Abstract
There is an increasing concern regarding elevated levels of Cr(VI) in the environment due to its higher mobility and toxicity compared to the trivalent form. Anomalous hexavalent chromium concentrations (up to 212 μg/L) were determined in irrigated groundwaters from the wider area of Thiva Basin (central Greece), frequently exceeding the permissible limit for human consumption (50 μg/L for total Cr). Based on the spatial distribution of Cr(VI) values, two groups of groundwater samples were distinguished, possibly reflecting different natural and/or anthropogenic factors that govern the levels of contamination. The first group is spatially located northwards of Thiva town and is consisted of concentrations that range from 13 to 212 μg/L (median 58 μg/L), while the second group is located near Mouriki village and Cr(VI) values range from <9 to 14 μg/L. The Cr(VI) chemical anomalies represent an important social problem because the agricultural products of this region are a major vegetable supply for Greece, bringing up the urgent need to evaluate the health effects associated with Cr(VI) exposure by ingesting the potentially contaminated foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chromium is an essential micronutrient for plant and animal metabolism (glucose metabolism, amino- and nucleic acid synthesis). However, when accumulated at high levels, it can generate serious trouble and diseases (nausea, skin ulcerations and lung cancer) and, as concentration reaches 0.1 mg/g body weight, it can ultimately become lethal (Ajmal et al. 1984). In groundwater systems, Cr is chiefly present in two oxidation states: Cr(III) and Cr(VI), depending on the redox conditions. The hexavalent form is considered to be the most toxic and is highly mobile and soluble compared to the trivalent one (Nikolaidis et al. 1999; Hellerich and Nikolaidis 2005); the latter is strongly adsorbed or precipitates as a solid (oxy)-hydroxide phase over the pH range of most natural waters (Peterson et al. 1996).
According to Peterson et al. (1996), the background concentrations of Cr in unpolluted groundwater are expected to be <10 μg/L, whereas in contaminated areas concentrations up to several hundred mg/L have been reported (Loyaux-Lawniczak et al. 2001). Due to the adverse health effects that may cause, there is an increasing concern regarding the presence of Cr(VI) in aquifers, where relatively high levels have been attributed to industrial pollution (e.g. Loyaux-Lawniczak et al. 2001) and/or to natural processes (e.g. Robles-Camacho and Armienta 2000; Gonzalez et al. 2005; Margiotta et al. 2012). Industry operations usually include steel works, leather tanning, pigmentation, plating and corrosion prevention, whereas geogenic elevated concentrations are the result of the weathering of Cr-rich minerals present in ultrabasic rocks. Based on toxicity, Cr(VI) is considered to be carcinogen, mutagen and terratogen after long exposure (ATSDR 2012), and can be absorbed by ingestion, inhalation and dermal contact. Although a portion of orally ingested Cr(VI) is converted to Cr(III) under the acid pH conditions of the human stomach, it still remains highly toxic upon inhalation and ingestion by entering the cells of several tissues resulting to DNA damage (Sedman et al. 2006). Thus, besides Crtotal determination, the speciation analysis is of particular significance due to diverse nature of biological interactions like toxicity, mobility, bio-availability and bio-accumulation.
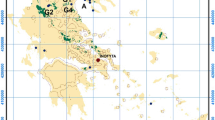
The study area of Thiva Basin (Fig. 1) is characterized by intense agricultural activities, mainly including the production of carrots, potatoes and onions. Recently, Kirkillis et al. (2012) investigated the heavy metal content of the agricultural products of Thiva Basin and pointed out that Cr concentrations are considerably higher than other uncontaminated control areas. Thus, the present follow-up study is aiming to get insight to the occurrence and speciation of Cr in the groundwater system of Thiva Basin, in relation to the potential sources of pollution, the main land use and the anticipating environmental impacts that may affect the food chain.
Materials and Methods
A total of 40 groundwater samples were collected in May 2010 from all the available boreholes under pumping conditions. Samples W1 to W25 were collected from the flat area located northwards of Thiva town, whereas W26 to W40 were collected from the area adjacent to the Mouriki village (Fig. 1). Groundwaters from these areas are mainly used for irrigation purposes. Unstable field parameters such as pH and Eh were measured onsite by means of portable instruments. The pH buffers with nominal values 7.01 and 8.01 were used for pH calibration. The water samples were immediately filtered in the field through conventional 0.45 μm membrane filters, acidified down to pH <2 with HNO3 in order to prevent metal precipitation and stored in new polyethylene bottles for Crtotal analyses. The bottles were rinsed three times with small amounts of the aqueous solution before being filled. One aliquot of the filtered sample was not acidified for Cr(VI) determinations.
Dissolved Cr(VI) concentrations were determined within 24 h of collection spectrophotometrically at a wavelength of 540 nm using the diphenylcarbazide colorimetric method (the color intensity is proportional to the Cr(VI) concentrations). Analyses of total chromium were performed by graphite furnace atomic absorption spectroscopy (Perkin Elmer 110B) using Cr standard solutions prepared by dilution from certified standard solutions of 1,000 mg/L up to μg/L levels. The detection limits in our study were defined as the concentration values that are numerically equal to three times the standard deviation of 15 replicate blank measurements. This process revealed 9 μg/l and 2 μg/L as detection limits for Cr(VI) and Crtotal respectively.
Results and Discussion
The analytical results of Cr(VI) in groundwater samples ranged from no detectable concentrations (<9 μg/L) to the elevated value of 212 μg/L (Table 1), denoting a significant impact to local hydrogeochemistry as may also be justified by their median concentration which is equal to 47.5 μg/L (Fig. 2). The pH conditions were slightly alkaline ranging from 7.5 to 8.2, whilst Eh values varied between −75.5 and −22.5 mV. Almost all dissolved Cr in groundwater samples was in the form of Cr(VI), as the average contribution of Cr(VI) to the Crtotal concentration was nearly 96 % with a Pearson correlation coefficient equal to 0.99, p < 0.01 (Fig. 3).
Based on the analytical results (Table 1) we can distinguish two individual groups of samples. The first one (group A) is distributed northwards of Thiva town and is consisted of elevated Cr(VI) concentrations (samples W1 to W25) ranging between 13 and 212 μg/L with a median value of 58 μg/L; Cr(VI) concentrations of this group are in most cases significantly higher than the 50 μg/L limit for human consumption as defined by the European Union for Cr regardless its speciation (Directive 98/83/EC 1998). The second one (group B) is located in the surrounding area of Mouriki village with significant lower concentrations ranging from values below detection limit (<9 μg/L) up to 14 μg/L. The median value of Cr(VI) for group A is higher than the mean values reported for geogenic enrichment derived from the occurrence of ultramafic rocks in Spain (12 μg/L – Robles-Camacho and Armienta 2000), Italy (39 μg/L – Fantoni et al. 2002) and USA (36 μg/L – Ball and Izbicki 2004). Additionally, the range of values for Cr(VI) in group A is similar for those reported (0–180 μg/L) few tens of kilometers southern of the study area (Asopos Basin), that were directly related with anthropogenic influences of industrial injection wells (Economou-Eliopoulos et al. 2011).
According to Reinmann and Filzmoser (2000), basic statistical attributes such as the p value (Anderson–Darling test) may be considered as a criterion for the assessment of normal distribution (when p > 0.005) of a statistical population. Going a step further, Appelo and Postma (2005) suggested that the distribution of a population may provide background details about the main process of its origin, thus a slow process (e.g. mineral dissolution) may be related with a normal (or log-normal) distribution, while an external factor (point-source pollution) may be related with abnormal distributions. Based on the above, it can be suggested that the source of Cr should be different regarding the enrichment of groundwater belonging to groups A and B. The difference among these groups, which was initially assessed by the analytical results, is also verified by the p values of their populations; it is clear that the origin of Cr(VI) for group B (p = 0.008 – normal distribution) is different than the origin for group A (p < 0.005 – abnormal distribution).
Accordingly, it should be pointed out that the hydrogeochemical conditions of the area concerning elevated chromium concentrations should be attributed to both geogenic and anthropogenic influences; depending on the origin of chromium, the order of values ranges relating the lower ones to geogenic sources and the higher to anthropogenic impact. This conclusion is also supported by the evaluation of the basic geological and geochemical data. The occurrence of ultrabasic formations is a potential source of Cr enrichment to both soils and groundwater. Elevated Cr concentrations, ranging from 134 to 856 mg/kg, were determined in agricultural surface soils of Thiva Basin (Antibachi et al. 2012), possibly signifying the presence of chemically labile sources of naturally-occurring Cr(III) in soils that undergo oxidation to Cr(VI). In soils and aqueous environments, Cr(III) adsorption by Mn-oxides is the first step toward its oxidation to Cr(VI); According to Tziritis (2009) and Antibachi et al. (2012) there are elevated values of Mn in soils from the wider area of Thiva, which may typically be attributed to the occurrence of Mn oxides, as a result of the weathering process of ultrabasic rocks, which are typically enriched in Mn (Tziritis et al. 2012). As described by Barlett and James (1979), Cr(III) typically accumulates on the surface of Mn and Fe-oxides and the undergoing process involves the oxidation of Cr(III) to Cr(VI) by the presence in the soil of oxidized Mn which serves as the electron acceptor in the reaction; oxidation rates tend to be higher with increasing pH (Eary and Ray 1986).
Additionally, the neighboring area of Asopos River Basin was recently found to be contaminated by Cr(VI), as result of the intensive industrial activities, such as plating metals, leather processing, stainless steel, that were discharged into the environment possibly through injection wells (Economou-Eliopoulos et al. 2011). The hydrogeological data till now are insufficient in order to prove or not a potential hydraulic connection and possible contaminant plume migration from Asopos to Thiva Basin, but the supplementary data (geological, statistical, geochemical, land use) so far suggest that these abnormal concentrations possible have their origin to anthropogenic influence.
Despite the origin of hexavalent chromium, an issue of great debate is whether and how much the aforementioned elevated concentrations affect directly or indirectly the human environment of the area. The direct impact as result of ingestion of contaminated water should be excluded because almost none of the collected groundwaters are used for drinking purposes. On the contrary, since the main water use is irrigation, there is a lot of concern whether the consumption of products from that area has a significant effect to humans. The main crops produced are carrots, potatoes and onions and are considered to be a major vegetable supply source for Greece. As can be seen in Fig. 1, all the samples with elevated Cr(VI) concentrations (group A) are located within the cultivated area, as may be suggested by the CORINE classification (EEA 1994), so the risk of bio-accumulation and impact to food chain is possible. Recently, Kirkillis et al. (2012) investigated the heavy metal content of the agricultural products of Thiva Basin and stated that Cr concentrations are considerably higher than other uncontaminated control areas. Future studies should focus on the possible effects on human health as result of the consumption of agricultural products that have been irrigated with such high Cr(VI) concentrations.
References
Ajmal N, Nomani A, Ahmad A (1984) Acute toxicity of chrome electroplating wastes to microorganism: adsorption of chromate and chromium (VI) on a mixture of clay and sand. Water Air Soil Pollut 23:119–127
Antibachi D, Kelepertzis E, Kelepertsis A (2012) Heavy metals in agricultural soils of the Mouriki-Thiva area (central Greece) and environmental impact implications. Soil Sediment Contam 21:434–450
Appelo C, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A.A Balkema Publishers, The Netherlands
ATSDR (2012) Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services, http://www.atsdr.cdc.gov/toxprofiles/tp7.html
Ball J, Izbicki JA (2004) Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Appl Geochem 19:1123–1135
Barlett R, James B (1979) Behavior of chromium in soils: III. Oxidation. J Environ Qual 8:31–35
Eary L, Ray D (1986) Kinetics of Cr(III) oxidation to Cr(VI) by reaction with Mangenese dioxide. Environ Sci Technol 21:1187–1193
EC (1998) Council Directive (98/83/EC) of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Commun, L330
Economou-Eliopoulos M, Megremi I, Vasilatos C (2011) Factors controlling the heterogeneous distribution of Cr(VI) in soil, plants and groundwater: evidence from the Assopos basin, Greece. Chem Erde 71:39–52
European Environmental Agency (1994) CORINE Land Cover. Commision of European Communities. http://www.eea.europa.eu/publications/COR0-part2
Fantoni D, Brozzo G, Canepa C, Cipolli F, Marini L, Ottonelo G, Zuccolini M (2002) Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environ Geol 42:871–882
Gonzalez AR, Ndung’u K, Flegal AR (2005) Natural occurrence of hexavalent chromium in the Aromas Red Sands aquifer, California. Environ Sci Technol 39:5505–5511
Hellerich LA, Nikolaidis NP (2005) Studies of hexavalent chromium attenuation in redox variable soils obtained from a sandy to subwetland groundwater environment. Water Res 39:2851–2868
Kirkillis C, Pasias IN, Miniadis-Meimaroglou S, Thomaidis NS, Zabetakis I (2012) Concentration levels of trace elements in carrots, onions, and potatoes cultivated in Asopos region, central Greece. Anal Lett 45:551–562
Loyaux-Lawniczak S, Lecomte P, Ehrhardt J (2001) Behavior of hexavalent chromium in a polluted groundwater: redox processes and immobilization in soils. Environ Sci Technol 35:1350–1357
Margiotta S, Mongelli G, Summa V, Paternoster M, Fiore S (2012) Trace element distribution and Cr(VI) speciation in Ca-HCO3 and Mg-HCO3 spring waters from the northern sector of the Pollino massif, southern Italy. J Geochem Explor 115:1–12
Nikolaidis NP, Hellerich LA, Lackovic JA (1999) Methodology for site-specific, mobility-based cleanup standards for heavy metals in glaciated soils. Environ Sci Technol 33:2910–2916
Peterson M, Brown G, Parks G (1996) Direct XAFS evidence for heterogenous redox reaction at the aqueous chromium/magnetite interface. Colloids Surf A 107:77–88
Reinmann C, Filzmoser P (2000) Normal and lognormal distribution in geochemistry: death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environ Geol 39:1001–1014
Robles-Camacho J, Armienta MA (2000) Natural chromium contamination of groundwater at León Valley, Mexico. J Geochem Explor 68:167–181
Sedman RM, Beaumont J, McDonald TA, Reynolds S, Krowech G, Howd R (2006) Review of the evidence regarding the carcinogenicity of hexavalent chromium in drinking water. J Environ Sci Health Part C 24:155–182
Tziritis E (2009) Groundwater and soil geochemistry of Eastern Kopaida region, (Beotia, central Greece). Cen European J Geosci 1:219–226
Tziritis E, Kelepertsis A, Fakinou G (2012) Geochemical status and interactions between soil and groundwater systems in the area of Akrefnio, Central Greece. Risk assessment, under the scope of mankind and natural environment. J Water Land Dev 15:127–144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tziritis, E., Kelepertzis, E., Korres, G. et al. Hexavalent Chromium Contamination in Groundwaters of Thiva Basin, Central Greece. Bull Environ Contam Toxicol 89, 1073–1077 (2012). https://doi.org/10.1007/s00128-012-0831-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0831-4