abundance in the muddy sand substrate and its economic interest (complex food chain up to the man). Very few studies that have examined contamination of this species by organochlorine pesticides and PCBs in Morocco and the Mediterranean (Benbakhta et al. 2007).
The sampling strategy was based on collecting a sufficient number (400 specimens) of living clams, for monthly analysis. In the laboratory, the samples were purified in seawater for 24 h and divided into two size classes, 20–30 mm and from 30 to 45 mm in length. Measurements were performed using a caliper (1/10 mm). Length is the largest dimension parallel to the axis of the hinge between the front edge of the rear edge of the shell. 20 individuals of each size class were then used for calculating the condition index CI = MDW/SDW × 1000, where MDW is meat dry weight (g) and SDW is the shell dry weight (g), following Beninger and Lukas (1984); Lucas and Beninger (1985); Orban et al. (2002); the remaining of the sample was peeled, crushed and stored at −18°C before being lyophilized. Representative samples of surface sediments from station 7 in the lagoon where clams are common were collected. The textual nature of these samples were considered to be representative of this area of the lagoon. Sediment sampled was homogenous being well mixed by biological and physical processes. Samples were combined into one bulk sample of approximately 1 kg. From which the <63 grain size fraction was extracted. This extraction was sub-sampled for analysis. Samples were collected by Eckman dredge at the same sampling point from where the clams were collected in October 2011. Samples were combined into a single sample of approximately 1 kg wet sediment that comprised the top ca. 2 cm of the surface substrate. The aim was to check for trapping of chlorinated pesticides in the sediments and to link the concentrations of PCBs compounds to those in the clam tissues. Samples were stored in aluminum foil and dried in an oven at a constant temperature of 60°C for 24 h. Chlorinated pesticides were analyzed in the clay–silt fraction (<63 μ) (Léaute 2008; Benbakhta et al. 2007). The studied organic contaminants are lipophilic and are stored in fatty tissues of marine organism filter feeders. Concentrations in the mollusc tissues were determined using the method of the International Atomic Energy Agency Monaco. It involves extraction of the lipids with a mixture of organic solvents hexane and acetone (90/10), using microwave field. The extract is filtered through cotton glass, and this phase is brought to dryness. The lipid content is determined gravimetrically (Phillips et al. 1997).
The protocol used was for biological samples loaded with lipids where quantities exceeded 25 % dry weight. Some methods require purification to remove the interference by fat to achieve a crystal clear extract and a ‘cleaner’ chromatogram. The extraction and purification protocol used therefore was Accelerated Solvent Extraction Dionex ASE 200, (Voisins le Bretonneux, France). This method reduces the number of purification steps compared to other methods required for these organochlorine compounds (Tapie 2006).
Prior to extraction, 22 mL cells of the Dionex ASE were prepared with cellulose filters, 2.5 g of silica covered with an acidified cellulose filter. The acidified silica was previously prepared by mixing sulfuric acid of high purity, with silica gel in the following proportions: 40–60 g of acid silica. The mixture is then placed at 200°C for 12 h. 0.5 g of samples were then introduced into the cell by gravimetry; glass beads added, then 20 μL of internal standard containing four PCBs (PCB30, PCB103, PCB155, PCB198) and 4,4′DDTd8 were gravimetrically added to the matrix prior to extraction. The sample was extracted with dichloromethane (Ultra resin Analyzed, Atlantic Lab, Blanquefort, France) according to the following procedure: 100 % dichloromethane, one cycle, heating time 5 min, static phase 8 min, flush volume 60 % purge time 60 s, pressure 130 bar, 100°C. The PCB congeners and the internal standards were obtained from Cluzeau info lab (Sainte Foy la Grande, France, purity 99 %).
The organic extract, already partially purified by silicic acid, is recovered and re-concentrated by an evaporator under vacuum (RapidVap Labconco, Kansas City, USA) after addition of 1 mL of isooctane. The evaporation conditions are: agitation rate 70 %, temperature 51°C, 20 min time re-concentration and 900 mbar vacuum and the extract was dissolved in iso-octane (0.5 mL) for purification. The extracted once re-concentrated (0.5 mL) was put in the column heading, and then eluted three times with 5 mL of pentane/dichloromethane (90:10, v:v). The eluate was collected, re-concentrated and dissolved in 90 µL of isooctane. A standard yield (octachloronaphtalène OCN) is added gravimetrically (25 mµ L) to calculate the extraction yield for each sample and thus validate each measurement. Once the sample was purified, it was injected into gas chromatograph type HP 5890 coupled to an electron capture detector (GC/ECD) and capillary column type HP-5MS (J & W Scientific, USA), length 30 m, internal diameter 0.25 mm, 0.25 μm thick film of 5 % Phenyl Methyl Siloxane as stationary phase. The chromatographic conditions for the analysis of chlorinated pesticides were: helium 1 mL/mn (Heb 5.6 quality, Linde, Toulouse, France), nitrogen at 60 mL/min (N2 gas Linde 5.0 quality, Toulouse, France), splitless (1 µL), injector temperature 250°C, detector temperature 280°C, the initial oven temperature at 60°C. The temperature program was 60°C for 2 min, 5°C/min up to 280°C and 10 min at 280°C.
For the analysis of PCBs, the chromatographic conditions were the same except for the temperature of the injector (280°C), the carrier gas (flow rate of 1.3 mL/min) and the initial oven temperature (80°C). The temperature program was also different using 80°C for 2 min, 5°C/min up to 320 and 320°C for 5 min.
The concentrations of HCB, γ-HCH, cis-chlordane and trans-nonachlor, DDT and its metabolites DDD and DDE, heptachlor and its epoxide, mirex and PCBs (CB 28 + 50, PCB 52, PCB 101, PCB 118, PCB 138, PCB 153, PCB 180) were measured by the following method. The response factor for the various compounds was determined by injecting a standard reference material SRM 2962 for PCBs and SRM 2961 for OCPs, provided by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA), the same solution spiked with internal standard PCB 30, PCB 103, PCB 155, PCB 198, which was used for the doping of the samples, in the same chromatographic conditions. Iso-octane was injected as a blank between each sample injection to ensure the cleanliness of the injector GC ECD. After injection, the chromatographic concentration results were calculated using GC ChemStation Software Version: G2090 BA Revision B 031.
In addition to white analysis (representing <10 % of the content of the sample) and in order to ensure the quality of the analyses, a double calibration was established at the level of quantization of PCBs and organochlorines. A performance standard of OCN (Octachloronaphtalène) was added to each sample to quantify the internal standards added initially and thus calculate an extraction yield for each sample. The extraction yields were constant over time and above 70 %. The validity of this method for the analysis of chlorinated pesticides and PCBs was confirmed by extraction and analysis of a certified matrix SRM 2977 (Mussel tissue, P. perna from Guanabara Bay, the Brazil) provided by NIST (Gaithersburg, MD, USA).
Analysis of variance (ANOVA F—1 HyperStat Online) was used to test variation between the analyzed compounds and according to season. The homogeneity of variance was tested by the Fisher–Snedecor test Fmax (p > 0.05).
Results and Discussion
Sediment Contamination
PCBs are presented as the sum of eight congeners (tPCB8) compounds used as indicators in the framework of the monitoring of the marine environment: these congeners are CB28 50, 52, 101, 118, 138, 153, 180. In general, hydrophobic organic contaminants (HOC) are mainly associated with the fine fraction <63 µm of marine sediments (Léaute 2008). This fraction was used in this study and, according to the results in Fig. 2, the level of contamination in the lagoon sediments by PCBs is relatively low. The concentrations of the eight congeners (tPCB8) are between 0.6 and 18.6 ng/g dw. This range is much lower than those observed in lagoons, estuaries and lakes of France and Spain values (Léaute 2008, Ifremer 2008). In these European coastal ecosystems, contamination of surface sediments, represented by the congener CB153, is generally considered a chemically persistent and major compound.
Congener PCB28 + 50 and PCB101 are well represented in the sediment sample with 18.6, 17.8 ng/g dw, respectively, followed by PCB52 with 10.8 ng/g dw. Their presence demonstrates industrial activities as the cause of the contamination and its dispersion in coastal marine ecosystems; industrial activities are only the source of these congeners. The contents of the other congeners vary between 2 and 4.2 ng/g dw. The PCB153 and PCB138 congeners are highly chlorinated compounds and are represented by very low levels in the Oualidia sediment sample. PCB180 shows negligible concentrations not exceeding 0.6 ng/g dw in the sample.
According to Pierard and Guarrigues (1996), the distribution profiles of PCBs levels are closely linked to the nature of the sediment (sand or mud). Congeners of lower chlorinated PCBs (3–4 Cl atoms) tend to settle on less sandy sediments and concentrate in muddy sediments. Given that the concentration of contaminants is linked to organic carbon and sediment grain size (Pierard and Guarrigues 1996), it is necessary to study more thoroughly the accumulation and bioavailability of these organic substances inthe lagoon sediments.
Tissue Contamination
The accumulation of PCBs in adipose tissue depends on the octanol—water relationship, log Kow (Kow = coefficient octanol/water: This is the relationship between the equilibrium concentration of a chemical in octanol and the same concentration of this substance in water. It is used to estimate indirectly the sorption of organic material in soil or bio concentration factor (BCF) in organisms.
Most organic pollutants have a strong tendency, when dispersed in water or after ingestion, to accumulate in animal fats (e.g. polychlorinated biphenyls in fat of fish and sea mammals cold) (Léaute 2008). This is due to the hydrophobic nature of these molecules. In practice, we consider that the octanol extraction well represents the animal lipid weight.
Kow will therefore provide an idea of the contaminant’s ability to accumulate in living organisms according to their lipid mass. It can be used to determine the behavior of the pollutant in the environment at the interface compartment with “biomass” in addition to information obtained at the interface between the various other compartments (water, air, soil) the Koc and partial pressures of the pollutant can be considered (Koc = partition coefficient organic carbon). It expresses the ratio between the amount of a compound adsorbed per unit weight of organic carbon in the soil or sediment and the concentration of the same compound in aqueous solution at equilibrium). Finally, modeling the behavior of the pollutant in the environment is helpful in assessing the probability of pollutant effects on humans. Toxicological data on the pollutant are used to estimate ecotoxicological risk for a given area. The potential for an organochlorine compound to be adsorbed onto soil depends on its physico-chemical properties and of the organic carbon content of the soil or sediment. This coefficient can be used to determine the distribution of a compound between the water and the solid) (Léaute 2008). Not all pollutants behave as described above (showing affinity for lipids), some have a so-called “pharmacological” effect due to their attachment to specific receptors in the body. In this case, they do not obey the model of oil/water distribution but the law of mass action is more applicable. Consequently, determining the bioaccumulation potential using the Kow statistic has limitations especially regarding certain water-soluble molecules (atrazine) (Léaute 2008).
The physico-chemical properties and toxicity of different congeners on organisms will be determined by the number and position of chlorine atoms on the molecule. PCBs, sparingly soluble in water, have a low Kow between 4–8 and tend to bioaccumulate significantly (Schwarzenbach et al. 2003). They are persistent and can thus be transported along the food chain. Their solubility varies depending on the position of the chlorine atoms and decreases when the number of chlorine atoms increases (Borja et al. 2005). When a mixture contains molecules more weakly chlorinated, the toxicity tends to increase. Bioaccumulation factor (BAF) is the ratio between the concentration of a substance in an organism and its concentration in water or sediment, given the direct absorption from the environment and food. Figure 3 shows the correlation between the bioaccumulation factor FBA and the values of Log Kow in the data from Oualidia Here there is a significant positive correlation between BAF and log Kow of eight congeners of PCBs (Fig. 3). This indicates a good balance between the concentrations of PCBs in the environment and concentrations in the tissues of the bivalve population. Profile contents PCBs, recorded in clams, is consistent with that of the distribution of the same congeners in surface sediments.
The occurrences and variations of PCBs concentrations in lagoon sediments concentrations can be explained by the very low rate of degradation of these compounds. Although not directly comparable the tPCB concentrations in (0, 63 µm) sediment and clam lipids were similar. The tPCB clam tissue concentration is noticeable less than that of the sediment. Extremely soluble in fat, PCBs bioconcentrate in organisms and accumulate in the food chain so posing serious problems of toxicity in the long term (Jayed 2011). Because of the ubiquitous nature of PCBs, their toxicity justifies classification of these compounds as persistent organic pollutants (POPs) and their inclusion as priority substances on the lists of the European Commission and the World Health Organization. Moroccan lagoons have not escaped from contamination by these pollutants and their presence in coastal ecosystems is of concern regarding environmental risk assessments (Léaute 2008).
Total concentrations of organochlorine compounds bioaccumulated in clam lipid tissue are illustrated in Fig. 4. tOCP includes the following compounds: Lindan HCB + Heptachlor + Heptachlor epoxide + Cis chlordane + Trans Nanochlor + Mirex. The occurrences of this set of contaminants are poorly represented in clams of the lagoon of Oualidia throughout the annual cycle.
Unlike and compared to tOCP (total organochlorine), the tDDT contents (2,4’-DDE + 2,4′DDD + 2,4’DDT + 4,4′DDE + 4,4’DDD 4.4’DDT) are significantly higher throughout the year (from 9.7 to 22.29 ng g−1 dw). There was a slight increase between July and October and a tendency to decrease from February to May. tPCB contamination (PCB28 + 50 + PCB52 + PCB101 + PCB118 + PCB153 + PCB138 + PCB180) is clearly demonstrated in the lagoon (Fig. 4). The maximum value is of the order of 50 ng/g in September and values are maintained between 25 and 35 ng/g between October and December. Between February and July, the concentrations are less important in this lagoon (12–22 ng/g dw). Concentrations fluctuate during the annual cycle by gradually decreasing from September. Standard deviations (Table 1) show clear significance of the seasonal fluctuations observed especially in the pesticides concentrations recorded in the bivalve tissues. The average concentrations of the tOCP, tDDT and tPCB are:
tOCP = 4.37 ng/g dw; tDDT = 14.26 ng/g dw and tPCB = 24.00 ng/g dw.
Average concentrations of three groups of target compounds are lower than those found in the lagoon of Moulay Bousselham (North West of Morocco). This latter site collects all the pesticide releases from agricultural drainage of the Rharb plain; the drainage water is loaded with pesticides and fertilizers (Jayed 2011). Benbakhta et al. (2007) confirmed this difference in levels between the stations studied in the latter lagoon and recorded levels of around 50 ng/g OCPs in May. Serghini et al. (2004) reported values ranging from 400 to 1800 ng/g tDDT and tOCP in eels issued from Moulay Bousselham lagoon. Thompson (2005) noted in clams from Arcachon in France, PCBs values ranging from 7.2 to 67.2 ng/g, and that tDDT content not exceed 8.7 ng/g. In the Egyptian coast of the Red Sea, Khaled et al. (2004) reported concentrations of tOCP ranging from 543 to 3356 ng/g in Brachiodontes sp. In the Gulf of Naples, Naso et al. (2005) reported a concentration of 177.2 ng/g in M. galloprovincialis and Stefanelli et al. (2004) cited a concentration of 144 ng/g in the same species in the Adriatic Sea. These levels show the considerable extent of bioaccumulation of these compounds by various marine organisms and indicate long-term health risks. The above authors agree bioaccumulation of OCPs and PCBs contaminants tend to bioaccumulate in fatty tissues of organisms (Khaled et al. 2004. Stefanelli et al. 2004; Naso et al. 2005; Léaute 2008). Lipid marine bivalves may in some cases be the cause of changes, affecting organochlorine residues in sediment observed during the annual cycle at the different sites. In this regard, we determined the seasonal levels of lipids in the whole freeze-dried flesh of clams. Figure 5 plots the monthly changes in lipid contents and condition index in clams.
Profiles of lipid contents vary slightly according to the annual cycle, the fat reserves in clams are maximum and minimum before breeding after spawning without being totally exhausted during gametogenesis (Fig. 5). The condition index changes confirm the main breeding season in the spring for the clam. Kamara et al. (2008) noted that the species breeds mainly in spring with an extension to the summer in the Oualidia lagoon. The lipid concentrations fluctuate between 0.014 and 0.06 g/g dw, demonstrating the presence of fat reserves in clams throughout the year. Comparison of the amounts of contaminants (tDDT, tPCB, tOCP) with lipid levels shows a low positive correlation (0.008–0.29). However the importance of the variance of organochlorine compounds concentrations in clams can be partially explained by the proportions of lipid. In order to check the difference between the clam BAF results, we split the sample into two size classes: Class 1 (T1) includes individuals from 20 to 30 mm (immature) and class 2 (T2) includes individuals from 30 to 45 mm, considered to be mature.
Clam body size may have a slight influence on the bioaccumulation factor (Thompson 2005), however the results here do not show any significant variation (p > 0.05) for all the families of contaminants (Fig. 6). Profiles levels are comparable between the two size classes. Comparing the bioaccumulation factor between the two size classes was also monitored monthly during the study period and again the results were not statistically significant. Thompson (2005) also showed an exponential correlation (R = 0.93) between levels of PCBs and the average class size of clams (20–25, 25–30, 30–35 and 35–40 mm) only for the month of September, no correlation was found in other months.
Other organochlorines (HCB, lindane, heptachlor, heptachlor epoxide, cis-chlordane, trans-nonachlor and mirex) are not accumulated in the adipose tissue of either size groups studied. They did not exceed 2.5 ng/g dw for all compounds except HCB which reached 4.5 ng/g dw. These are very low compared to those observed in other lagoons in the Mediterranean levels (Naso et al. 2005; Benbakhta et al. 2007).
Conclusion
POP oncentrations in the Oualidia lagoon vary according to the three groups of compounds (OCP, DDT and PCBs). The lagoon is particularly contaminated by tPCB, followed by tDDT while tOCP contents are very low. The cyclopentadiene compounds (aldrin, heptachlor epoxide and heptachore) are present in trace amounts or are even absent in some samples implying that exposure to these products has ceased. The size of the clams did not appear to influence the accumulation factor in this case study. Indeed, the results obtained for the mature and immature size groups did not show a significant variation (p > 0.05) for any contaminants. The concentration profiles for the two size classes do not show any statistically significant variations.
Contamination levels in the Oualidia lagoon are however low overall and generally below those identified in other parts of Morocco and the Mediterranean for bivalves and fish. Indeed, levels are below the thresholds of residues allowed in the standards set by international organizations: Aldrin + Dieldrin <10 mg/kg, Chlordane <50 mg/kg, DDT + DDD + DDE <200 mg/kg, Heptachlor <10 mg/kg, PCBs (total) <10 mg/kg (WHO, FAO, UNEP and the EU). Thus, the risk to consumers of shellfish remains low in the current state; nevertheless, it is prudent for the users of these toxic materials, such as farmers and industrialists, to minimize usage to reduce effects on organisms and to maintain environment awareness about public health. The currently low contamination of bivalves by OCPs and PCBs can be explained by the prohibition of the use of certain products in Morocco since 1984. But the fact that they are still detected, could be explained by the illegal use of some old stocks of these compounds in different regions of Morocco.
This study confirms the occurrence of the main compounds of organochlorine pesticides in the Oualidia Lagoon and attributes this to industrial and agricultural activities. At present, the ranges of values appear to be acceptable within known limits and almost all the contamination levels are lower than in the Sidi Moussa Lagoon (Morocco). However, more monitoring will be programmed in the future for surveillance purposes. Furthermore, it would be useful to extend this to other coastal areas of Morocco. The results presented here are the first extensive report concerning contamination of bivalves by organochlorine compounds in Oualidia Lagoon.
References
Benbakhta B, Fekhaoui M, El Abidi A, Idrissi L, Lecorre P (2007) Résidus de pesticides organochlorés chez les bivalves et les poissons de la lagune de Moulay Bousselham (Maroc). Afrique Science 03:146–168
Beninger PG, Lucas A (1984) Seasonal variations in condition, reproductive activity, and gross biochemical composition of two species of adult clam reared in a common habitat: tapes decussatus L. (Jeffreys) and Tapes philippinarum (Adam and Reeves). J Exp Mar Biol Ecol 79:19–37
Borja J, Taleon DM, Aurasenia J, Gallardo S (2005) Polychlorinated biphényls and their biodegradation. Process Biochem 40:1999–2013
Custer T, Custer C, Gray B (2010a) Polychlorinated biphenyls, dioxins, furans, and organochlorine pesticides in belted kingfisher eggs from the Upper Hudson River Basin, New York, USA. Environ Toxicol Chem 29(1):99–110
Custer CM, Custer T, Dummer P (2010b) Patterns of organic contaminants in eggs of an insectivorous, an omnivorous, and a piscivorous bird nesting on the Hudson River, New York, USA. Environ Toxicol Chem 29(10):2286–2296
Ifremer OG (2008) Qualité de l’eau et contaminations: Contamination par les PolyChloroBiphényles (PCB) dans l’estuaire de la Seine. GIP, Seine Aval, pp 1–6
Jayed M (2011) Evaluation des concentrations en pesticides organochlorés et polychlorobiphényles dans les gisements coquilliers du littoral marocain. Thèse de doctorat en Sciences de l’Environnement et Valorisation des ressources. Faculté des Sciences de l’Université Chouaîb Doukkali, El Jadida. p 159
Jayed M, Chafik A, Benbrahim S, Vale C, Bakkas S, Pereira P, Ana Ferreira M (2010) Polychlorinated biphenyls and chlorinated pesticides in the mussel Mytilus galloprovincialis sampled along the Moroccan Atlantic Coast. J Oceanogr Mar Sci 1(5):93–98
Kamara A, Rharbi N, Ramdani M, Berraho A (2005) Etude comparative du cycle sexuel de la palourde Ruditapes decussatus (L.) issue de trois milieux paraliques des côtes marocaines. Mar Life 15(1–2):43–50
Kamara A, Rharbi N, Ramdani M, Berraho A (2008) Recherches préliminaires au développement de l’élevage de la palourde européenne (Ruditapes decussatus L.) sur les côtes marocaines et au repeuplement des sites surexploités. Bull Soc Zool Fr 133(1-3):189–198
Kelly SM, Eisenreich KM, Baker JE, Rowe CL (2008) Accumulation and maternal transfer of polychlorinated biphenyls in snapping turtles of the Upper Hudson River, New York, USA. Environ Toxicol Chem 27(12):2565–2574
Khaled A, El Nemr ATO, El-Sikaily SA, Abd-Alla AMA (2004) Polychlorinated biphenyls and chlorinated pesticides in mussels from the Egyptian Red Sea coast. Chemosphere 54:1407–1412
Léaute F (2008) Biogéochimie des contaminants organiques HAP, PCB et pesticides organochlorés dans les sédiments de l’étang de Thau. Doctorat de l’Université Pierre et Marie Curie, Paris, p 255
Lucas A, Beninger PG (1985) The use of physiological condition indices in marine bivalve aquaculture. Aquaculture 44:187–200
Naso B, Perrone D, Ferrante MC, Bilancione M, Lucisano A (2005) Persistent organic pollutants in edible marine species from the Gulf of Naples, Italy. Sci Total Environ 343:83–95
Orban E, Di Lena G, Nevigato T, Casini I, Marzetti A, Caproni R (2002) Seasonal changes in meat content, condition index and chemical compostion of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem 77:57–65
Phillips KM, Tarragó-Trani MT, Grov TM, Grün I, Lugogo R, Harris RF, Stewart KK (1997) Simplified gravimetric determination of total fat in food composites after chloroform-methanol extraction. J Am Oil Chem Soc 74(2):137–142
Pierard C, Guarrigues P (1996) Grain size distribution of polychlorobiphenyl in coastal sediment. Environ Sci Technol 30:2776–2778
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. Wiley, New Jersey
Serghini A, Mehdaoui O, Fekhaoui M, Venant Y (2004) Le rôle des lipides dans l’accumulation des organochlorés. Cas de l’anguille. Biologie & Santé 4(1):35–42
Stefanelli P, Di Muccio A, Ferrara F, Attard Barbini D, Generali T, Pelosi P, Amendola G, Vanni F, Di Muccio S, Ausili A (2004) Estimation of intake of organochlorine pesticides and chlorobiphenyls through edible fishes from the Italian Adriatic Sea during 1997. Food Control 15:27–38
Tapie N (2006) Contamination des écosystèmes aquatiques par les PCB et PBDE : Application à l’estuaire de la gironde. Thèse de doctorat, Université Bordeaux 1:233p
Thompson S (2005) Détermination des composés aromatiques et organochlorés dans l’environnement marin. Thèse de doctorat, Université Bordeaux 1:151p
Acknowledgments
The authors would like to thank the reviewers for their comments and express their sincere gratitude to Laurent Pluhet from Physico-Toxico-chemistry Laboratory - Bordeaux1 University, for his useful help in the technical analyses. Many thanks to all colleagues of the monitoring network and the safety of the Oualidia station, for providing the biological samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayed, M., Benbrahim, S., Bakkas, S. et al. Accumulation of Organochlorines in the European Clam (Ruditapes decussatus) and Sediment of the Oualidia Lagoon (Morocco). Bull Environ Contam Toxicol 94, 614–621 (2015). https://doi.org/10.1007/s00128-015-1517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1517-5