Abstract
Nine endosulfan degrading bacterial strains were isolated by soil enrichment with endosulfan. Bacterial strain M3 was the most efficient degrader. Endosulfan degradation was accompanied by a decrease in pH of the medium and an increase in chloride ion concentration. The bacterium was tested for its ability to degrade endosulfan at different concentrations in broth and soil. Maximum degradation occurred at concentrations of 50 μg/ml of broth and 100 μg/g of soil. Values of Ks and Vmax were different for (α)- and (β)-endosulfan in broth. The kinetic indices (Vmax/Ks) for α-endosulfan and β-endosulfan were 0.051 and 0.048 day−1 respectively, indicating that (α)-endosulfan was more rapidly degraded. Bacterial strain M3 was identified as Klebsiella sp. M3 on the basis of 16S rDNA sequence similarity (GenBank accession number JX273762).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endosulfan, a sulphurous acid ester of a chlorinated cyclic diol, is a mixture of two stereo isomers, (α)- and (β)-endosulfan, in a ratio of 7:3. Endosulfan is a broad-spectrum insecticide used in a wide variety of food and non-food crops. It is known for persistence in nature. Endosulfan contamination has been detected in soil, water, air, and food products because of its abundant usage and persistence in nature (Rao and Murty 1980). Endosulfan is also well known for its non-targeted toxicity to fishes and aquatic invertebrates (Michael et al. 2005) and has been implicated in mammalian toxicity (Saiyed et al. 2003).
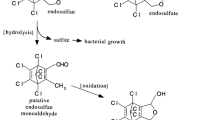
These environmental and health issues have led us to investigate the detoxification of endosulfan present in the environment by different bioremediation strategies. Endosulfan detoxification in a single enzymatic reaction makes it an ideal candidate for bioremediation. Two pathways for single step detoxification of endosulfan have been reported. One pathway involving hydrolysis (enzymatic and chemical) removes the sulphur moiety. The resultant hexachloride endosulfan diol (Li et al. 2009) poses little threat to the environment. Another pathway involves oxygenation of endosulfan, resulting in the formation of endosulfan monoaldehyde (Sutherland et al. 2002). Microbial metabolism of endosulfan also often results in the formation of endosulfan sulfate via oxidation (Shivaramaiah and Kennedy 2006). The aim of this study was to find an efficient microbial degrader of endosulfan, and determine the possible pathways for degradation of endosulfan in nature. The gathered information can be used in the development of bioremediation strategies for decontamination of the endosulfan contaminated environment.
Materials and Methods
Soil was collected from experimental fields of Punjab Agricultural University, Ludhiana, India. Topsoil was collected from the first 15 cm, air dried, sieved and stored at 4°C until used for experiments. Endosulfan degrading bacteria were isolated by soil enrichment with endosulfan and followed by shake flask enrichment in NSM (non sulfur medium) broth (Sutherland et al. 2000). Initially, bacterial strains were selected based on their distinct colony morphological features, such as shape, size, colour, margin, surface, etc. These colonies were purified by further streaking on the NSM agar plate. All isolated bacterial strains were identified using 16S rDNA sequence analysis (Goswami and Singh 2009).
The endosulfan degradation study was conducted in 100 ml of NSM supplemented with 50 ppm of endosulfan. Experimental flasks were inoculated with 1 ml of LB grown culture which was centrifuged and washed with phosphate buffer (pH 7, 0.05 M). Uninoculated flasks, spiked with endosulfan were set up as a control. All flasks were incubated on a microbial shaker at 30°C with 150 rpm for 15 days. To monitor the degradation of endosulfan and generation of metabolites, 3 ml of culture were extracted using ethyl acetate. The pH and ODλ595nm of the broth were also monitored. Analysis was performed by HPLC (high performance liquid chromatography unit, Perkin Elmer series 200) with a UV/Vis detector. The separation column was Pecosphere 5CR C18, 0.5 × 15 cm (Perkin Elmer, Waltham, Massachusetts, USA). HPLC grade water and acetonitrile in a combination ratio of 35:65 were used as mobile phase at a flow rate of 1.0 ml/min. The column was kept at ambient temperature. LOD (limit of detection) and LOQ (limit of quantification) were estimated for endosulfan isomers and their metabolites in HPLC using their calibration curves (Table 1). Chloride ion concentration was estimated using the method described by Verma et al. (2006).
Technical grade endosulfan concentrations ranging from 10 to 100 ppm were maintained in 100 ml NSM broth. As preliminary studies with 9 isolated strains demonstrated strain M3 as most efficient degrader of endosulfan, it was chosen for further studies. Strain M3 was identified as belonging to the genus Klebsiella. All flasks were inoculated with Klebsiella sp. strain M3. Flasks were incubated under the same conditions. A control flask without endosulfan was also incubated. Aliquots were withdrawn for analysis of growth and endosulfan degradation. The kinetic constants Ks (affinity constant), Vmax (theoretical maximum activity) and Vmax/Ks (kinetic index) were also calculated using the Michaelis–Menten model at the concentrations corresponding to which maximum degradation was obtained.
Biodegradation of endosulfan in soil by Klebsiella sp. M3 was tested in a microcosm as described by Li et al. 2009. Soil samples were collected from an agricultural field of the Department of Zoology, University of Delhi, India as described previously. The soil had never been treated before with endosulfan. Soil samples were dried, mixed and then sieved through a 2 mm mesh size strainer and stored at 4°C. Half of the soil samples were sterilized. The endosulfan was added in the sterilized and non-sterilized soil sample to give the concentrations of 10, 50 and 100 mg/kg dw of soil, and were mixed well. One set of non-sterilized and sterilized soil was inoculated with the strain M3 at rate of 2 × 108 cells/g dw of soil. A corresponding set of uninoculated soil served as a control. Experimental soil samples in glass flasks were incubated at 30°C in a BOD incubator. Experimental samples were collected at intervals of 30 days from day 0 to day 150. Degradation was studied by gas chromatography (GC) using a Shimadzu instrument (Model GC-17 AAF, Tokyo, Japan) equipped with a Ni electron capture detector. Nitrogen was used as carrier gas. The oven temperature was programmed as follows: an initial temperature of 180°C (hold for 2 min), followed by elevation to 250°C (hold 2 min) at a rate of 10°C/min and followed by a final raise to 270°C at a rate of 10°C/min with a hold of 4 min. Temperature of the injector port and detector block were maintained at 250 and 300°C respectively. The LOD and LOQ were also calculated for GC (Table 2).
The inoculated strain M3 was isolated from soil by serial dilution on LB agar plate containing vancomycin hydrochloride (30 μg/ml), amoxicillin (10 μg/ml) and ampicillin (100 μg/ml). For identification, the isolated colonies were analysed by ERIC-PCR according to the method of Kumar et al. (2008).
The values from triplicate samples were analyzed using analysis of variance (ANOVA) with Sigma Plot Version 12.0. p values less than 0.05 were considered significant.
Results and Discussion
Out of nine strains isolated following enrichment, the strain designated as M3 showed maximum degradation of endosulfan with 74.5 ± 2.26 % of (α)-endosulfan and 67.5 ± 1.59 % of (β)-endosulfan degraded after 15 days in broth. Other strains designated as M1, M2, M4, M5, M6, M7, M8, and M9 showed corresponding degradation percentages of 52.4 ± 2.38, 41.2 ± 2.54, 20.5 ± 4.08, 42.8 ± 1.70, 64.1 ± 1.36, 11.8 ± 1.70, 41.6 ± 2.84 and 63.2 ± 1.9 % for (α)-endosulfan; and 30.6 ± 2.87 %, 34.9 ± 2.94 %, 35.5 ± 3.5 %, 48.5 ± 3.54 %, 66.7 ± 1.20 %, 18.5 ± 2.82 %, 34.4 ± 1.96 % and 48.9 ± 3.67 % for (β)-endosulfan respectively. Previous studies have also reported strains having different degrading potential with their varied degradation pathways (Sutherland et al. 2002; Kumar and Philip 2006; Weir et al. 2006; Verma et al. 2006). This could be due to the different endosulfan metabolizing potentials of different strains and due to differences in the enzyme system responsible for endosulfan degradation (Sutherland et al. 2002; Weir et al. 2006). Even in the uninoculated control flask, (α)- and (β)-endosulfan decreased by 9.4 ± 1.63 % and 7.47 ± 1.32 % respectively. This degradation in control flasks was attributed to abiological losses. On the basis of 16S rDNA sequence analysis and BLAST search, the strain M3 was provisionally named as Klebsiella sp. M3. 16S rDNA sequence has already been submitted in GenBank with accession number JX273762.
Endosulfan degradation was also accompanied by an increase in ODλ595 nm of culture media by 0.659 and a decrease in pH by 2.5 units. This increase in OD is an indicator of utilization of endosulfan by Klebsiella sp. M3 for its growth, as there was no increase in OD in the control flasks without endosulfan present. An increase in bacterial culture OD in the presence of endosulfan was also shown by bacterial culture in a previous study (Sutherland et al. 2000). Degradation of endosulfan accompanied by decreased pH was also reported by Verma et al. (2006). They documented that decreased pH could be due to the release of chloride ions, leading to the formation of HCl. The pH of the medium supported the fact that the degradation of endosulfan was not via chemical hydrolysis.
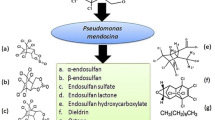
Degradation of endosulfan by Klebsiella sp. M3 was accompanied by appearance of metabolites like endosulfan diol, hydroxyether, lactone and sulfate (Table 3). Formation of these metabolites during endosulfan degradation has also been reported previously (Weir et al. 2006; Shivaramaiah and Kennedy 2006). Shivaramaiah and Kennedy (2006) have shown endosulfan sulfate and lactone as the terminal end products, as was observed for Klebsiella sp. M3 in the present study. The amount of endosulfan that was dissipated could not be accounted for by the concentration of metabolites. This suggests that complete mineralization of some fraction of endosulfan may have occurred (Table 3) (Kumar and Philip 2006). An increase in chloride ion concentration by 6 × 10−6mol during endosulfan degradation also clearly indicated extensive degradation of endosulfan by Klebsiella sp. M3 (Verma et al. 2006). The probable pathway of endosulfan degradation by Klebsiella sp. M3 depicted on the basis of our obtained chromatograms is given in Fig. 1.
Sigmoidal growth curves were obtained at all concentrations, with maximum growth at 50 ppm followed by 25, 10, 75, and 100 ppm. The ODλ595nm of NSM supplemented with 10, 25, 50, 75, and 100 ppm increased by 0.25, 0.512, 0.659, 0.417, and 0.317 respectively. In NSM with no endosulfan ODλ595 nm decreased by 0.037. Inhibition of growth at higher concentration could be due to cytotoxicity of endosulfan, as stated by Awasthi et al. (2000).
To determine the kinetics of degradation of endosulfan by Klebsiella sp. M3, the Michaelis–Menten kinetics model was selected to fit the experimental data. The modified form of MM kinetic relation was used:
where, dS/dt is the rate of degradation, S is the substrate concentration, and Vmax is the maximum degradation rate. The constant Ks (affinity constant) has been used in place of Km because the activities of bacterial cells towards endosulfan degradation are measured using the whole cell rather than purified enzymes (Futamata et al. 2005). The integration of Eq. (1) yields Eq. (2).
where, So is the initial concentration of substrate, S is the concentration of substrate at time t, t is the period for which degradation was studied. Kmax and Ks have already been designated in Eq. (1). By plotting 1/t lnSo/S (Y-axis) against So-S/t (X-axis), the inverse of the slope (m) of the best fit straight line will give Ks and the intercept will give Vmax/Ks (Fig. 2a, b). Lower values of Ks and higher values of Vmax indicate a higher affinity of the bacterial community towards degradation of substrate. The obtained results here have shown that the (β)-isomer (Ks 17.2 mg/L) has a higher affinity to its degrading enzyme as compared to the (α)-isomer (Ks 32.2 mg/L), but Vmax for the α-isomer (1.66 mg/L/day) was greater than that for the β- isomer (0.286 mg/L/day). Instead of considering Ks and Vmax individually, the ratio Vmax/Ks (kinetic index/specific affinity) was considered. It actually links enzymatic substrate uptake and the microbial growth. It is considered a better approach to assess the competition for nutrients at low concentration. Kinetic index values for (α)-endosulfan and (β)-endosulfan were 0.051 and 0.048 respectively, indicating better degradation capability for α-endosulfan as compared to β-endosulfan by Klebsiella sp. M3. These enzyme kinetic characteristics are dependent upon the cell’s history, the organism’s intrinsic characteristics and the existing environmental parameters. As these parameters are subject to change, these kinetic characteristics can also change (Kovarova-Kovar and Egli 1998).
Degradation of endosulfan in soil has been reported to be somewhat different from degradation in water (Li et al. 2009). Endosulfan degradation was maximum in soil treated with 100 μg/g dw of soil followed by 50 and 10 μg/g dw of soil, but the remaining residues of endosulfan in soil increased with increasing concentrations of applied endosulfan (Tables 4, 5). Increased degradation of endosulfan generally occurred in inoculated soils as compared to the uninoculated control soils (Table 5) (Li et al. 2009; Kumar et al. 2008). An exception was observed for (β)-endosulfan in non-sterilized soil, where slightly more degradation occurred in the uninoculated control soil. This indicated the endosulfan degradation capability of Klebsiella sp. M3 in soil. Endosulfan degradation in inoculated soil was accompanied by an increase in the population of Klebsiella sp. M3 (Fig. 3). Besides this, detection of endosulfan monoalcohol (a degradation product of endosulfan) only in inoculated sterilized and non-sterilized soil again supported our assumption that Klebsiella sp. M3 played an important role in the endosulfan degradation, along with other indigenous strains of soil microorganisms.
Growth curves were plotted after confirming the colony identity through ERIC-PCR. Among all treatments given to soil, maximum growth of Klebsiella sp. M3 was recorded in sterilized soil receiving amendments of 100 μg/g dw of soil, and minimal growth in non-sterilized soil receiving endosulfan amendments of 10 μg/g dw of soil (Fig. 3), indicating the positive relation between endosulfan concentration and growth of strain M3.
On the basis of the obtained results, Klebsiella sp. M3 may be considered as an important enzymatic source for detoxification of endosulfan contaminated sites (soil and water). Klebsiella sp. M3 may be useful in the development of bioprocess technologies for bioremediation.
References
Awasthi N, Ahuja R, Kumar A (2000) Factors influencing biodegradation of soil applied endosulfan isomers. Soil Biol Biochem 32:1697–1705
Futamata H, Nagano Y, Watanabe K, Hiraishi A (2005) Unique kinetic properties of phenol-degrading Variovorax strains responsible for efficient trichloroethylene degradation in a chemostat enrichment culture. Appl Environ Microbiol 71:904–911
Goswami S, Singh DK (2009) Biodegradation of α and β endosulfan in broth medium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20:199–207
Kovarova-Kovar K, Egli T (1998) Growth kinetics of suspended microbial cell: from single substrate-controlled growth to mixed substrate kinetics. Microbial Mol Biol Rev 62:646–666
Kumar M, Philip L (2006) Endosulfan mineralization by bacterial isolates and possible degradation pathway identification. Biorem J 10:179–190
Kumar M, Lakshmi V, Khanna S (2008) Biodegradation and bioremediation of endosulfan contaminated soil. Bioresour Technol 99:3116–3122
Li W, Dai Y, Xue B, Li Y, Peng X, Zhang J, Yan Y (2009) Biodegradation and detoxification of endosulfan in aqueous medium and soil by Achromobacter xylosoxidans strain CS5. J Hazard Mater 167:209–216
Michael TW, Jen-NI K, Craig B, Grant S, Graham VA, John P (2005) Toxicity of alpha-, beta-, (alpha + beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ Toxicol Chem 24:1146–1154
Rao DMR, Murty AS (1980) Persistence of endosulfan in soils. J Agric Food Chem 28:1099–1101
Saiyed H, Dewan A, Bhatnagar V, Shenoy U, Shenoy R, Rajmohan H, Patel K, Kashyap R, Kulkarni P, Rajan B, Lakkad B (2003) Effect of endosulfan on male reproductive development. Environ Health Perspect 111:1958–1962
Shivaramaiah HM, Kennedy IR (2006) Biodegradation of endosulfan by a soil bacterium. J Environ Sci Health Part B 41:895–905
Sutherland TD, Horne J, Lacey MJ, Harcourt R, Russell RJ, Oakeshott JG (2000) Enrichment of an endosulfan-degrading mixed bacterial culture. Appl Environ Microbiol 66:2822–2828
Sutherland TD, Horne I, Russell RJ, Oakeshott JG (2002) Gene cloning and molecular characterization of a two-enzyme system catalyzing the oxidative detoxification of β-endosulfan. Appl Environ Microbiol 68:6237–6245
Verma K, Agrawal N, Farooq M, Misra RB, Hans RK (2006) Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicol Environ Saf 64:377–381
Weir KM, Sutherland TD, Horne I, Russell RJ, Oakeshott JG (2006) A single monooxygenase, Ese, is involved in the metabolism of the organ chlorides endosulfan and endo sulphate in an Arthrobacter sp. Appl Environ Microbiol 72:3524–3530
Acknowledgments
We are highly thankful to the funding agency, Indian Council of Agricultural Research (ICAR) for providing funds under NFBSFARA (NFBSFARA/WQ-2023/2012-13). Ms. Madhu is thankful to CSIR, Delhi for providing the fellowship as JRF/SRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, M., Singh, D.K. Biodegradation of Endosulfan in Broth Medium and in Soil Microcosm by Klebsiella sp. M3. Bull Environ Contam Toxicol 92, 237–242 (2014). https://doi.org/10.1007/s00128-013-1168-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1168-3