Abstract
Homing pigeons (Columba livia) were used as a model to assess the effects of chlorpyrifos and aldicarb on flight times at sub-lethal, environmentally relevant concentrations. A significant increase in flight times of birds dosed with aldicarb and with chlorpyrifos was observed. Plasma cholinesterase activity was measured over time following exposure to either compound. The results suggest that the time of peak inhibition would correlate with migratory flight activity after exposure. In total, the results of these studies show that sub-lethal exposure to cholinesterase-inhibiting pesticides can affect the flying ability of non-target avian species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since the banning of organochlorine pesticides in the 1970’s in the U.S., cholinesterase (ChE) inhibiting compounds have become widely used on agricultural and residential areas to control insect infestations. Although these compounds are effective in controlling the targeted insects, there have been numerous documented poisoning events to non-target species. Among the most vulnerable of non-target species are birds, in particular migratory birds. These species have the potential to be exposed to harmful contaminants over vast geographic areas as they breed and winter in drastically different locations, some even on different continents (Gard and Hooper 1995). Because of the conversion of many migratory bird habitats to agriculture, these species of birds have been forced to use farmland as their feeding and roosting sites thereby increasing the potential of exposure to ChE inhibiting compounds (Iko et al. 2003). Two of the most widely used ChE inhibiting insecticides in the U.S. are chlorpyrifos (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate), an organophosphate, and aldicarb (2-methyl-2-(methylthio) propionaldehyde O-methylcarbamoyloxime), a carbamate. The National Pesticide Use Database reports that in 2002, chlorpyrifos and aldicarb were the second and fourth most applied insecticides in the U.S., respectively (Gianessi and Reigner 2006).
There is growing concern that exposure to ChE inhibiting insecticides may have negative effects on avian populations by affecting physiological and behavioral responses. When northern bobwhites (Colinus virginianus) and mourning doves (Zenaida macroura) received an acute dose of aldicarb, they were limited in their cover seeking ability due to the rapid onset of physical impairment (Hawkes et al. 1996). Additionally, northern bobwhites exposed to chlorpyrifos applied at a rate of 6.7-kg active ingredient/hectare exhibited significant behavioral anomalies such as drooping wings, staggering gait, and failure to respond to auditory stimuli (Booth et al. 2005). There is, however, limited information describing the effects of low-level, environmentally relevant concentrations of ChE inhibitors on the flying ability of avian species. When white-throated sparrows (Zonotrichia albicollis) were dosed with an environmentally relevant concentration (256 ppm) of the organophosphate acephate, they were unable to establish a preferred migratory orientation and exhibited random activity (Vyas et al. 1995). The comparison of differences in flight time in pigeons may provide insights into potential impacts on migratory birds as well as other avian species. With the understanding that compromised flying efficiency and orientation may lead to negative effects on avian migration, homing pigeons (Columba livia) were used to assess the effects of chlorpyrifos and aldicarb on flight times and related ChE inhibition at sublethal, environmentally relevant concentrations.
Materials and Methods
Pigeons were housed and trained at the campus approved homing pigeon research facility located at the University of Nevada, Reno Agricultural Experiment Station. These studies were conducted under an approved protocol by the university’s animal care and use committee. Training of birds occurred in a manner similar to that in the sport of pigeon racing. In general, pigeons are trained to home to their respective lofts prior to race day. Pigeons are judged on average flight speed during the race based on time of release, time of arrival, and distance of flight. In the current study, birds were released in groups of 10 or fewer and trained to sequentially longer distances until a final distance of 150 miles was reached. The birds were then banded with an electronic chip ring on the left leg to be timed for homing to the loft with a Benzing Atis Top timer and scanner (Munich, Germany). Prior to transport, the home timer and release point clocks were synchronized. Releases occurred within the same 2 h window to aid in orientation.
Three control flights with no gavage were conducted with every bird included in the study. Birds were then ranked according to average flight time and divided into 5 groups of 7 in order to ensure an even distribution of flight times between groups. Birds were not fed or given water the morning of the experimental flights because crop contents could affect the absorption of the toxin. Pure analytical grade chlorpyrifos and aldicarb were purchased from Chem Service, Inc. (West Chester, PA). The chemicals were weighed into 10 ml dose equivalents, chlorpyrifos at 3 and 5 mg/kg and aldicarb at 0.25 and 0.5 mg/kg, and then they were dissolved in 10 ml polyethylene glycol (PEG) prior to being suspended in 40 ml of deionized water. The blank vehicle control (0.0 mg/kg) contained 10 ml PEG and 40 ml deionized water. Dosed flights, including vehicle control, were conducted approximately 1 week apart, allowing time for chemical clearance and a rest period between flights. Upon arrival at the release point, the birds were dosed according to their weight. Once dosed, they were then allowed to sit for 10 min prior to their release. The 5 groups of 7 birds were released in a randomly selected order for each flight.
Cholinesterase inhibition by these compounds was determined by repeated sampling of subjects over a time course. The birds were divided into 9 groups of 5 birds each. Each treatment group received a different concentration of chlorpyrifos at 1.0, 2.5, 5.0, and 7.5 mg/kg or aldicarb at 0.1, 0.25, 0.5, and 1.0 mg/kg. Control birds received the blank vehicle (0.00 mg/kg). Pesticide solution preparation was as described for the flight study. Blood samples were collected at 0, 1, 4, 8, 24, and 48 h after dosing. Whole blood (up to 300ul) was collected by puncturing the brachial vein with a 26G-1/2” needle and filling capillary blood collection tubes containing lithium heparin (Ram Scientific, Yonkers, NY). Blood was stored on ice, and then centrifuged at 4,000g at 4°C for 6 min to separate plasma from erythrocytes. Plasma was stored undiluted at −70°C until analysis. Plasma ChE activity was determined colorimetrically using the Ellman method (Ellman et al. 1961) as modified by Nostrandt et al. (1993) for use with carbamate inhibited ChE and analyzed with an automated microplate reader (BioTek Instruments Inc., Winooski, VT). Enzyme activity was expressed as a percent of initial ChE activity.
For the flight study, One-Way Analysis of Variance (ANOVA) with Tukey’s post test and t-tests were performed to determine the effects of dosage/concentration on flight times and differences in flight times with repeated dosing among and within treatment groups. For the ChE inhibition study, comparisons between time points for each concentration and comparisons among concentrations at each time point were performed using ANOVA with a Dunnett’s Multiple Comparison post test and Student’s t-tests. All statistics were performed using Prism 3.0 software (GraphPad, San Diego, CA).
Results and Discussion
The pigeons were allowed adequate training time to demonstrate consistent flight times prior to dosing. Because undosed (no gavage) control flights were initiated prior to any dosed flights, all birds were able to serve as their own controls. Dosed flights included dosing birds with either chlorpyrifos or aldicarb at non-lethal concentrations or vehicle control. The dosed vehicle control group (0.0 mg/kg) flight times were not statistically different from the average undosed flight times for any group of birds (Table 1). These results demonstrate that there were no differences in flight times between groups when untreated. Comparison of the control undosed and control dosed flight times (Table 1) also demonstrated that there was no statistical difference in flight times due to gavage. The flight times of chlorpyrifos treated birds were not significantly different from their undosed flight times (Table 1). Gavaged vehicle controls were, however, 12 min faster than their un-gavaged (undosed) flights (Table 1). This non-statistically significant difference in flight times between gavaged and un-gavaged control groups resulted in the 3.0 mg/kg chlorpyrifos group becoming significantly different when compared to the 0.00 mg/kg gavaged group (Fig. 1). The fact that birds exposed to chlorpyrifos (3.0 mg/kg) exhibited significantly slower flight times when compared to vehicle controls (0.00 mg/kg) flying under the same weather conditions substantiates the toxic effect of the pesticide. The aldicarb dosed groups resulted in significantly slower flight times when compared to both vehicle controls (0.00 mg/kg, Fig. 1) and their respective undosed return times (Table 1). Thus the effect of both pesticides was considerably greater than the effect of gavage. Aldicarb at sub-lethal levels had a greater impact on flight time than chlorpyrifos. These results are consistent with previous studies from this lab which showed that carbamates have a greater effect than organophosphates on flight times (Brasel et al. 2007).
Comparison of flight times from gavaged vehicle controls (0.00 mg/kg) and treated birds (error bars = mean ± SEM, n = 7 for 0.00 mg/kg group, n = 6 for 0.25 mg/kg aldicarb group, n = 5 for 0.5 mg/kg aldicarb group, n = 7 for 3.0 mg/kg chlorpyrifos group, and n = 6 for 5.0 mg/kg chlorpyrifos group). ** p < 0.01 and * p < 0.05 versus 0.00 mg/kg, ANOVA with Tukey’s Multiple Comparison Test
Migratory birds, as well as pigeons, rely on magnetic compass orientation as the basis of their navigational system (Wiltschko and Wiltschko 2003). It has been demonstrated that orientation may be affected when birds are exposed to an external magnetic pulse which alters neurologically based processes (Walcott et al. 1979). Cholinesterase inhibition from organophosphate or carbamate compounds, in theory, would also interfere with this system given that neurotransmission would be compromised. Thus, ChE levels were assessed to correlate results from the flight study.
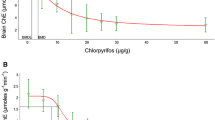
The dosages chosen for this study were based on previous studies in our laboratory in which sub-lethal levels of organophosphate and carbamate insecticides were administered to homing pigeons (Brasel et al. 2007), the oral LD50 values of both compounds, and other studies in which anticholinesterase compounds were tested on birds at sub-lethal concentrations. Exposure to either chlorpyrifos or aldicarb at these environmentally relevant dosages resulted in a dramatic decrease in plasma ChE activity. Chlorpyrifos dosed birds exhibited a dose dependent decrease in activity at the initial time measurements (1 and 4 h) with full recovery at all dosage levels by 48 h. Birds exposed to the highest concentration (7.5 mg/kg) took much longer to fully recover. Full recovery was observed by 48 h post-dosing whereas all other groups had recovered by 8 h (Fig. 2a). Aldicarb exposure resulted in slightly different results. All dosage groups showed extreme plasma ChE depression, to around 10% of initial activity, at the first post-dose measurement (1 h). All groups of aldicarb exposed birds completely recovered by 24 h whereas chlorpyrifos exposed birds had not fully recovered in that time period. Additionally, aldicarb dosed birds exhibited a significant increase in ChE activity at 24 and 48 h post exposure (Fig. 2b). Recovery of ChE levels after exposure to a ChE-inhibiting pesticide is the result of two separate processes, the first being spontaneous reactivation of the inhibited enzyme by hydrolysis. The second is new enzyme synthesis (Blaber and Creasey 1960). In the case of inhibition from carbamate exposure, reactivation of the carbamyl-cholinesterase intermediate to regenerate an active enzyme is a rapid process (Kwong 2002). The differing rates of reactivation of organophosphates and carbamates may explain results seen in the current study.
a Plasma ChE activity after an oral dose of chlorpyrifos (n = 5 for each dosage group, error bars = mean ± SEM). Significant difference from vehicle control (0.00 mg/kg) values: at 1 h p < 0.0001 from all groups except 1.0 mg/kg; at 4 h p < 0.0001 from all groups except 1.0 mg/kg; at 8 h p < 0.001 from 7.5 mg/kg group; at 24 h p < 0.05 from 7.5 mg/kg group. b Plasma ChE activity after an oral dose of aldicarb (n = 5 for each dosage group, error bars = mean ± SEM). Significant difference from control (0.00 mg/kg) values: at 1 h p < 0.001 all groups; at 4 h p < 0.001 all groups; at 8 h p < 0.05 for 0.1, 0.25, and 0.5 mg/kg groups and p < 0.001 for 1.0 mg/kg group. Significant rebound from initial values: at 24 h p < 0.05, p < 0.001, p < 0.001 from the 0.25, 0.5, and 1.0 mg/kg groups, respectively; at 48 h p < 0.05, p < 0.001, p < 0.001 from the 0.25, 0.5, and 1.0 mg/kg groups, respectively
Reductions in ChE activity have been shown to elicit a number of sub-lethal, behavioral effects on avian species, both in laboratory and field studies. The inhibition of ChE caused by exposure to anticholinesterase insecticides has resulted in an alteration of nesting behavior (Busby et al. 1990), increased susceptibility to predation (Hunt et al. 1992), and decreased food consumption (Grue et al. 1997). The time of peak inhibition observed after ChE activity analysis would also correlate with behavioral differences in migratory flight activity after exposure to a contaminated food or water source, as seen in the flight study.
The levels of pesticides chosen for these studies represented realistic environmental exposure levels of non-target avian species drinking from contaminated irrigation water or eating vegetation or pesticide granules on farmlands after application (Hill and Camardese 1984; Booth et al. 2005). The implications of these studies for migratory birds are important due to their frequent use of agricultural areas where cholinesterase inhibiting pesticides are extensively applied. The repercussions of these potential migratory effects from pesticide exposure on population levels require further investigation.
References
Blaber LC, Creasey NH (1960) The mode of recovery of cholinesterase activity in vivo after organphosphorus poisoning. Biochem J 77:597–604
Booth GM, Mortensen SR, Carter MW, Schaalje BG (2005) Hazard evaluation for northern bobwhite quail (Colinus virginianus) exposed to chlorpyrifos-treated turf and seed. Ecotoxicol Environ Saf 60:176–187
Brasel JM, Collier AB, Pritsos CA (2007) Differential toxic effects of carbofuran and diazinon on time of flight in pigeons (Columba livia): potential for pesticide effects on migration. Toxicol Appl Pharmacol 219:241–246
Busby DG, White LM, Pearce PA (1990) Effects of aerial spraying of fenitrothion on breeding white-throated sparrows. J Appl Ecol 27:743–755
Ellman GL, Courtney KD, Valentino Andres J, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Gard NW, Hooper MJ (1995) An assessment of potential hazards of pesticides and environmental contaminants. In: Martin TE, Finch DM (eds) Ecology and management of neotropical migratory birds. Oxford University Press, New York
Gianessi L, Reigner N (2006) Pesticide use in U.S. Crop Production: 2002. Crop Protection Research Institute, pp 1–36
Grue CE, Gibert PL, Seeley ME (1997) Neurophysiological and behavioral changes in non-target wildlife exposed to organophosphate and carbamate pesticides: thermoregulation, food consumption, and reproduction. Am Zool 37:369–388
Hawkes AW, Brewer LW, Hobson JF, Hooper MJ, Kendall RJ (1996) Survival and cover seeking response of northern bobwhites and mourning doves dosed with aldicarb. Environ Toxicol Chem 15:1538–1543
Hill EF, Camardese MB (1984) Toxicity of anticholinesterase insecticides to birds: technical grade versus granular formulations. Ecotoxicol Environ Saf 8:551–563
Hunt KA, Bird DM, Mineau P, Shutt L (1992) Selective predation of organophosphate-exposed prey by american kestrels. Animal Behav 43:971–976
Iko WM, Archuleta AS et al (2003) Plasma cholinesterase levels of mountain plovers (Charadrius montanus) wintering in Central California, USA. Environ Toxicol Chem 22(1):119–125
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149
Nostrandt AC, Duncan JA, Padilla S (1993) A modified spectrophotometric method appropriate for measuring cholinesterase activity in tissue from carbaryl-treated animals. Toxicol Sci 21:196–203
Vyas NB, Kuenzel WJ, Hill EF, Sauer JR (1995) Acephate affects migratory orientation of the white-throated sparrow (Zonotrichia albicollis). Environ Toxicol Chem 14:1961–1965
Walcott C, Gould JL, Kirschvink JL (1979) Pigeons have magnets. Science 205:1027–1029
Wiltschko R, Wiltschko W (2003) Avian navigation: from historical to modern concepts. Animal Behav 65:257–272
Acknowledgments
These studies were funded in part by Nevada Agricultural Experiment Station Hatch Grant 0731.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moye, J.K., Pritsos, C.A. Effects of Chlorpyrifos and Aldicarb on Flight Activity and Related Cholinesterase Inhibition in Homing Pigeons, Columba livia: Potential for Migration Effects. Bull Environ Contam Toxicol 84, 677–681 (2010). https://doi.org/10.1007/s00128-010-0020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-010-0020-2