Abstract
Perfluoroalkyl acids (PFAAs) are found globally in environmental samples and have been studied in various species. In this study, we compare the sensitivity of three avian species to the toxic effects of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). Eggs of great cormorant (Phalacrocorax carbo sinensis), herring gull (Larus argentatus) and the domestic White Leghorn chicken (Gallus gallus domesticus) were exposed in ovo by injection into the air sac. Effects on embryo survival were observed following exposure to PFOS and PFOA in chicken and herring gull. Chicken was found to be the most sensitive species with 50 % reduced embryo survival at 8.5 μg/g egg for PFOS and 2.5 μg/g egg for PFOA. Cormorant was shown to be the least sensitive species. The difference in sensitivity between chicken and herring gull was a factor of 2.7 for PFOS and 3.5 for PFOA. Between chicken and great cormorant, the sensitivity difference was 2.6 for PFOS and 8.2 for PFOA. Effects on embryo survival were seen at egg injection doses of PFOS close to levels found in environmental samples from wild birds, indicating that PFOS could be having effects in highly exposed populations of birds. This study also shows that there are differences in species sensitivity to PFOS and PFOA that should be taken into consideration in avian wildlife risk assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) belong to a group of chemicals called perfluoroalkyl acids (PFAAs) that have been used for over half a century in numerous applications such as water-, oil- and dirt-resistant treatments for textiles, oil-resistant paper coatings, polishes and fire-fighting foams (Kissa 2001). These chemicals are resistant to degradation (Key et al. 1998) which makes them persistent and together with their numerous applications ubiquitous in wildlife and the environment. PFAAs are present in arctic snow, freshwater lakes, sea water, tap water, fish, birds, mammals, and humans (Boulanger et al. 2004; Ericson et al. 2008; Giesy and Kannan 2001; Kannan et al. 2005; Kärrman et al. 2007; Schuetze et al. 2010; Taniyasu et al. 2003). In eggs of great cormorant (Phalacrocorax carbo sinensis) and herring gull (Larus argentatus) from Lake Vänern, Sweden, PFOS has been found in concentrations of 419–1163 ng/g (median 552 ng/g) and 38.5–771 ng/g (median 292 ng/g), respectively (Nordén et al. 2013). Also, guillemot eggs (Uria aalge) from the Baltic Sea have high levels of PFOS (Holmström et al. 2005). The median PFOA levels in Lake Vänern cormorants and herring gulls were 4.05 and 0.50 ng/g, respectively. PFOA is not as common as PFOS in wildlife samples, but in livers from cormorants in the Mediterranean Sea, PFOA has been found at 29–450 ng/g (mean 95 ng/g) (Kannan et al. 2002).
Studies on hatching or pipping success of chicken embryos (Gallus gallus domesticus) after exposure via egg injections have reported median lethal dose (LD50) values for PFOS at 4.9 and 93 μg/g (Molina et al. 2006; O’Brien et al. 2009a). Other bird species such as mallard and northern bobwhite quail have also been studied, but to our knowledge, no toxicity studies on avian fish-eating top predators have been published (Newsted et al. 2007). PFOA has also been studied on the developing chicken embryo (O’Brien et al. 2009b; Yanai et al. 2008), but studies on other avian species are scarce. The mode-of-action of PFOS and PFOA on embryo development is not clear. In studies on gene expression following PFOS and PFOA exposure in rat and chicken liver, regulated genes were found in the categories of metabolism and transport of fatty acids among others (Guruge et al. 2006; Hu et al. 2005; Yeung et al. 2007). One suggested mechanism for these effects on gene expression has been the peroxisome proliferator activated receptor-alpha (PPARα) to which both PFOS and PFOA are agonists (Shipley et al. 2004; Maloney and Waxman 1999) although some studies are indicating that only PFOA is working through this mechanism (Abbott et al. 2007, 2009; Corsini et al. 2011). PFOS has been shown to induce chicken embryo hepatic β-oxidation at doses lower than reported environmental avian levels which could be a downstream effect of PPARα activation (Nordén et al. 2012).
As there can be big differences in species sensitivity to environmental pollutants, it is important that studies are done on a variety of species and especially on those with high risk of exposure. In this study, we are comparing the species sensitivity to PFOS and PFOA in eggs from wild bird populations to domestic White Leghorn chicken (G. gallus domesticus) which is a commonly used model organism in avian toxicology studies. The wild species are great cormorant (P. carbo sinensis) and herring gull (L. argentatus) from bird colonies in Lake Vänern, Sweden. Embryo survival, body weight and liver and heart weights were the effect variables selected.
Materials and methods
Chemicals for exposure experiments
The perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) injection solutions were made by dissolving the potassium salt of PFOS (Chemica 98 %, Lot 77,282, about 21 % branched isomer) or the carboxylic acid of PFOA (Aldrich 96 %, Lot 17,146-8) at different concentrations in dimethyl sulfoxide (DMSO) and diluting with sterile water to 5 % DMSO (for the PFOS solutions) or 2.5 % DMSO (for the PFOA solutions) (v/v). The two PFAAs used were of a technical mixture and therefore a mixture of branched and linear isomers. A water solution with 5 or 2.5 % (v/v) DMSO was used as control. The reason for the higher DMSO concentration in the PFOS experiments was due to difficulties in getting PFOS dissolved in small DMSO volumes.
Eggs
Fertilized, unincubated White Leghorn chicken (G. gallus domesticus) eggs were purchased from Ova production, Vittinge, Sweden, and kept at 10–12 °C until incubation. Fertilised eggs from great cormorant (P. carbo sinensis) and herring gull (L. argentatus) colonies were collected from nests on the small islands Kabbaskären (N58° 43.831′, E13° 48.260′), Midskär (N58° 44.352′, E13° 48.492′), and Kroppholmen (N58° 43.707′, E13° 44.165′) in Lake Vänern, Sweden, in 2007 and 2008. The wild eggs were collected shortly after the egg laying had begun. After collection, the eggs were transported warm (ambient temperature did not get below 20 °C) to the lab and placed in an incubator within 4 h. Immediately after incubation, wild eggs were sorted according to developmental stage and randomly assigned to different dose groups. All eggs were incubated in ventilated chambers at 37.5 °C, 60 % relative humidity with automatic egg turning every second hour. Eggs were candled every 1 to 3 days to monitor viability. The experimental protocol was approved by the Swedish Animal Welfare Agency.
Exposure and studies of effects
The chicken eggs were injected on day 4 of incubation. Cormorant and herring gull eggs were candled to determine developmental stage and were injected at the developmental stage equivalent to the chicken embryo developmental stage at day 4 of incubation. All eggs were randomly divided into dose and control groups. After egg swiping with ethanol, a 1- to 2-mm hole was drilled in the shell above the air sac and 1 μL injection solution/g egg was injected through the hole and onto the inner shell membrane. The dose groups (Table 1) for the PFOS study in chicken were control (n = 20), 0.1 (n = 10), 0.3 (n = 10), 1 (n = 10), 3 (n = 10) and 10 μg/g egg (n = 15). For the PFOA study, the doses were control (n = 32), 0.16 (n = 10), 0.5 (n = 10), 1.6 (n = 27) and 5 μg PFOA/g egg (n = 28) and an uninjected control group (n = 10). For the cormorant study, the dose groups were control (n = 14) and 10 μg/g egg for PFOS (n = 13) and control (n = 26), 3.5 (n = 11) and 10 μg/g egg (n = 15) for PFOA. The dose groups for the herring gull were control (n = 20) and 10 μg/g egg (n = 20) for PFOS and control (n = 37) and 10 μg/g egg (n = 33) for PFOA. The true doses of the anions when corrected for the impurities of the chemicals and the mass of the cation were 90.9 % of the potassium salt dose (PFOS) and 95.8 % of the carboxylic acid dose (PFOA). After injection, the hole was sealed with paraffin and the incubation was continued. The chicken eggs were candled every 1 to 3 days for survival monitoring. Chicken embryos were sacrificed and dissected on day 19 of incubation, and gull and cormorant eggs were sacrificed and dissected when each egg was pipping. Pipping is when the chick makes the first crack in the shell and is the beginning of the hatching process, and it occurs about a day before hatching. For cormorant and herring gull, pipping occurs on about day 26 of incubation. For chicken, it occurs earlier and hatching occurs around days 20–21 (Hamburger and Hamilton 1951). The reason for terminating the experiment at pipping was to get as uniform timing for the termination of the experiment as possible. Determining developmental stage on the wild species was difficult due to the pigmented egg shells, especially later in development when the embryo was large. This approach enabled us to end the experiments in all species at approximately the same stage of development. After dissection, embryos, livers and hearts were weighed.
Chemical analysis of liver concentrations of PFAAs
For the chicken experiments, three randomly selected livers from each of the two highest dose groups and one liver from the control group were analysed. PFOS concentrations were determined in PFOS control and exposure experiments, while PFOA was analysed in PFOA control and exposure samples. For cormorant and herring gull, three livers from the 10 μg/g PFOS and PFOA dose groups and three control livers were analysed. The livers were analysed for the injected compound (PFOS or PFOA, respectively) to determine uptake. For sample extraction and clean-up, the method described by Verreault et al. (2007) was applied with the following modifications. Aliquots of approximately 0.5 g (control livers) or 0.05 g (exposed livers) of the homogenised samples were taken for extraction. 13C4-PFOS and 13C4-PFOA were used as internal standards (ISTDs) for PFOS and PFOA analysis, respectively. A total amount of 10 or 100 ng of each ISTD was spiked to control or exposed samples, respectively. Control samples were processed as described by Verreault et al. (2007), while raw extracts of exposed samples were adjusted with acetonitrile to 10 mL and an aliquot of 1 mL was taken for clean-up on graphitized carbon. A total amount of 10 ng of 3,5-bis(trifluoromethyl)phenyl acetic acid was added as volumetric standard to all extracts before instrumental analysis. Instrumental analysis and quantification was done by high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC/MS/MS) using the instrumentation and internal standard quantification method as described by Holmström and Berger (2008). For quality control, method detection and quantification limits were determined on the basis of procedural blank extractions, which were performed with every batch of samples. Method detection limits for the control samples were 0.3 and 0.5 ng/g for PFOS and PFOA, respectively, while method quantification limits were 1.0 and 1.5 ng/g, respectively. Recoveries of the ISTDs were calculated for all samples (n = 25) and were (mean ± 1 standard deviation) 85 ± 17 % for 13C4-PFOS and 88 ± 14 % for 13C4-PFOA. Five duplicate sample extractions were performed (three for exposed livers and two for control livers) to assess precision. Quantified concentrations for PFOS and PFOA varied with ≤9 % between the duplicate samples. A fish sample previously investigated in an international interlaboratory comparison study was analysed together with the cormorant and herring gull control samples for accuracy testing. The results for PFOS and PFOA deviated with 8 and 13 %, respectively, from the median values obtained in the interlaboratory comparison study (van Leeuwen et al. 2009).
Statistical analysis
The benchmark doses (BMDs) and the benchmark dose lower confidence limit (BMDL) were calculated using Benchmark Dose Software 2.1.2 supplied by the U.S. Environmental Protection Agency (http://www.epa.gov/ncea/bmds/index.html). The benchmark dose is the dose on the dose-response curve where a 10 % effect is seen (BMD10) and the benchmark dose lower confidence limit is the dose at which 10 % effect is seen on the lower 95 % confidence interval curve (BMDL10). The BMD/BMDL is less dependent on sample size and the choice and interval of doses than the no observed effect level (NOEL)/lowest observed effect level (LOEL) approach. A LogProbit model was best suited for the data and was used to model the dose-response curve for the chicken experiment. The LD50 with 95 % confidence interval (CI) was calculated using U.S. Environmental Protection Agency probit analysis program (Version 1.5, http://www.epa.gov/nerleerd/stat2.htm). The Student t test was used to test significance on continuous data, and Fisher’s exact test was used to test significance on the categorical embryo survival data. All BMD/BMDL, NOEL/LOEL and LD50 values are stated as the anion doses and are corrected for chemical impurities of the salts. To compare the survival of the wild species with chicken and to take into account the lower control survival seen in the wild species, a relative survival was calculated by dividing the fraction of surviving embryos in the dose groups with that of the control groups.
Results
Embryo survival
Chicken embryo survival was reduced in the 10 μg/g PFOS (p = 0.0019), 1.6 μg/g PFOA (p < 0.0001) and 5 μg/g PFOA (p < 0.0001) dose groups (Fig. 1). A tendency for lower survival was seen in the 3-μg/g PFOS dose, although this effect was not statistically significant. The LD50 was 8.5 μg/g (17.1 nmol/g, 95 % CI = 3.9–1657 μg/g) for PFOS and 2.5 μg/g (6.0 nmol/g, 95 % CI = 1.9–3.3 μg/g) for PFOA. The LOEL was 0.9 and 1.5 μg/g for PFOS and PFOA, respectively. The PFOS and PFOA NOEL were 2.73 and 0.48 μg/g, respectively. The BMD10 and BMDL10 were 1.26 and 0.42 μg/g, respectively, for PFOS and 1.01 and 0.60 μg/g, respectively, for PFOA. Most embryo deaths occurred on day 1 after injection when exposed to PFOA and between days 6 and 9 in embryos exposed to PFOS (Fig. 2).
In the wild species, the survival of the control groups was lower than for chicken (Table 1) and therefore the relative control-adjusted survival was used for the species sensitivity comparison. For cormorant, the relative control-adjusted embryo survival was 72 % (p = 0.208) in the 10 μg/g PFOS dose and 86 % (p = 0.186) and 87 % (p = 0.135) in the 3.5 and 10 μg/g PFOA groups, respectively. For herring gull, the relative control-adjusted embryo survival was 59 % (p = 0.047) for 10 μg/g PFOS and 46 % (p < 0.0001) for 10 μg/g PFOA. As seen in chicken, the timing of the mortalities was different following PFOS and PFOA exposure in herring gull (Fig. 2).
To compare species sensitivity, the relative control-adjusted survival for the wild species was compared to the chicken dose-response curves. For PFOS, herring gull was 1.6 times less sensitive and cormorant was 2.6 times less sensitive. For PFOA, herring gull was 3.5 times less sensitive and cormorant was 2.8 (and up to 8.1 times for the 10 μg/g dose) times less sensitive.
Body and organ weights
In chicken and herring gull, no statistically significant changes in liver somatic index and heart somatic index were seen in the exposed groups compared with the control groups (data not shown). After 10 μg/g PFOS exposure in cormorant, an 18 % increase in liver somatic index was seen compared to control (data not shown, p = 0.032). Herring gulls that were exposed to 10 μg/g PFOS had an 11 % increased body weight compared to the control (data not shown, p = 0.0066). Herring gulls exposed to 10 μg/g PFOA showed a decreased body weight by 10 % compared to the control (data not shown, p = 0.0022). Chicken and cormorant did not show any statistically significant changes in body weight after exposure.
Hepatic concentration of PFOS and PFOA
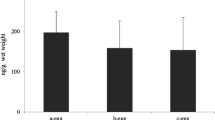
The liver concentration of PFOS and PFOA and liver accumulation factor in all three species is shown in Table 2. The chicken liver concentration was linearly correlated to the given dose with a slope of 0.79 for PFOS (y = 0.79 × +0.31, r 2 = 0.935, p = 0.0004) and 1.83 for PFOA (y = 1.83 × +0.088, r 2 = 0.9948, p < 0.0001). The hepatic concentrations of PFOS and PFOA in the exposed groups were in all cases close to expected based on the doses given to the eggs and assuming uniform distribution in the egg. High concentrations of PFOS were observed in the cormorant control livers (mean 713 ng/g) and herring gull control livers (mean 600 ng/g).
Discussion
In agreement with other studies, no effects on body weight and relative organ weights were observed following PFOS (Molina et al. 2006; Peden-Adams et al. 2009) and PFOA (O’Brien et al. 2009b) exposure in chicken. Herring gull body weight was affected by both chemicals but towards a higher weight after PFOS exposure and towards a lower weight after PFOA exposure. The mechanisms behind these weight changes are not known but the fact that the two chemicals induce changes in different directions could be an indication of differing toxic mechanisms between PFOS and PFOA. This effect is however only seen in the herring gull.
Molina et al. (2006) reported a PFOS LD50 for White Leghorn chickens of 4.9 μg/g egg wt (95 % CI = 0.28–297.12 μg/g) based on hatch rates, Peden-Adams et al. (2009) reported that their data did not support this in the White Leghorn chicken but indicated the LD50 was higher than 5 mg/kg egg (μg/g), and O’Brien et al. (2009a) reported a LD50 of 93 μg/g (95 % CI = 4 μg/g–673,000 μg/g) based on pipping success. The present study yielded a PFOS LD50 in chicken of 8.5 μg/g egg (17.1 nmol/g) though, as in the studies of Molina and O’Brien, the confidence interval was high. There seems to be the best agreement with the Molina study, which was based on hatch rates. In contrast with our findings, O’Brien et al. (2009b) observed no effects on survival in chicken after exposure to PFOA. The reasons for differing results may be due to the selected exposure methods and the various endpoints related to viability, e.g. hatch rates, pipping success, survival at incubation day 19 and in the case of the PFOA study of O’Brien et al., also the isomer composition of the studied chemical. The hatching process is very energy requiring and therefore it is likely that the effective dose in our study would have been lower if the experiment had been terminated after hatching. Therefore, we consider the LD50 presented in this study to be a conservative (high) estimate when considering ecological risks of PFOS and PFOA to avian fauna.
The LD50 of PFOS in chicken was 2.9 times higher than that of PFOA when compared on a molar basis. In cormorants, the toxicity of PFOS and PFOA appeared to be quite similar and this was also the case for the herring gull. It was also clear that herring gulls were more sensitive to PFOS and PFOA than cormorants. For the chicken embryos, embryo mortalities occurred earlier in PFOA exposure (1 day after exposure) than in PFOS exposure (7–9 days after exposure), which could indicate that these chemicals have different mechanisms of toxicity. The same pattern was seen in herring gull but could not be established in cormorant, possibly due to the higher embryo survival.
Of the three species studied, chicken was the most sensitive to both chemicals. For the species comparison, relative survival was used to account for the lower survival in the wild species control groups. The reason for the lower control survival in the wild species is most likely due to (a) the transport time between field collection and incubation in the lab and perhaps (b) a generally lower embryo survival in the wild species possibly affected by a pre-existing burden of environmental toxins or other environmental factors. The average time for the transport was 4 h, and the temperature during the transport was not below 20 °C. The wild species are not under the same control as the chicken which is of a controlled and low genetic variation has a controlled environment and a standardised diet. It was not possible to standardise egg quality in the field collected eggs to the same level as chicken eggs; and therefore, we had to accept a lower control survival.
The mechanisms causing the toxic effects of PFOS and PFOA are still unclear. Some studies have shown that PPARα might be a pathway for the toxicity of PFOA but not for PFOS (Abbott et al. 2007, 2009; Corsini et al. 2011). Thus, a basis for the differences in the timing of mortality in the chicken embryo development, with PFOA causing mortality earlier than PFOS, could be that the earlier mortality by PFOA is mediated by PPARα pathways, while PFOS toxicity could have other causes. It can however not be excluded that differences in toxicity between PFOS and PFOA can be due to differences in toxicokinetics. Another toxic mechanism for PFOS is the induction of fatty acid β-oxidation observed in chicken liver in vitro after in ovo exposure to PFOS which may be mediated through PPARα (Nordén et al. 2012). However, the effects occur at doses lower than the ones causing reduced survival in the current study which suggests that other mechanisms are responsible for the reduced survival.
A good correlation between the injected amount of PFOS and PFOA and the concentration in liver was observed (Table 2), verifying that within the dose range used in this study, the internal doses cohered well to the injected doses. In chicken, the accumulation of the chemicals by the liver in relation to the injected dose was twice as high for PFOA compared to PFOS. Corresponding differences in accumulation were not seen in herring gull and cormorant.
The NOEL value of PFOS for chicken embryo survival (2.7 μg/g anion dose) is close to concentrations that have been reported for the same populations of cormorant and herring gull in Lake Vänern as well as levels found in eggs of guillemots from the Baltic Sea (Nordén et al. 2013; Holmström et al. 2005). Even though the sensitivity of chicken and the wild birds in this study differs, the margin of exposure compared to potentially toxic levels in the most PFOS polluted environments, like the Baltic Sea, could be small for other wild species or when other PFAAs are taken into consideration. The highest levels found in the cormorants and herring gulls in Lake Vänern and guillemot eggs from the Baltic Sea and liver levels are almost the same as, or higher than, the predicted BMD10 for chicken. The median egg and liver levels in cormorant as well as several individual samples from herring gull exceeded the BMDL10 for chicken. For PFOA the NOEL and BMDL10 are close to the highest concentration of PFOA found in the Mediterranean cormorant livers and five to sixfold higher than the mean concentration in the study by Kannan et al. (2002). Populations of birds with high PFOA exposure and accumulation could reach levels where effects occur in chicken. The generally more elevated environmental levels of PFOS yield a lower margin of exposure for PFOS than that of PFOA for the avian fauna. These results indicate that the environmental burden of PFOS could be affecting embryo survival in some bird species. For tree swallows (Tachycineta bicolor), negative effects on hatching have been observed at egg PFOS levels of 150–200 ng/g wet weight, which is lower than the LOEL of 0.9 μg/g observed for chicken in our study. The authors suggest that the high sensitivity could be due to behavioral effects and other factors that are not accounted for in laboratory studies or that tree swallows are unusually sensitive to PFASs (Custer et al. 2014). Thus, it seems as if there can be bird species that could potentially be at high risk from PFAS exposure due to such factors as these authors suggest.
The high levels of PFOS found in the livers from the wild birds in our study could come from both the breeding ground and other locations to which the birds migrate. There is some evidence of PFOS contamination in the aquatic food web in Lake Vänern. In pooled samples of perch, which is a predatory fish and also included in the great cormorant diet (Engström 2001), PFOS levels were found at around 110–120 ng/g which is about tenfold higher than in a neighbouring large lake (Vänerns vattenvårdsförbund 2012; Berger et al. 2009). Therefore, the Lake Vänern environment could be a contributing factor to the PFAA contamination in cormorants and herring gulls. Further studies on levels in water and food web from Lake Vänern or the migration destinations would give more insight to where the main exposure occurs.
Whether the levels of PFAAs in the environment are currently increasing or not depends on the specific PFAA, the location and the species. In polar bears from East Greenland, levels of PFOS, PFOA and several other PFAAs were increasing in the latest samples in a study by Dietz et al. (2008) whereas Harbour Seals from 1999 to 2008 from the German Bight indicated decreasing levels of PFOS, PFOA and other PFAAs and increasing levels of perfluorodecane sulfonate (PFDS) (Ahrens et al. 2009). In eggs of fish-eating birds, the PFOS levels show signs of levelling out in samples from the 2000s (Holmström et al. 2005; Verreault et al. 2007).
Conclusions
Embryo survival was affected in chicken and herring gull after exposure to PFOS and PFOA. Also, a tendency for lower embryo survival, though not statistically significant, was observed in cormorant. Chicken was more sensitive to PFOS and PFOA than the other species. Cormorant and herring gull could tolerate 1.6–2.6 times higher dose of PFOS than chicken to reach the same effect on embryo survival. The differences were bigger for PFOA. At the same effect level, cormorant could tolerate approximately eight times higher dose of PFOA than chicken. Even though herring gull was less sensitive than chicken, populations with high PFAA burden could be at risk of effects on embryo survival. Furthermore, the risk of sublethal effects is still present. PFOA was more toxic than PFOS but the lower environmental levels of PFOA give a larger margin of exposure. It is worth noting that the environmental exposure to PFAAs in wildlife comprises of various mixtures of PFAAs and other contaminants rather than single substances. Little is known of how these combinations affect birds and other wildlife. In summary, our study shows that risk assessment of PFOS and PFOA in avian wildlife should take into consideration that there may be large interspecies differences in sensitivity and that continued monitoring of the environmental levels of PFAAs as well as PFAA toxicity is of importance.
References
Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, Nakayama S, Lindstrom AB, Strynar MJ, Lau C (2007) Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci 98:571–581
Abbott BD, Wolf CJ, Das KP, Zehr RD, Schmid JE, Lindstrom AB, Strynar MJ, Lau C (2009) Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR[alpha]) in the mouse. Reprod Toxicol 27:258–265. doi:10.1016/j.reprotox.2008.05.061
Ahrens L, Siebert U, Ebinghaus R (2009) Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere 76:151–158. doi:10.1016/j.chemosphere.2009.03.053
Berger U, Glynn A, Holmström KE, Berglund M, Ankarberg EH, Törnkvist A (2009) Fish consumption as a source of human exposure to perfluorinated alkyl substances in Sweden—analysis of edible fish from Lake Vättern and the Baltic Sea. Chemosphere 76:799–804. doi:10.1016/j.chemosphere.2009.04.044
Boulanger B, Vargo J, Schnoor JL, Hornbuckle KC (2004) Detection of perfluorooctane surfactants in great lakes water. Envrion Sci Technol 38:4064–4070. doi:10.1021/es0496975
Corsini E, Avogadro A, Galbiati V, dell’ Agli M, Marinovich M, Galli CL, Germolec DR (2011) In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 250:108–116. doi:10.1016/j.taap.2010.11.004
Custer CM, Custer TW, Dummer PM, Etterson MA, Thogmartin WE, Wu Q, Kannan K, Trowbridge A, McKann PC (2014) Exposure and effects of perfluoroalkyl substances in tree swallows nesting in Minnesota and Wisconsin, USA. Arch Environ Contam Toxicol 66(1):120–138. doi:10.1007/s00244-013-9934-0
Dietz R, Bossi R, Rigét FF, Sonne C, Born EW (2008) Increasing perfluoroalkyl contaminants in East Greenland polar bears (Ursus maritimus): a new toxic threat to the Arctic bears. Envrion Sci Technol 42:2701–2707. doi:10.1021/es7025938
Engström H (2001) Long term effects of cormorant predation on fish communities and fishery in a freshwater lake. Ecography 24:127–138. doi:10.1034/j.1600-0587.2001.240203.x
Ericson I, Nadal M, Bavel B, Lindström G, Domingo JL (2008) Levels of perfluorochemicals in water samples from Catalonia, Spain: is drinking water a significant contribution to human exposure? Environ Sci Pollut Res 15:614–619. doi:10.1007/s11356-008-0040-1
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Envrion Sci Technol 35:1339–1342. doi:10.1021/es001834k
Guruge KS, Yeung LWY, Yamanaka N, Miyazaki S, Lam PKS, Giesy JP, Jones PD, Yamashita N (2006) Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol Sci 89:93–107. doi:10.1093/toxsci/kfj011
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Holmström KE, Berger U (2008) Tissue distribution of perfluorinated surfactants in common Guillemot (Uria aalge) from the Baltic Sea. Envrion Sci Technol 42:5879–5884. doi:10.1021/es800529h
Holmström KE, Järnberg U, Bignert A (2005) Temporal trends of PFOS and PFOA in Guillemot eggs from the Baltic Sea, 1968–2003. Envrion Sci Technol 39:80–84. doi:10.1021/es049257d
Hu W, Jones PD, Celius T, Giesy JP (2005) Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol Phar 19:57–70. doi:10.1016/j.etap.2004.04.008
Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP (2002) Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean seas. Envrion Sci Technol 36:3210–3216. doi:10.1021/es020519q
Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated compounds in aquatic organisms at various trophic levels in a great lakes food chain. Arch Environ Contam Toxicol 48:559–566. doi:10.1007/s00244-004-0133-x
Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, Lignell S, Lindström G (2007) Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115:226–230. doi:10.1289/ehp.9491
Key BD, Howell RD, Criddle CS (1998) Defluorination of organofluorine sulfur compounds by Pseudomonas Sp. Strain D2. Envrion Sci Technol 32:2283–2287. doi:10.1021/es9800129
Kissa E (2001) Fluorinated surfactants and repellents, 2nd edn. CRC Press, New York
Maloney EK, Waxman DJ (1999) trans-Activation of PPAR[alpha] and PPAR[gamma] by structurally diverse environmental chemicals. Toxicol Appl Pharm 161:209–218. doi:10.1006/taap.1999.8809
Molina ED, Balander R, Fitzgerald SD, Giesy JP, Kannan K, Mitchell R, Bursian SJ (2006) Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ Toxicol Chem 25:227–232. doi:10.1897/04-414R.1
Newsted JL, Coady KK, Beach SA, Butenhoff JL, Gallagher S, Giesy JP (2007) Effects of perfluorooctane sulfonate on mallard and northern bobwhite quail exposed chronically via the diet. Environ Toxicol Phar 23:1–9
Nordén M, Westman O, Venizelos N, Engwall M (2012) Perfluorooctane sulfonate increases β-oxidation of palmitic acid in chicken liver. Environ Sci Pollut Res 19:1859–1863. doi:10.1007/s11356-012-0869-1
Nordén M, Berger U, Engwall M (2013) High levels of perfluoroalkyl acids in eggs and embryo livers of great cormorant (Phalacrocorax carbo sinensis) and herring gull (Larus argentatus) from Lake Vänern, Sweden. Environ Sci Pollut Res Int. doi:10.1007/s11356-013-1567-3
O’Brien JM, Carew AC, Chu S, Letcher RJ, Kennedy SW (2009a) Perfluorooctane sulfonate (PFOS) toxicity in domestic chicken (Gallus gallus domesticus) embryos in the absence of effects on peroxisome proliferator activated receptor alpha (PPAR[alpha])-regulated genes. Comp Biochem Physiol C: Toxicol Pharmacol 149:524–530. doi:10.1016/j.cbpc.2008.11.009
O’Brien JM, Crump D, Mundy LJ, Chu S, McLaren KK, Vongphachan V, Letcher RJ, Kennedy SW (2009b) Pipping success and liver mRNA expression in chicken embryos exposed in ovo to C8 and C11 perfluorinated carboxylic acids and C10 perfluorinated sulfonate. Toxicol Lett 190:134–139. doi:10.1016/j.toxlet.2009.07.004
Peden-Adams MM, Stuckey JE, Gaworecki KM, Berger-Ritchie J, Bryant K, Jodice PG, Scott TR, Ferrario JB, Guan B, Vigo C, Boone JS, McGuinn WD, DeWitt JC, Keil DE (2009) Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS). Reprod Toxicol 27:307–318. doi:10.1016/j.reprotox.2008.10.009
Schuetze A, Heberer T, Effkemann S, Juergensen S (2010) Occurrence and assessment of perfluorinated chemicals in wild fish from Northern Germany. Chemosphere 78:647–652. doi:10.1016/j.chemosphere.2009.12.015
Shipley JM, Hurst CH, Tanaka SS, DeRoos FL, Butenhoff JL, Seacat AM, Waxman DJ (2004) Trans-activation of PPARα and induction of PPARα target genes by perfluorooctane-based chemicals. Toxicol Sci 80:151–160. doi:10.1093/toxsci/kfh130
Taniyasu S, Kannan K, Horii Y, Hanari N, Yamashita N (2003) A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Envrion Sci Technol 37:2634–2639. doi:10.1021/es0303440
Van Leeuwen SPJ, Swart CP, van der Veen I, de Boer J (2009) Significant improvements in the analysis of perfluorinated compounds in water and fish: results from an interlaboratory method evaluation study. J Chromatogr A 1216:401–409. doi:10.1016/j.chroma.2008.11.029
Vänerns vattenvårdsförbund (2012) Undersökning av stabila organiska ämnen och metaller i abborre och gädda 2010–2011 Rapport nr 71. Vänerns vattenvårdsförbund
Verreault J, Berger U, Gabrielsen GW (2007) Trends of perfluorinated alkyl substances in herring gull eggs from two coastal colonies in northern Norway: 1983–2003. Envrion Sci Technol 41:6671–6677. doi:10.1021/es070723j
Yanai J, Dotan S, Goz R, Pinkas A, Seidler FJ, Slotkin TA, Zimmerman F (2008) Exposure of developing chicks to perfluorooctanoic acid induces defects in prehatch and early posthatch development. J Toxicol Env Health 71:131. doi:10.1080/15287390701613280
Yeung LWY, Guruge KS, Yamanaka N, Miyazaki S, Lam PKS (2007) Differential expression of chicken hepatic genes responsive to PFOA and PFOS. Toxicology 237:111–125
Acknowledgments
Funding for this project was provided by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas). We would like to thank Sten-Gunnar Steensson and Ola Westman for their help with the egg collection; Katrin Holmström, Anne-Sofie Kärsrud and Marko Filipovic for the analysis and result interpretation and Krister Halldin for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Nordén, M., Berger, U. & Engwall, M. Developmental toxicity of PFOS and PFOA in great cormorant (Phalacrocorax carbo sinensis), herring gull (Larus argentatus) and chicken (Gallus gallus domesticus). Environ Sci Pollut Res 23, 10855–10862 (2016). https://doi.org/10.1007/s11356-016-6285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6285-1