Abstract
Chromite ore processing residue (COPR) is generated by the roasting of chromite ores for the extraction of chromium. Leaching of carcinogenic hexavalent chromium (Cr(VI)) from COPR dumpsites and contamination of groundwater is a key environmental risk. The objective of the study was to evaluate Cr(VI) contamination in groundwater in the vicinity of three COPR disposal sites in Uttar Pradesh, India, in the pre-monsoon and monsoon seasons. Groundwater samples (n = 57 pre-monsoon, n = 70 monsoon) were taken in 2014 and analyzed for Cr(VI) and relevant hydrochemical parameters. The site-specific ranges of Cr(VI) concentrations in groundwater were <0.005 to 34.8 mg L−1 (Rania), <0.005 to 115 mg L−1 (Chhiwali), and <0.005 to 2.0 mg L−1 (Godhrauli). Maximum levels of Cr(VI) were found close to the COPR dumpsites and significantly exceeded safe drinking water limits (0.05 mg L−1). No significant dependence of Cr(VI) concentration on monsoons was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is a widely used element in various industrial activities such as metal plating and leather tanning. (Kimbrough et al. 1999). It is a redox-sensitive transition metal and exists in the environment in either the trivalent (Cr(III)) or hexavalent (Cr(VI)) oxidation states. Its chemical properties and environmental behavior are directly related to the dominant valence state. While Cr(III) is considered to be essential to mammals for the maintenance of normal carbohydrate, lipid, and protein metabolism (Pechova and Pavlata 2007), Cr(VI) is known to be carcinogenic when inhaled (Holmes et al. 2008) or ingested, e.g., with drinking water (Sun et al. 2015). However, the most widely known reaction of Cr(VI) is sensitization and elicitation of allergic contact dermatitis (Shelnutt et al. 2007). Furthermore, Cr(VI) compounds are also highly toxic to plants and are detrimental to their growth and development (Nagajyoti et al. 2010). Due to its toxicity, the World Health Organization determined a guideline value of 0.05 mg L−1 for total Cr in drinking water (WHO 2011).
Extensive groundwater contamination with Cr has been reported in the area of Kanpur (Uttar Pradesh, North India), which hosts a prominent cluster of leather-processing industries. The geochemical behavior and distribution of Cr in aquifers have been studied in industrial areas of Kanpur (Ansari et al. 1999, 2000; Dwivedi et al. 2015; Singh et al. 2009; Srinivasa Gowd et al. 2010), Unnao (Dwivedi et al. 2015; Srinivasa Gowd et al. 2010; Srivastava and Singh 2014), and Rania (Prakash et al. 2011; Singh et al. 2013). Apart from groundwater contamination caused by industrial effluents, the illegal disposal of Cr-containing waste is a primary Cr source. This waste originates from production sites of basic chromium sulfate (BCS) located in Rania and in Chhiwali, 30 and 40 km away from Kanpur, respectively. Production of BCS occurs via the high-lime process through which the insoluble Cr(III) present in the ore is converted to soluble Cr(VI) and extracted by aqueous leaching (Burke et al. 1991). The waste of this process is the so-called chromite ore processing residue (COPR), which is dumped at uncontrolled landfills close to the factories or in adjacent villages. Besides the large landfills, COPR is found elsewhere in these areas, because it is commonly used in road construction, in berms, and as foundation and backfill. Residual Cr contents of 81 and 74 g kg−1 have been measured in the COPR at two manufacturing sites, of which 20 and 13%, respectively, were present as Cr(VI) (Matern et al. 2016). Deposited COPR is not stable and undergoes weathering, resulting in destabilization and dissolution of Cr(VI)-bearing minerals (Chrysochoou et al. 2010; Geelhoed et al. 2002; Geelhoed et al. 2001). Leaching of Cr(VI) from COPR was evident from the strong yellow stain of well water. Field test kits indicated Cr(VI) concentrations of up to 80 mg L−1 in piezometers close to the main dumpsite in Rania (Singh et al. 2013). Leaching of Cr(VI) from the COPR waste is directly linked to the incidence of seepage water, expected to occur mainly during the monsoon season (July–September). It was, therefore, hypothesized that Cr(VI) influx to the underlying aquifers would vary seasonally.
Chromium(VI) contamination of groundwater affected by COPR is not only an Indian issue but also a key environmental risk arising at COPR sites worldwide. Concentrations up to 91 and 6.7 mg L−1 Cr(VI) have been measured in Scottish COPR-affected groundwater and tributary stream, respectively (Farmer et al. 2002). Burke et al. (1991) detected Cr(VI) concentrations up to 30 mg L−1 in groundwater samples in New Jersey COPR-affected areas. Groundwater Cr contamination has also been reported in China, Baltimore (Maryland), and Japan (Burke et al. 1991; Li et al. 2011; Sun et al. 2014).
The objectives of this study were (i) to identify and map the COPR waste sites in three industrial areas, (ii) to investigate the geochemical characteristics of groundwater in the surroundings of the COPR sites, (iii) to asses and classify the groundwater quality with respect to Cr(VI) contamination, and (iv) to elucidate the influence of monsoonal precipitation on Cr(VI) mobilization from COPR sites. The findings of this study describe the spatial dimension of Cr(VI) contamination for three COPR-contaminated sites, which is fundamental for the evaluation of environmental risks and remediation strategies. This is important, since the local population uses the groundwater not only for livestock rearing and irrigation but also for drinking.
Material and methods
Study area
The present study was carried out in the villages of Rania, 30 km to the west of Kanpur (district Kanpur Dehat) as well as in Chhiwali and Godhrauli, 40 and 47 km, respectively, to the southeast of Kanpur (district Fatehpur), in the State of Uttar Pradesh, North India (Fig. 1). These areas contain sites of historic or current production of the BCS used in the leather tanning process. In Rania, the production of BCS commenced in the late 1980s with the first manufacturing unit. In 1992, four manufacturing units were already operating. These works were closed in 2005 due to raised environmental concerns. However, COPR from the production of BCS has been disposed of in surrounding fields. In Chhiwali, two Cr works were established in 2004, which are still in operation. Several illegal waste dumps of unknown area and thickness were found near Chhiwali and its neighboring village, Godhrauli.
Geology and climate
Geologically, the study area is located in the central alluvial Ganga plain that was formed in response to the development of the Himalayas (Rao 1973; Singh 1996) and remains an important link between Himalayan Orogen and Indian Ocean since the early Quaternary period until now (Sinha et al. 2010). The modern landscape of the region was primarily formed due to the late Glacial/early Holocene incisions associated with an intensified monsoon. The area has an almost flat topography gently running from northwest to southeast (Singh et al. 2006b). These regions have a distinct stratigraphy. As opposed to earlier belief that contribution of sediments responsible for these plains has been predominantly from Himalayas during most of the Quaternary period, recent findings suggest that a contribution of sediments from cratonic sources was also significant during the late Quaternary period (Sinha et al. 2010). Such competition between Himalayan and cratonic sources of sediments is evident in the subsurface alluvial stratigraphy in the region. The alluvial deposits of the Ganga plains are predominantly constituted of micaceous sand, silt, clay, gravel, and kankar, the maximum thickness of alluvium being ∼500 m above bedrock of Bundelkhand granite (Srivastava et al. 2013). In Nauraiya Khera (Kanpur), which is ∼30 km east of the present study area, wells were constructed to provide information about the subsurface lithology of the region up to a depth of 50 m bgl using piezometers. These investigations indicated that sediments in the area consist of distinct layers of clay, fine sand, clay, medium sand, clay and kankar, fine sand, and clay and kankar with varying thicknesses (Singh et al. 2009). These authors also developed fence plots of the subsurface lithology, which indicated the wedge shaped and nonuniform nature of sandy clay as well as sand layers.
In the study area, a three-tier aquifer system exists. A shallow (upper) aquifer including the phreatic zone extends to varying depths of 25 to 130 m, a middle aquifer is located between 90 and 240 m, and a lower aquifer extends to depth between 180 and 450 m (Singh et al. 2006a). The upper aquifer forms the main source of water supply to the dug wells and shallow tube wells (Singh et al. 2006a) and has been observed to be most contaminated with hexavalent chromium (Singh et al. 2009).
The study area is situated in the humid subtropical climate zone characterized with cool (November–February), hot (April–June), and monsoon (July–September) seasons. Heavy rainfall typically occurs during the monsoon season in July, August, and September, accounting for almost 70 to 80% of the total precipitation (Chowdhury et al. 1982). Since the monsoonal rain normally arrives in this area as early as June, two periods are determined for the convenience of description, namely pre-monsoon period (March–May), a time before the occurrence of monsoonal rain and monsoon period (June–September). From 2009 to 2013, the average rainfall was 689 mm in the Kanpur Dehat district and 681 mm in the Fatehpur district (India Meteorological Department (IMD 2015a)), with about 85 and 89%, respectively, taking place during monsoon (IMD 2015a).
Sampling
Altogether, 75 wells were investigated, from which 57 groundwater samples were collected in the pre-monsoon period in April 2014 and 70 samples at the end of the monsoon period in September 2014. The intention was to sample identical wells in the two sampling campaigns. However, some wells were not available during the second sampling campaign. In this case, alternative wells, close to the original ones, were chosen. Overall, samples were taken from 52 wells in both seasons. Groundwater samples were collected across the entire study area from local hand pumps (LHP), India Mark-II hand pumps, so-called common tube wells (CTW), open dug wells (ODW), and others (two submersible pumps, one deep tube well, one irrigation well, and one pond). Since the depth of the wells (Rania: LHP and ODW 4 to 15 m, CTW 30 to 62 m; Chhiwali: ODW 7 to 11 m, LHP and CTW 31 to 76 m; Godhrauli: CTW 22 to 46 m) is relatively uniform for the well types, conclusions for the aquifers can be made. Concerning the objective of this study, three aspects were especially considered in the selection of the wells: (i) to assess the extent of the pollutant plumes, (ii) to study the influence of sampling depth, which conforms to different types of wells, and (iii) to identify local specifics. The hand pumps were sampled from the outlets after flushing of about 30 L to remove stagnant water. The samples were immediately filtered through a 0.45-μm cellulose acetate filter (Merck) and collected in acid-cleaned (conc. HNO3, supra pure, Merck) polyethylene tubes without headspace. Sample aliquots were analyzed in situ for Cr(VI) using a field test kit (Merck Millipore), while other parameters like electric conductivity (EC) and speciation were recorded on unacidified samples in the laboratory. Although the samples were not acidified immediately on site, no precipitation occurred prior to the measurements. For each sample, well depth, well owner, and geographic coordinates were recorded. Additionally, the owners of the wells were consulted regarding the color and taste of the water during the course of the year.
Analyses of the physical and chemical composition of groundwater
Groundwater pH was measured potentiometrically after calibration using an ion-sensitive glass electrode (Aquatrode Plus, Metrohm). The EC was determined using a conductivity probe (SevenEasy, Mettler-Toledo). For the determination of alkalinity, 5 mL of each sample was titrated with 0.1 M HCl to pH 8.2 and 4.3 using a Metrohm 775 Dosimat and a Metrohm 728 Stirrer. Chromium(VI) concentration was determined spectrophotometrically at 540 nm (Lambda 25 UV/Vis Spectrometer, PerkinElmer) according to the diphenylcarbazide method (US EPA 1992). The detection limit for Cr(VI) in the samples was 0.005 mg L−1. The concentrations of F−, Cl−, SO4 2−, PO4 3−, NO2 −, and NO3 − were determined by ion chromatography (ICS-1000, Dionex). Concentrations of Ca, K, Mg, and Na were measured by flame atomic absorption spectrometry (AAS, iCE 3500, Thermo Scientific) with an air-acetylene flame, and total Cr (>1 mg L−1) was determined with a nitrous oxide-acetylene flame. Nineteen elements (Si, Sr, Al, As, Ba, Cd, Co, Cr (<1 mg L−1), Cu, Fe, Mn, Mo, Ni, Pb, Sb, Se, U, V, and Zn) were analyzed by inductively coupled plasma mass spectrometry (ICP-MS, X-Series II, Thermo Scientific).
Statistical evaluation
Descriptive statistics were performed with the measured values of the samples collected in the pre-monsoon period and at the end of the monsoon period using Origin 8.5 (OriginLab). Statistical data evaluation was performed by the software IBM SPSS Statistics (Version 21). Basically, the nonparametric Spearman rank correlation coefficient was determined as a measure of the statistical dependency between variables, since some of the variables were not normally distributed. Significance was considered in circumstances where p < 0.01. A two-sample t test assuming equal variances was applied to investigate if log-transformed Cr(VI) levels of the pre-monsoon and monsoon sampling campaigns differed significantly. The test was applied to the whole sample collectively and to subsample groups that accounted for the degree of contamination.
Results and discussion
Mapping of COPR dumpsites
Many of the COPR waste sites in Rania, Chhiwali, and Godhrauli are located in densely populated residential areas, in industrial or commercial areas, and in the vicinity of agricultural land (Fig. 1). Details of the coordinates of these sample locations, the estimated area, and the starting date and duration of landfill operations are provided in the supplementary material (Table S1). In Rania, the main COPR dumpsite (#E) covers an area of about 55,800 m2 and is located opposite to a closed BCS manufacturing unit. The thickness of the COPR heaps is highly variable and is visually estimated to be between 0.5 and 1.5 m. The factory site itself also hosts huge quantities of COPR waste. Besides the main dumping site, several small waste sites are located along the road in Umran, where the COPR was partially used as a base material in road construction (#A). However, no COPR dumps could be identified in Khan Chandpur. In Chhiwali, the main COPR dumpsites have an area of about 2100 m2 (#H) and 8100 m2 (#K). Furthermore, several small dumpsites (<350 m2) are dispersed in this area either along the Kanpur–Allahabad highway or next to the side roads. Notably, in the case of dumpsite #H, parts of the village are directly located on the waste heap, which affects the people living there on an unimaginable scale. In Godhrauli, five COPR dumpsites with areas ranging from 100 to 5200 m2 could be identified. The dumpsites are located outside the village in an area of intensive agriculture. During the monsoon season, Cr is leached out from the COPR waste and flushed over the fields. Matern and Mansfeldt (2016) reported a Cr content of 0.54 g kg−1 in an agricultural soil close to the dumpsite #Q, of which about 9% was present in the mobile or exchangeable form.
Geochemical characteristics of groundwater
Table 1 shows the descriptive statistics of the investigated groundwater samples. For all analyzed solutes, except F−, NO3 −, As, Cr, Mo, Ni, and U, the measured maximum values were below the drinking water guidelines set up by the WHO (2011) and are hence not discussed further. This also holds for Ni, which slightly exceeded the guideline value in one sample only (5% above WHO standard). Important parameters of the investigated wells in the study area (Table S2) as well as detailed element concentrations (Tables S3 and S4) are listed in the SM.
Fluoride contamination
Consumption of water with elevated F− causes fluorosis, which is the most prevalent groundwater-related disease in India (Mumtaz et al. 2015). Fluoride concentrations were found to be high in the study area, with maximum values of 6.3 mg L−1 (Rania), 33 mg L−1 (Chhiwali), and 10.9 mg L−1 (Godhrauli). Overall, 75% of the wells exceeded the guideline value of 1.5 mg L−1 (WHO 2011). No differences in contamination were observed between the different types of wells. Altogether, F− concentration in groundwater varies without any obvious trend, indicating a sporadic occurrence. Fluoride contamination has been found elsewhere in Uttar Pradesh (CGWB 2010) and has been related to the weathering of the Indo-Gangetic alluvium (Kumar and Saxena 2011). Raju et al. (2012) reported concentrations up to 6.7 mg L−1 in wells of the Sonbhadra district, Uttar Pradesh.
Nitrate contamination
Nitrate is considered to be relatively nontoxic. However, there is a risk of methemoglobinemia particularly to infants arising from elevated NO3 − concentrations in drinking water (Fewtrell 2004). Maximum nitrate concentrations in the study area were 230 mg L−1 (Rania), 4481 mg L−1 (Chhiwali), and 135 mg L−1 (Godhrauli), and the highest NO3 − values (>500 mg L−1) were found in ODWs. In total, 25% of the wells exceeded the guideline value of 50 mg L−1 (WHO 2011) with wells in Chhiwali having higher incidence (71%) and severity of contamination. Elevated NO3 − concentrations up to 375 mg L−1 have also been reported for Fatehpur district (CGWB 2010). Sankararamakrishnan et al. (2008) reported NO3 − concentrations up to 735 mg L−1 in Kanpur Dehat district. Nevertheless, NO3 − concentrations as high as measured in our study area have not been observed before in Uttar Pradesh. Nitrate contamination may arise from runoff from agricultural fields after excess application of nitrogen fertilizers, the disposal of untreated human or animal waste (Chakraborti et al. 2011), or the oxidation of organic nitrogen compounds in soil by chromate.
Arsenic contamination
Groundwater As contamination in the Ganga Plain is primarily observed in the floodplains, since its sources are the rivers originating from the Himalaya Mountains (Chakraborti et al. 2009). However, As concentrations in the upper Ganga plain are lower than reported for the middle and lower Ganga plain. Arsenic is classified by the International Agency of Research on Cancer (IARC) as a class I carcinogen that exhibits acute and chronic toxicity depending on the type of exposure (Hettick et al. 2015). Hence, the WHO (2011) has set up a guideline value of 10 μg L−1. Arsenic concentrations were elevated in 11% of the wells with maximum concentrations of 51 μg L−1 (Rania), 75 μg L−1 (Chhiwali), and 13 μg L−1 (Godhrauli) and predominantly present in the upper aquifer up to a depth of 12 m. Chakraborti et al. (2009) reported As concentrations up to 707 μg L−1 for the adjacent Allahabad district. In the same study, 66% of the wells contained As above the WHO guideline value and As contamination mostly occurred in shallow hand tube wells (depth 7.5 to 40 m).
Molybdenum contamination
Molybdenum is an essential trace element for plants, animals, and humans (Gupta 1997); however, ruminants may develop molybdenosis, a Mo-induced copper deficiency at higher consumption (Underwood 1976). Furthermore, chronic oral exposure can result in gastrointestinal disturbances, growth retardation, anemia, hypothyroidism, bone and joint deformities, sterility, and liver and kidney abnormalities (Afkhami et al. 2009). Molybdenum concentrations were elevated in 16% of the investigated wells with maximum concentrations of 364 μg L−1 (Rania), 1900 μg L−1 (Chhiwali), and 35 μg L−1 (Godhrauli), compared to the health-based guideline value being 35 μg L−1 (WHO 2011). Elevated Mo levels were restricted to ODWs. To the best of our knowledge, elevated Mo concentrations have not been reported previously for any parts of Uttar Pradesh. Elevated Mo concentrations are possibly derived from weathering of black shales of the Himalayas (Rahaman et al. 2010).
Uranium contamination
Exposure to U can cause both radiological and chemical toxicities, e.g., nephrotoxicity, although the chemical toxicity is more relevant here (Kurttio et al. 2006). Uranium concentrations were found to be high in the study area, with maximum values of 115 μg L−1 (Rania), 350 μg L−1 (Chhiwali), and 108 μg L−1 (Godhrauli). Altogether, 59% of the wells exceeded the WHO guideline value of 30 μg L−1. These values are distinctly higher than those reported by Kumar et al. (2015) who found U concentrations up to 63 μg L−1 in Uttar Pradesh, with the maximum U value for Fatehpur being 36 μg L−1. Higher U concentrations were found in the state of Punjab, North India, with maximum U values of 579 μg L−1 (Bajwa et al. 2015). The reason for elevated U concentrations is unexplained, but local mineralogical composition may be the contributing factor (Kumar et al. 2015).
Problem of salinity
Groundwater salinity is a growing problem in several Indian states (Chakraborti et al. 2011). In Uttar Pradesh, instances of salinity have been reported previously, resulting mainly from excessive groundwater use for irrigation (Chakraborti et al. 2011). The WHO has not established a guideline value for salinity, but water with an EC up to 2.5 mS cm−1 is drinkable according to European Union (Drinking Water) Regulations (2014). The Bureau of Indian Standards (BIS 2009) has recommended a drinking water standard for salinity of 0.75 mS cm−1 that can be increased to 3.0 mS cm−1 in case of no alternate source. Relatively high values of EC were found in the study area with a maximum of 4.6 mS cm−1 (Rania), 16.2 mS cm−1 (Chhiwali), and 2.6 mS cm−1 (Godhrauli), although the ODWs were more affected than the other wells. Wells with elevated EC values higher than 2.5 mS cm−1 are marked in blue in Fig. 1. Overall, 17% of the wells showed EC values above the guideline value. However, 70% of the affected wells were placed in Chhiwali. Thus far, only local spots with EC values above 3.0 mS cm−1 have been reported for some districts in Uttar Pradesh (CGWB 2010). Elevated values of EC are not attributed to the COPR dumps, since they are also observed in wells located in areas unaffected by COPR. Indeed, high Cr(VI) concentrations lead to elevated EC values from 1 to 2 mS cm−1; the highest EC values (2.5 to 16 mS cm−1) were found in samples being totally uncontaminated with Cr.
Main cations and anions
Solutes like Cl−, SO4 2−, Ca, K, Mg, and Na are not a significant risk for human health even at higher concentrations as long as the salinity does not exceed critical values. However, they are important parameters for identifying the groundwater characteristics and tracking any temporal changes in the chemical composition of groundwater. The highest values of the aforementioned elements were found in wells in Chhiwali (Table S4). ODWs with depths around 11 m were more affected than other wells, although one CTW (55 m) and one submersible pump (91 m) also showed high Cl−, SO4 2−, and Na values.
Cr(VI) distribution in the groundwater
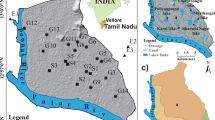
Figure 1 and Table S3 of the SM show the Cr(VI) distribution in the investigated samples. The samples were classified according to their total Cr concentrations as highly contaminated (Cr >1 mg L−1, red), moderately contaminated (0.05 mg L−1 < Cr < 1 mg L−1, orange), and uncontaminated (Cr <0.05 mg L−1, green).
Chromium(VI) concentrations in Rania ranged from below the detection limit (low detection limit (LDL)) up to 35 mg L−1. High Cr(VI) concentrations were mainly present in LHPs and CTWs but not in OWDs. However, even deeper aquifers (well no. 29) seem to be contaminated, with Cr(VI) concentrations up to 2.47 mg L−1 at 175-m depth. Overall, 21% of the wells were moderately contaminated and 28% were highly contaminated, with the highest Cr(VI) concentrations being found close to main COPR dumping site (23 mg L−1), northward along the street in Umran (35 mg L−1) and in the village Khan Chandpur (30 mg L−1) lying approximately 2 km south of the main dumping site (#E). Chromium(III) concentrations were found to be distinctly lower (up to 5.6 mg L−1) and were correlated with Cr(VI) concentrations. Since Cr(III) is not leached from COPR in significant amounts (data not shown), incidence of Cr(III) may point to a partial Cr(VI) reduction in the vadose zone or in the groundwater as well as the oxidation of organic material in soils by Cr(VI). Farmer et al. (2002) reported that Cr(VI) reduction by humic substances and the formation of Cr(III)–humic complexes in COPR affected groundwater in Glasgow. However, Cr(III) associated with organic material in groundwater was site-specific in the Glasgow study.
The villages Prasidhpur (1 km east of the dumpsite) and Bilsarayan (3 km south of the dumpsite) and the western part of Umran were uncontaminated. The high Cr(VI) levels in the wells south of the highway and north of Khan Chandpur could not be related to the presence of COPR dumpsites located in direct vicinity. We therefore hypothesize that the well contamination stems from a Cr(VI) plume spreading in south-southeastern direction from the main dumpsite north of the highway. If this were the case, the plume would have covered a distance of 1 km approximately since the operation of the dumpsite was initiated. The direction of groundwater flow and the rate of plume spreading were confirmed by the determination of the hydraulic gradient. Therefore, we used the piezometric levels and GPS coordinates of ODWs ∼3 and ∼1.5 km apart from one another, with one ODW located in the village of Khan Chandpur (Table S5). Evaluation of the hydraulic triangle gave a regional maximum hydraulic gradient of 0.000537 m m−1 with a south-southeastern groundwater flow direction. Assuming a saturated hydraulic conductivity of 10−3 m s−1 and a porosity of 0.5 m3 m−3, this yields a pore water velocity of 0.093 m day−1. Neglecting both adsorption of chromate and anion exclusion, this is equivalent to a 30-year traveling distance of 1 km approximately. Since COPR production in Rania started in the late 1980s, this provides a strong indication for the dumpsite as the cause of an advancing Cr(VI) plume. The main dumpsite is considered to be in the center of the plume, showing the highest Cr(VI) concentrations up to 80 mg L−1, measured through a piezometer installed at the dumpsite (Singh et al. 2013). Most probably, the contamination north of Umran could be considered disconnected from this plume as some smaller heaps were found along the roadside. Singh et al. (2013) investigated 21 wells in this area and reported a similar Cr(VI) distribution in Rania, but Cr(VI) concentrations northward along the street in Umran never exceeded 1.5 mg L−1. In general, Cr(VI) distribution varied on a small scale; e.g., two LHPs with the same depth, which were located in the northern part of Umran and separated by ∼10 m, had Cr(VI) concentrations of 0.21 (moderately contaminated) and 3.95 mg L−1 (highly contaminated).
In Chhiwali, Cr(VI) concentrations ranged from LDL to 115 mg L−1, although highly contaminated wells were only found adjacent to the dumping sites. Chromium(III) concentrations in groundwater were also distinctly lower with a maximum value of 6.3 mg L−1. Overall, 17% of the wells were moderately contaminated and 8% of the wells were highly contaminated. Furthermore, Cr(VI) contamination seems to be restricted to the upper aquifers, since no elevated Cr(VI) concentration was found below 40-m depth (Table S3 of the SM). Small-scale variability of the contamination was also observed here, but as opposed to Rania, no plume could be identified. Wells in the northeast and southwest of the dumpsite (#K) did not have elevated Cr(VI) concentrations; however, one well had a slightly elevated Cr(III) concentration (0.085 mg L−1). No Cr(VI) contamination was observed in the southeastern part of the study area of Chhiwali. Even the CTW located adjacent to the BCS manufacturing unit was uncontaminated. Contamination in the northwestern parts of Chhiwali seems bounded by a river, and an inflow of Cr(VI)-contaminated water into the river cannot be excluded.
In Godhrauli, 13% of the wells were moderately contaminated and 38% were highly contaminated with Cr(VI) concentrations ranging from LDL to 2.0 mg L−1. As reported by the villagers, visually, the Cr(VI) concentration of the moderately contaminated wells seems to increase at higher water usage. The data suggests that wells installed at a depth of ∼22 m were more contaminated than deeper wells (>30 m), although the number of samples is too low for any definitive conclusion. As described for Chhiwali, Cr(VI)-contaminated water was found close to the dumping sites and not elsewhere in this area.
Altogether, the Cr(VI) concentrations in the three investigated areas are in the range of the concentration reported for Glasgow and New Jersey (Burke et al. 1991; Farmer et al. 2002). However, the maximum value found in Chhiwali is, according to our knowledge, distinctly higher than that reported elsewhere in the world.
Correlation of selected parameters
Correlation coefficient of selected parameters indicated strong positive correlation between Cr(VI) and Cr(III) in the entire study area (Table 2). Chromium(VI) inflow arises from leaching of COPR dumpsites and is potentially at least partly reduced to Cr(III) by soil compounds during percolation through the subsurface. The association between As-Mo-U arises from the geogenic source of these metals, since there is no known anthropogenic source, which can contribute to this type of association in the study area. No significant correlations that were valid for the entire study area were found for F− and NO3 −. However, a high correlation exists in the Godhrauli area for NO3 − and Cr(VI), as well as Cr(III). Although this correlation is difficult to explain, some works reported a link between Cr(VI) reduction and the presence of NO3 −. Han et al. (2010) found an increase in chromate reduction when denitrification was high presumably because metabolites increased. These metabolites are used by chromate-reducing bacteria as electron donator, which seemed to be the link between nitrate and chromate. It should be noted that in the study area, many specifics exist and overlap, which makes correlation, in general, difficult.
Influence of monsoon precipitation
Results of the t test revealed no significant effect of the pre-monsoon and monsoon periods on groundwater contamination by Cr(VI). This is supported by the fact that Cr(VI) concentration increased in some wells in the monsoon season, while in others, it decreased (Tables 3 and S3). Median values were relatively unaffected by sampling season (Table 3). This holds for wells with Cr(VI) concentrations above the guideline value, where 11 wells had higher and 10 wells had lower Cr(VI) concentrations in the monsoon compared to the pre-monsoon season.
In the design of the study, we hypothesized that monsoon precipitation drives the influx of Cr(VI) from the COPR dumpsites to the center of contaminant plumes and possibly has a diluting effect at the plume fringes. However, this could not be confirmed. A possible reason could be the weak southwest monsoon season rainfall over India in 2014 (IMD 2015b). Seasonal rainfall was lower than normal in most parts of the country. Uttar Pradesh received less than 42% of the long-term average from 01 June 2014 to 30 September 2014 (IMD 2015b). Precipitation varied regionally resulting in even lower rainfall percentages in the districts Fatehpur (−68%) and Kanpur Dehat (−73%)(IMD 2015b). Furthermore, the timescales of infiltration and onward transport to the sampled locations may be larger than the 2-month monsoonal period considered in this study. In the investigated period, the seasonality of Cr(VI) levels may thus rather be attributed to other factors including (i) diurnal variations, (ii) irrigation effects, and (iii) replacement of water in ODWs. Diurnal variations in the individual wells seem to be dependent on water consumption. Some villagers reported that the water is apparently clean in the morning (colorless) and turns to be contaminated (yellowish) after longer use in the evening. This effect could bias the sample data since it was not possible to collect the samples at the same time of the day in the two sampling periods. The effects of irrigation are closely related to diurnal variations since irrigation greatly increases the actual water demand. In response to visually discernible contamination, in some cases, the villagers replaced the water in the ODWs. Obviously, water was only initially clean but turned contaminated within weeks. Under such conditions, observed Cr(VI) levels may not be representative depending on the time span between water replacement and sampling Cr(VI) levels. Altogether, it can be assumed that hydraulic windows exist between the different groundwater stories, e.g., when well drilling was inappropriate. Thus, wells providing water from uncontaminated aquifers may deliver contaminated water when consumption is high and contaminated aquifers are tapped.
It is noticeable that most of the wells showed decreased Ca and increased Mg concentrations during the monsoon season (Table S6), for which we do not have any explanations yet. However, no significant effect of the pre-monsoon and monsoon could be observed for Cl−, SO4 2−, K, and Na.
Conclusion and recommendations
Field sampling results showed that 95% of the sampled wells of the study area do not provide safe drinking water. Most samples exhibited multiple contamination with various elements and compounds exceeding international threshold values. Although F−, NO3 −, As, Mo, and U were found to be elevated in most of the investigated wells, Cr(VI) constitutes the most prominent hazard due to its acute toxicity and exceptionally high concentrations. The severity of Cr contamination could be gauged from the incidence of 19% of the wells that were classified as moderately contaminated and 23% as highly contaminated. The measured concentration of 115 mg L−1 in Chhiwali was higher than for COPR-affected groundwater bodies elsewhere in the world. A total of 17 COPR dumps were identified as sources of Cr(VI) groundwater contamination. Although the influence of monsoon precipitation on Cr(VI) mobilization from the dumpsites could not be established, this study clearly highlights the necessity of immediate monitoring of the identified contaminants. Monitoring of major components (Na, K, Ca, Mg, Cl−, SO4 2−, and HCO3 −) as well as hydrogen and oxygen stable isotopes may also be useful in order to identify the geochemical characteristics in water, including the temporal variability, and the connection between surface water and groundwater. There is an urgent need to inform the villagers about the danger of drinking the contaminated water. Color coding of the contaminated wells may be helpful in this context. Remediation of the contaminated groundwater may provide clean water to the local population on the medium term only. Therefore, interim solutions should be sought for. These could range from simple, yet robust, water treatment methods applicable at the level of individual households to the provision of safe drinking water by pipelines. The main COPR dumpsites must be remediated either in situ by encapsulation/stabilization or by disposal to a hazardous waste landfill.
This study is a first in-depth report on the reach of groundwater pollution at the COPR dumpsites around Kanpur based on a screening of potentially affected wells. Given this, some aspects remain tentative regarding the hydrogeological setting that should be addressed in future research. The use of multilevel sampling techniques and a better characterization of the downgradient transport are among the primary issues asides a solution for the safe water supply to the residents.
References
Afkhami A, Madrakian T, Amini A (2009) Mo(VI) and W(VI) removal from water samples by acid-treated high area carbon cloth. Desalination 243:258–264
Ansari AA, Singh IB, Tobschall HJ (1999) Status of anthropogenically induced metal pollution in the Kanpur-Unnao industrial region of the Ganga Plain, India. Environ Geol 38:25–33
Ansari AA, Singh IB, Tobschall HJ (2000) Role of monsoon rain on concentrations and dispersion patterns of metal pollutants in sediments and soils of the Ganga Plain. India Environ Geol 39:221–237
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2015) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci, in press. 1–9
BIS (2009) Draft Indian standard drinking water—specification. Second Revision of IS 1050. Bureau of Indian Standards. New Delhi, India.
Burke T, Fagliano J, Goldoft M, Hazen RE, Iglewicz R, McKee T (1991) Chromite ore processing residue in Hudson County, New Jersey. Environ Health Perspect 92:131–137
CGWB 2010 Ground water quality in shallow aquifers of India, Central Ground Water Board. Ministry of Water Resources, Government of India, Faridabad, India
Chakraborti D, Ghorai SK, Das B, Pal A, Nayak B, Shah BA (2009) Arsenic exposure through groundwater to the rural and urban population in the Allahabad-Kanpur track in the upper Ganga plain. J Environ Monitor 11:1455–1459
Chakraborti D, Das B, Murrill MT (2011) Examining India’s groundwater quality management. Environ Sci Technol 45:27–33
Chowdhury MI, Safiullah S, Iqbal Ali SM, Mofizuddin M, Enamul Kabir S (1982) Carbon transport in the Ganges and the Brahmaputra: preliminary results. Mitt Geo-Paläont Inst Univ Hamburg 52:457–468
Chrysochoou M, Dermatas D, Grubb DG, Moon DH, Christodoulatos C (2010) Importance of mineralogy in the geoenvironmental characterization and treatment of chromite ore processing residue. J Geotech Geoenviron Eng 136:510–521
Dwivedi AK, Vankar PS, Sahu RS (2015) Geochemical trends of heavy metal in aquifer system of Kanpur Industrial Zone, Uttar Pradesh (India): a case study. Environmental Earth Sciences 73:7287–7296
European Union (Drinking Water) Regulations (2014) European Commission
Farmer JG, Thomas RP, Graham MC, Geelhoed JS, Lumsdon DG, Paterson E (2002) Chromium speciation and fractionation in ground and surface waters in the vicinity of chromite ore processing residue disposal sites. J Environ Monitor 4:235–243
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect 112:1371–1374
Geelhoed JS, Meeussen JCL, Lumsdon DG, Hillier S, Roe MJ, Thomas RP, Bewley RJF, Farmer JG, Paterson E (2001) Modelling of chromium behaviour and transport at sites contaminated with chromite ore processing residue: implications for remediation methods. Environ Geochem Health 23:261–265
Geelhoed JS, Meeussen JCL, Hillier S, Lumsdon DG, Thomas RP, Farmer JG, Paterson E (2002) Identification and geochemical modeling of processes controlling leaching of Cr(VI) and other major elements from chromite ore processing residue. Geochim Cosmochim Acta 66:3927–3942
Gupta UC (1997) Symptoms of molybdenum deficiency and toxicity in crops. In: Gupta UC (Editor), Molybdenum in agriculture. Cambridge University Press, pp. 160–170
Han R, Geller JT, Yang L, Brodie EL, Chakraborty R, Larsen JT, Beller HR (2010) Physiological and transcriptional studies of Cr(VI) reduction under aerobic and denitrifying conditions by an aquifer-derived pseudomonad. Environ Sci Technol 44:7491–7497
Hettick BE, Canas-Carrell JE, French AD, Klein DM (2015) Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J. Agric Food Chem 63:7097–7097
Holmes AL, Wise SS, Wise JP (2008) Carcinogenicity of hexavalent chromium. Indian J Med Res 128:353–372
IMD (2015a) Indian Meteorological Department, Ministry of Earth Sciences, Government of India. http://hydro.imd.gov.in/hydrometweb/(S(spdjkny1vy412l55oyixsg3c))/DistrictRaifall.aspx. Accessed 3 Dec 2015
IMD (2015b) Annual report 2014. Indian Meteorological Department, Ministry of Earth Sciences, Government of India. www.imd.gov.in/section/nhac/monsoon_report_2014.pdf. Accessed 3 Dec 2015
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Kumar S, Saxena A (2011) Chemical weathering of the Indo-Gangetic alluvium with special reference to release of fluoride in the groundwater, Unnao District, Uttar Pradesh. J Geol Soc India 77:459–477
Kumar MP, Prerna S, Akash K, Prasad MK (2015) Uranium in ground water of eastern Uttar Pradesh, India: a preliminary study. Inter Res J Environ Sci 4:70–74
Kurttio P, Harmoinen A, Saha H, Salonen L, Karpas Z, Komulainen H, Auvinen A (2006) Kidney toxicity of ingested uranium from drinking water. Am J Kidney Dis 47:972–982
Li XB, Xu WB, Zhou QS, Peng ZH, Liu GH (2011) Leaching kinetics of acid-soluble Cr(VI) from chromite ore processing residue with hydrofluoric acid. J Cent S Univ Technol 18:399–405
Matern K, Kletti H, Mansfeldt T (2016) Chemical and mineralogical characterization of chromite ore processing residue from two Indian disposal sites. Chemosphere 155:188–195
Matern K, Mansfeldt T (2016) Chromium release from a COPR-contaminated soil at varying water content and redox conditions. J Environ Qual 45:1259–1267
Mumtaz N, Pandey G, Labhasetwar PK (2015) Global fluoride occurrence, available technologies for fluoride removal, and electrolytic defluoridation: a review. Crit Rev Environ Sci Technol 45:2357–2389
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Pechova A, Pavlata L (2007) Chromium as an essential nutrient: a review. Veterinarni Medicina 52:1–18
Prakash R, Srivastava SK, Singh M, Rastogi R, Singh K, Bhartariya KG (2011) Metallic contamination in ground water of Rania industrial area, Kanpur, UP. National Workshop on chemistry our life, our future. Lucknow, India
Rahaman W, Singh SK, Raghav S (2010) Dissolved Mo and U in rivers and estuaries of India: implication to geochemistry of redox sensitive elements and their marine budgets. Chem Geol 278:160–172
Raju NJ, Dey S, Gossel W, Wycisk P (2012) Fluoride hazard and assessment of groundwater quality in the semi-arid Upper Panda River basin, Sonbhadra district, Uttar Pradesh, India. Hydrol Sci J 57:1433–1452
Rao MBR (1973) The subsurface geology of the Indo-Gangetic plains. J Geol Soc India 14:217–242
Sankararamakrishnan N, Sharma AK, Iyengar L (2008) Contamination of nitrate and fluoride in ground water along the Ganges alluvial plain of Kanpur district, Uttar Pradesh, India. Environ Monit Assess 146:375–382
Shelnutt SR, Goad P, Belsito DV (2007) Dermatological toxicity of hexavalent chromium. Crit Rev Toxicol 37:375–387
Singh IB (1996) Geological evolution of Ganga plain—an overview. J Palaeontol Soc India 41:99–137
Singh KP, Malik A, Mohan D, Singh VK, Sinha S (2006a) Evaluation of groundwater quality in northern indo-Gangetic alluvium region. Environ Monit Assess 112:211–230
Singh KP, Singh VK, Malik A, Basant N (2006b) Distribution of nitrogen species in groundwater aquifers of an industrial area in alluvial Indo-Gangetic Plains—a case study. Environ Geochem Health 28:473–485
Singh RK, Sengupta B, Bali R, Shukla BP, Gurunadharao VVS, Srivatstava R (2009) Identification and mapping of chromium (VI) plume in groundwater for remediation: a case study at Kanpur, Uttar Pradesh. J Geol Soc India 74:49–57
Singh RK, Sachan VK, Ansari MQ, Pandey DS, Kamyotra JS (2013) Groundwater pollution due to chromium rich hazardous waste disposal in Rania-Khanchandpur area, Distt Kanur Dehat(R), U.P., India: a case study. Uttar Pradesh State biodiversity board. Lucknow, India
Sinha R, Tandon SK, Gibling MR (2010) Shallow sub-surface stratigraphy of the Ganga basin, Himalayan foreland: present status and future perspectives. Quat Int 227:81–86
Srinivasa Gowd S, Ramakrishna Reddy M, Govil PK (2010) Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J Hazard Mater 174:113–121
Srivastava A, Singh S, Prakash R, Srivastava S (2013) Impact of tanneries on groundwater quality in Kanchandpur area, Kanpur dehat district, UP. J Inst Public Health Eng 2:19–26
Srivastava A, Singh S (2014) Industrial impact of chromium, fluoride & salinity in Unnao UPSIDC area, Unnao, Uttar Pradesh. I J Sci Res Develop 1:2596–2600
Sun H, Brocato J, Costa M (2015) Oral chromium exposure and toxicity. Curr Environ Health Rep 2:295–303
Sun T, Chen J, Lei X, Zhou C (2014) Detoxification and immobilization of chromite ore processing residue with metakaolin-based geopolymer. J Environ Chem Eng 2:304–309
Underwood EJ (1976) Molybdenum in biological nitrogen fixation. Molybdenum in the environment. Marcel Dekker Inc., New York
US EPA (1992) SW-846, method 7196A, chromium, hexavalent (colorimetric). US Environmental Protection Agency, Washington
WHO (2011) Guidelines for drinking-water quality. Fourth ed. vol. 1 Recommendations. Geneva
Acknowledgements
This research was supported by the German Research Foundation (DFG) under contract no. Ma 2143/14-1 and Dr. Hohmann-Förderung of the Gesellschaft für Erdkunde zu Köln. The authors wish to thank the Founder and President of the Kanpur-based NGO “Eco Friends,” Mr. R. K. Jaiswal, for his constant support both from afar and (especially) on site. We also appreciate the valuable support of the Geoinformatics Lab in the Department of Civil Engineering at the IIT Kanpur. Further special thanks are owed to Mr. Imran Siddiqui of Super Tanneries Ltd., Kanpur.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kenneth Mei Yee Leung
Electronic supplementary material
ESM 1
(DOCX 116 kb)
Rights and permissions
About this article
Cite this article
Matern, K., Weigand, H., Singh, A. et al. Environmental status of groundwater affected by chromite ore processing residue (COPR) dumpsites during pre-monsoon and monsoon seasons. Environ Sci Pollut Res 24, 3582–3592 (2017). https://doi.org/10.1007/s11356-016-8110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8110-2