Abstract
Total Mercury (Hg) concentrations were determined in soil, river sediments and six (6) species of fish from the River Pra Basin in southwestern Ghana by Cold Vapour Atomic Absorption Spectrometry. Mercury concentration (μg g−1) ranged from 0.042 to 0.145 for soil: from 0.390 to 0.707 for sediments and from <0.001 to 0.370 for fish. All the fish samples had Hg concentration below the World Health Organisation (WHO) permissible limit of 0.5 μg g−1 whereas all the sediment samples had levels higher than the US-EPA value of 0.2 μg g−1. The results obtained from this study showed that fish from River Pra Basin are unlikely to constitute any significant mercury exposure to the public through consumption. No apparent trend of increasing mercury concentration along the main river as it flows downward toward the sea was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury is a highly toxic metal and its contamination of the environment has become a major issue of global concern. Mercury is a natural component of the earth crust, and it does not have any role in the human body. Mercury comes from a range of natural sources such as volcanoes, soils, mercury rich geological zones and forest fires, as well as from fresh water, lakes, rivers and the ocean (Renzoni et al. 1998). Increased use of mercury in gold recovery in mining operations in many developing countries in recent years has raised world concern over the release of this toxic metal into the environment. Previous experiences of human mercury poisoning in Manamata Bay in Japan (Irukayama 1977) and in Iraq (Bakir et al. 1973) indicate the dangers associated with mercury contamination. Recent research activities on the environmental impact of mercury from gold mining have concentrated mainly on Latin America, particularly the Brazilian Amazon. As a result, several research papers and scientific reports have been published on mercury contamination and health effects in the Amazon (Pfeiffer and Larceda 1988; Larceda and Salomons 1991; Nriagu et al. 1992; Akagi et al. 1995) Some work, however, has been undertaken to study environmental mercury contamination in Ghana where an increasing large number of people are engaged in artisanal gold mining using mercury amalgamation technique. Studies on assessment of mercury levels in water, sediments, soil, food crops, fish and some human tissues have been carried out in different areas including River Ankobra and Pra basins. (Amonoo-Neizer et al. 1996; Adimado and Baah 2002; Golow and Adzei 2003; Bannerman et al. 2003; Bonzongo et al. 2003; Babut et al. 2003; Golow and Mingle 2002; Donkor et al. 2006). But most of these studies did not attempt to link levels of mercury in the different compartments to the artisanal gold mining activities and therefore did not suggest that gold mining activities contribute mercury to the aquatic environment. Limited number of samples was also tested. Mercury released into river systems during gold ore-processing is likely to be transformed into the highly toxic methylmercury and becomes accumulated through biomagnification in aquatic food chains, particularly in fish. In spite of the considerable global concern about mercury contamination of fish and fishery products, information on mercury contamination of fish in freshwaters in Ghana is scanty. This research sought to determine the levels of mercury in different fish species, soil and surface sediments from the River Pra Basin in Southwestern Ghana and attempt to determine if any trend of increasing mercury concentrations along the river as it flows downstream could be established due to contributions from the tributaries where there is a concentration of small scale mining activities.
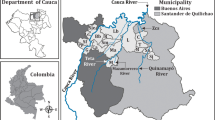
River Pra is one of the main rivers in which intense fishing activities are carried on. Topographical features demonstrate that river Pra has its source from the confluence of river Ofin and river Brim. Dunkwa, a small mining town, is located along river Ofin just before it enters river Pra. After this confluence, the river Pra then proceeds through Awisam, Twifo Praso, Twifo Mampong in the Central Region and down through Daboase and Beposo in the Western Region and finally joins the Gulf of Guinea at Shama also in the Western Region of Ghana (Fig. 1). Since artisanal gold mining activities using mercury are carried out close by the rivers it is likely that mercury could be carried along the river Pra. Aquatic species like fish may be affected by this contamination and eventually affecting living organisms that may also feed on these aquatic species.
Materials and Methods
Fish samples were obtained from River Pra at Awisam, Twifo Praso, Twifo Mampong, Daboase and Beposo between August 2005 and January 2006 from fishermen. Soil and sediments were also collected within the same period. Surface sediments were collected by the grab method and soil samples were taken about 200 m away from the bank of the river in each town. The samples were placed in clean plastic bags. Fish and sediment samples were stored on ice in an ice chest. All the samples were immediately transported to the laboratory. Sediment samples were sieved through 2 mm mesh, air-dried and homogenized by grinding using mortar and pestle. Soil samples were air-dried and sieved through 2 mm sieve. The fish samples were stored in the freezer after they had been sorted out and identified at the Theoretical and Applied Biology Department of the Kwame Nkrumah University of Science and Technology. They were later removed and allowed to thaw. The weight and total length of each fish were measured. A portion of edible muscle tissues were removed from the dorsal portion and stored in a freezer until analysis.
All glassware were soaked overnight in a detergent solution; rinsed and soaked in 10% (v/v) HNO3 solution overnight. They were rinsed with distilled water followed by 0.5% KMnO4 solution and rinsed again finally with distilled water before use. All reagents used were of analytical reagent grade (BDH Chemicals Ltd, Poole, England) unless otherwise stated. Double distilled water was used for the preparation of all solutions. Fish, sediments and soil samples were digested for total mercury determination by an open flask procedure developed at the National Institute for Minamata Disease (NIMD) in Japan by Akagi and Nishimura (1991) and reported earlier (Voegborlo et al. 2004). Total mercury concentrations were determined in all the digests by cold vapour atomic absorption spectrophotometry using an automatic Mercury Analyzer Model HG-5000 (Sanso Seisakusho Co., Ltd., Japan) developed at NIMD. The reducing reagent used in mercury analysis was 0.5 mL of 10% (w/v) SnCl2·2H2O in 1 M HCl. Quality assurance samples analyzed included procedural blanks, replicate samples and post-digestion spikes. The accuracy of the procedure was determined by analysis of certified reference material (Dogfish muscle, DORM-2) from the National Research Council (NCR) in Canada and Fish Homogenate Certified Reference Material IAEA-407 from International Atomic Energy Agency, Vienna. Recovery studies were performed by adding increasing amounts of mercury chloride standard solution to samples of two different fish species, which were taken through the digestion procedure. The resulting solutions were analyzed for mercury concentration.
Results and Discussion
Total Hg concentrations were determined in soil, sediments and fish species from River Pra at Awisam, Twifo Praso, Twifo Mampong, Daboase and Beposo. A total of one hundred and sixty (160) fish samples covering six (6) different species, five (5) soil and five (5) sediment samples were analysed. Results of mercury (Hg) concentration, fresh weight of fish are presented in Table 1. Mean results for soil and sediments are given in Table 2.
The validity of the method used in this study has been proved by the agreement between the measured concentration (mean = 4.67 μg−1, n = 3) which lies within the certified (range of 4.38–4.90 μg g−1) concentrations in the dogfish muscle (DORM-2) and concentrations obtained for the measured (mean = 0.215 μg g−1, n = 3) was in agreement with the certified values (0.216–0.228 μg g−1) for IAEA-407.
Recoveries were between 98.08% and 103.8%. Mercury concentrations in fish from Twifo Praso ranged from 0.040 to 0.083 μg g−1 wet weight for Labeo coubie (mean: 0.057), 0.033–0.011 μg g−1 wet weight for Tilapia zilii (mean: 0.059), from 0.093 to 0.257 μg g−1 wet weight for Chrysichthys sp. (mean: 0.187), from 0.143 to 0.187 μg g−1 wet weight for Heterobranchus longifillis (mean: 0.165) and from 0.025 to 0.069 μg g−1 wet weight for Alestis imberi (mean: 0.041). The highest Hg concentration was recorded in Chrysichthys sp. (0.257 μg g−1 wet weight) and the lowest Hg concentration was recorded in Alestis imberi (0.025 μg g−1 wet weight). Burger et al. (2001) reported site-specific differences in muscle tissue mercury levels in fish from the Savannah River and found that mercury concentrations generally reflected trophic levels. Fish species at high trophic levels showed higher Hg concentrations whereas those at lower trophic levels recorded low Hg levels. A similar trend was observed in this study. Hg concentrations increased with trophic levels. Fish species have been categorized into numerical trophic values where top predators were assigned a value of 5 and above; between 4.00 and 4.99 fish are classified as high level carnivores; middle level carnivores are assigned 3.00 to 3.99. The omnivores, herbivores or dentritivores are assigned values between 2.00 and 2.99 (Froese and Pauly 2009). Thus from Twifo Praso, Labeo coubie at trophic level 2.0 (Froese and Pauly 2009) recorded Hg concentration that ranged between 0.040 and 0.083 μg g−1 wet weight (mean: 0.057) whereas Chrysichthys sp., at trophic level 3.4 (Froese and Pauly 2009) recorded Hg concentration range of 0.092–0.257 μg g−1 wet weight (mean: 0.187). Lange et al. (1994) also showed that mercury concentration varies with fresh weight of fish and total length. High correlation between mercury concentration and total length and fresh weight of fish are normally observed among carnivorous species whereas poor correlations are observed among herbivorous species. Among the fish species studied at Twifo Praso, there was a poor correlation between Hg concentration and fresh weight of fish for Labeo coubie (r 2 = 0.1076, trophic level = 2.0), Tilapia zilli (r 2 = 0.1219, trophic level = 2.0), Chrysichthys sp. (r 2 = 0.1456, trophic level = 3.4), Heterobranchus longifillis (r 2 = 0.3864, trophic level = 3.7) and Alestis imberi (r 2 = 0.0042, trophic level = 3.2). Mercury concentrations ranged between 0.018 and 0.057 μg g−1 (mean: 0.042) wet weight for Alestis imberi from Twifo Mampong and between below 0.001 and 0.136 μg g−1 (mean: 0.071) wet weight for Labeo coubie from Awisam. There was poor correlation between mercury concentration and total length (r 2 = 0.0404) and fresh weight (r 2 = 0.0453) for Alestis imberi. Labeo coubie from Awisam showed good correlation between mercury concentration and fresh weight (r 2 = 0.697). This is in agreement with other authors who reported similar findings for carnivorous species (Lange et al. 1994). Mercury concentrations in the muscle tissue of fish from Beposo ranged from 0.014 to 0.216 μg g−1 (mean: 0.065) wet weight for Alestis imberi, from 0.104 to 0.370 μg g−1 (mean: 0.152) wet weight for Chrysichthys sp., from 0.035 to 0.043 μg g−1 (mean: 0.039) wet weight for Labeo coubie and from 0.019 to 0.163 μg g−1 (mean: 0.052) wet weight for Tilapia multifasciata. Hg concentrations were higher for fish at higher trophic levels. For samples from Daboase, Hg concentration ranged from 0.020 to 0.037 μg g−1 wet weight for Labeo coubie (mean: 0.029), 0.019–0.041 μg g−1 wet weight for Tilapia zilli (mean: 0.028), 0.013–0.042 μg g−1 wet weight for Tilapia multifasciata (mean: 0.026) and 0.069–0.315 μg g−1 wet weight for Chrysichthys sp. (mean: 0.165). There was good correlation between Hg concentration and fresh weight (r 2 = 0.8038) and total length (r 2 = 0.8449) for Tilapia zilli. There was however, poor correlation between Hg concentration and total length and fresh weight for Labeo coubie, Tilapia multifasciata and Chrysichthys sp. The mean Hg concentration recorded for river sediments ranged from 0.390 to 0.707 μg g−1 with the highest being recorded at Daboase and the least at Twifo Praso whereas for soils the levels ranged from 0.042 to 0.145 μg g−1 with the highest being recorded at Beposo and the lowest at Twifo Praso.
In an earlier study of mercury levels in sediments and soil within the river Pra basin, lower concentrations were reported (Donkor et al. 2006). For sediments a range of 0.007 to 0.057 μg g−1 (mean = 0.026) was reported for the lower Pra basin and a range of 0.013 to 0.023 μg g−1 (mean = 0.018) for the upper Pra basin. For soil, mercury levels ranged 0.003–0.202 (mean = 0.076) and 0.012–0.034 (mean = 0.025) for the lower and upper Pra basins respectively. The difference could be attributed to different sampling periods, seasons, locations with respect to the mining areas, volume and flux of waterways and particle size distribution of the sediments (Larceda and Salomons 1991). When our results were compared with levels reported for elsewhere, differences were observed. The levels obtained for sediment from the Brazilian Madeira river (0.030–0.350 μg g−1; mean = 0.130) and from Brazilian Tapajos river (0.170–0.430 μg g−1; mean = 0.290) were lower than values reported in this study. Far higher values were however reported for sediments from Philippines Mindanao island (0.920–66.470; mean = 21.030). On the other hand mercury levels reported for soil in this study were lower than levels reported for Brazil Madeira river (0.270–0.540 μg g−1; mean = 0.390) and for Brazil Tapajos river (0.180–0.360 μg g−1). Recent mercury accumulation rate in sediments is reported to increase with concentrations in fish. Hunter et al. (1987) reported that more than 90% of the surface sediments of the Onondaga Lake contained mercury concentrations greater than 0.1 ppm. In the same year, mercury levels in fish were also found to exceed 0.5 ppm, the maximum permissible levels established by the World Health Organisation (WHO).
The levels obtained for fish by Hunter et al. (1987) are higher than the levels recorded in the fish species under this study even though high levels were obtained for the sediments. Mercury concentrations in bed sediments are not necessarily correlated with concentrations in fish tissues (Rose et al. 1999). The results of this study appeared to follow a similar trend as poor correlation was observed between mercury concentration in fish and sediments at all the sampling sites. The relationship between mercury concentrations of fish and sediments vary as a function of factors that affect sediment methylation rates and mercury bioavailability. Although some studies have shown that sediments can be a sink for mercury (Rudd and Turner 1983; Sorensen et al. 1990), mercury accumulation by fish depends on the combined effect of the abundance of available inorganic mercury in sediments/water column, trophic interaction and the rate at which microflora transform mercury into methylmercury in addition to the species-specific accumulation and seasonal variations (Jackson 1990). That mercury concentrations in soil from similar areas were far lower than concentrations in sediments suggests that atmospheric deposition was not significant.
River Pra has three main tributaries namely, River Anum, River Oda and Fumso River. Small scale artisanal gold mining activities are widespread along these rivers. The low mercury concentration in all the species studied in River Pra can be ascribed to low input of Hg into the environment from artisanal gold mining which involves the use of Hg; and factors affecting methylation of mercury were probably not favourable. Human activities such as agriculture that employs the use of mercurial compounds could be minimal. An attempt to determine any trend of mercury accumulation along the main river Pra will require the use of an indicator obtained at all the sampling locations. But, no specie of fish was obtained at all the sampling locations. Labeo coubie was available at four out of the five locations and was therefore used as the indicator. Even though generally, good correlation between mercury concentrations and fish fresh weight was not obtained, mercury concentration was normalized in order to reduce any effect of variable size composition of the samples. Labeo coubie did not show any consistent trend along the main river as it flows downward toward the sea (Awisam = 0.290 μg; Twifo Praso = 0.277 μg; Daboasi = 0.073 μg; Beposo = 0.115 μg). Similarly, no trend was exhibited for the sediments. All the fish samples from the five locations along the Pra River recorded mercury concentrations below the World Health Organisation’s threshold value of 0.5 μg g−1. The results obtained in this study therefore showed that fish from River Pra does not constitute any significant methylmercury exposure to the public through fish consumption from the studied areas. Whereas the mean values for soil in this study were below the US-EPA value of 0.2 μg g−1, the mean values for sediment exceeded it.
References
Adimado AA, Baah DA (2002) Mercury in human blood, urine, hair, nail and fish from the Ankobra and Tano river Basin in South Western Ghana. Bull Environ Contam Toxicol 68:339–346
Akagi H, Nishimura H (1991) Speciation of mercury in the environment. In: Suzuki T, Imura N, Clarkson TW (eds) Advances in mercury toxicology. Plenum Press, New York, USA, pp 53–76
Akagi H, Malm O, Branches FJP, Kinjo Y, Kashima Y, Guimaraes JRD, Oliveira R, Haraguchi BK, Pfeiffer WC, Takizawa Y, Kato H (1995) Human exposure to mercury due to gold mining in the Papajos river basin, Amazon, Brazil: speciation of mercury in human hair, blood and urine. Water Air Soil Pollut 80:85–94
Amonoo-Neizer EH, Nyamah D, Bakiamoh SB (1996) Mercury and arsenic pollution in soil and biological samples around the mining towns of Obuasi, Ghana. Water Air Soil Pollut 91:363–373
Babut M, Sekyi R, Rambaud A (2003) Improving the environmental management of small scale gold mining in Ghana: a case study of Dumasi. J Cleaner Prod 11:215–221
Bakir F, Jamluji SF, Amin-Zaki L, Murtadha M, Khalidi NY, Al-Rawi S, Tikriti HI, Dhahir TW, Clarkson JC, Doherty RA (1973) Methylmercury poisoning in Iraq. Science 181:230–241
Bannerman W, Potin-Gautier M, Amouroux D, Tellier S, Rambaud A, Babut M, Adimado A, Beinhoff C (2003) Mercury and arsenic in the gold mining regions of the Ankobra river basin in Ghana. J Phys IV 107:107–110
Bonzongo JC, Donkor AK, Nartey VK (2003) Environmental impacts of mercury related to artisanal gold mining in Ghana. J Phy IV 107:217–220
Burger J, Gaines KF, Boring CS, Stephens WL Jr, Snodgrass J, Gochfeld M (2001) Mercury and selenium in fish from Savannah River: species, trophic levels and differences. Environ Res 87:108–118
Donkor AK, Bonzongo JC, Nartey VK, Adotey DK (2006) Mercury in different environmental compartments of the Pra River Basin, Ghana. Sci Total Environ 368:164–176
Froese R, Pauly D (2009) Editors 2009. Fish Base World Wide Web electronic publication www.fishbase.org version (10/2009)
Golow AA, Adzei EA (2003) Mercury in the surface soil and cassava crop near an alluvial goldmine at Dunkwa-on-Offin, an alluvial goldmine, Ghana. Bull Environ Contam Toxicol 69:228–235
Golow AA, Mingle LC (2002) Mercury in river water and sediments in some rivers near Dunkwa-on-Offin, an alluvial goldmine, Ghana. Bull Environ Contam Toxicol 70:379–384
Hunter DB, Leatherland JF, Stokes PM (1987) The effects of polychlorinated biphenyls and methyl mercury, singly adding combination on mink II. Reproduction and kit development. Arch Environ Contam Toxicol 16:449–454
Irukayama K (1977) Case history of minamata. In: Tsubaki T, Irukayama K (eds) Minamata desease, methylmerury poisoning in minamata and niigata. Japan, Kodansah Ltd. (Tokyo) and Elsevier Science. Publ. Co., Amsterdam, pp 2–56
Jackson TA (1990) Biological and Environmental control of mercury accumulation by fish in lakes and reservoirs of northern Manitoba, Canada. Can J Fish Aquat Sci 48:2449–2470
Lange TR, Royals HE, Connor LL (1994) Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida. Lake Fla Game Freshw Fish Comm 32:727
Larceda LD, Salomons W (1991) Mercury in the amazon: a chemical time bomb? A report sponsored by the Dutch ministry of housing. Phys Plann Environ, 47 pp
Nriagu JO, Pfeiffer WC, Malm O, Mierle G (1992) Mercury pollution in Brazil. Nature 356:389
Pfeiffer WC, Larceda LD (1988) Mercury input into the Amazon region, Brazil. Environ Technol Lett 9:325–330
Renzoni A, Zino F, Franchi E (1998) Mercury levels along the food chain and risk for exposed populations. Environ Res 77:68–72
Rose R, Hutcheson MS, West CR, Pancorbo O, Hulme K, Cooperman A, DeCesare G, Isaac R, Screpetis A (1999) Fish mercury distribution in Massachusetts, USA lakes. Environ Toxicol Chem 18:1370–1379
Rudd JWM, Turner MA (1983) Suppression of mercury and selenium bioaccumulation by suspended and bottom sediments. Can J Fish Aquat Sci 40:2218–2227
Sorensen JA, Glass GE, Schmidt KW, Huber JK, Rapp GR Jr (1990) Airborne mercury deposition and watershed characteristics in relation to mercury concentrations in water, sediments, plankton and fish of eighty northern Minnesota lakes. Environ Sci Technol 24:1716–1727
Voegborlo RB, Baah DA, Kwaansa-Ansah EE, Adimado AA, Ephraim JH (2004) Mercury concentrations in fish species from the Gulf of Guinea, Ghana. Bull Environ Contam Toxicol 73:1057–1064
Acknowledgments
The authors would like to thank Mr. Munir Abdullah Dawood of Pure and Applied Biology Department, Faculty of Biosciences, Kwame Nkrumah University of Science and Technology for identifying the fish species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oppong, S.O.B., Voegborlo, R.B., Agorku, S.E. et al. Total Mercury in Fish, Sediments and Soil from the River Pra Basin, Southwestern Ghana. Bull Environ Contam Toxicol 85, 324–329 (2010). https://doi.org/10.1007/s00128-010-0059-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-0059-0