Abstract
The effects of aqueous extracts from Microcysts aeruginosa strains (both microcystin-producers and non-microcystin producers) on germination and root growth were investigated for three economically important plant species: Festuca rubra L., Lolium perenne L., and Lactuca sativa L. There was a clear inhibition of root growth for L. sativa exposed to strains containing microcystins (5.9–56.4 μg L−1). The strain that produced the most pronounced effects contained the lowest concentration of microcystin suggesting that other cellular compounds may also affect growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The demand for intensive agriculture from a rapidly increasing human population has lead to the eutrophication of aquatic ecosystems through the excess accumulation of nutrients, namely phosphorus and nitrogen. In many cases, these waterbodies are used for recreational activities as well as sources for potable water. Environmental conditions such as increased temperature and radiation accompanied with low rates of vertical mixing of the water column and slow flow rates produce conditions conducive to the growth of many species of toxic cyanobacteria. These organisms can accumulate at the water surface forming scums that cause deoxygenation of the water column, changes in organoleptic characteristics, and the mass release of toxins. Among the most common hepatotoxic species found in the freshwater environment are Microcystis, Anabaena, Nodularia, Oscillatoria, Nostoc and Cylindrospermopsis (Carmichael 1992). The most common hepatotoxins found in freshwater lakes and rivers are the microcystins (MCs) and over 60 different chemical forms have been described (Sivonen and Jones 1999). These compounds have been implicated in several outbreaks of sickness and death in humans as well as the intoxication of domestic and wild animals (Kuiper-Goodman et al. 1999). The toxic effect of MCs is due to their inhibitory effects on protein phosphatases 1 and 2A (PP1 and PP2A) (Mackintosh et al. 1990). These enzymes are important for normal cell growth and metabolism. Although initial studies on the toxicity of MCs have focused on the effects of purified MCs on humans and other animals, it has also been suggested that these compounds might have much wider ecological impacts (Pflugmacher 2002).
Studies carried out by Mitrovic et al. (2004) showed that concentrations in the range from 10 to 15 μg MC-LR mL−1 lead to reduced growth in Lemna minor L. and Wolfia arrhiza L. Kurki–Helasmo and Meriluoto (1998) showed that seed germination of Sinapsis alba L. was significantly reduced in the presence of MC-RR at a concentration of 5 μg mL−1. Higher concentrations, in the range of 20–40 μg mL−1, lead to a decrease in root growth. McElhiney et al. (2001) showed that photosynthesis in Phaseolus vulgaris L. was inhibited by three different MC variants at concentrations between 1.6 and 7.7 μg mL−1. Hamvas et al. (2003) reported that MC-LR caused reduced growth and productivity of Sinapsis alba L. Tests carried out with the aquatic duckweed Lemna minor L. showed a reduction in total chlorophyll content as well as growth inhibition following exposure to MC-RR (Weiss et al. 2000). Pflugmacher (2002) reported that the growth rate of the macrophyte Ceratophyllum demersum L. was severely reduced in response to absorption of MC-LR. A decrease in the number and size of leaves of Spirodela oligorrhiza L. was found to result from exposure to MC (Romanowska-Duda and Tarczynska 2002). Exposure to MCs also been shown to cause reduced growth rates in some plant species cultivated for human consumption such as Lactuca sativa L. These compounds can accumulate in the tissues and be transferred along food chains (Codd et al. 1999).
To date, there are no conclusive studies demonstrating the role of cyanotoxins such as MC, although there have been some reports indicating that they provide chemical defence against herbivory and have allelopathic effects on other aquatic organisms. Pflugmacher (2002) showed that MCs display allelopathic effects against the aquatic macrophyte C. demersum L. causing growth inhibition and reduced photosynthetic oxygen production. Leblanc et al. (2005) found that MC-LR produced by M. aeruginosa did not inhibit growth of the macrophyte Lemna gibba L., however, the authors demonstrated a wide variation in responses to MC between different species of aquatic macrophytes. There seems to be a difference on species sensitivity to microcystins and there are suspicions that other compounds from cyanobacteria apart from the toxins may have negative effects on germination and growth of many plant species.
In this work we investigate the effects of cell extracts from MC-producing and non-MC-producing strains of Microcystis aeruginosa on germination and root growth of two grass species (Festuca rubra L. and Lolium perenne L.) and lettuce (Lactuca sativa L.).
Materials and Methods
Cyanobacteria extracts were prepared using Microcystis aeruginosa strains isolated from several different lakes and reservoirs in Portugal (Table 1). Test solutions were prepared with 1 mg of lyophilised cyanobacteria in 10 mL of distilled water. The mixture was homogenized and ultrasonicated on ice for 15 min to break the cell walls and facilitate the release of MCs. Extracts were then centrifuged at 4,000g for 8 min to remove cell debris, before filtering (0.45 μm).
Germination and root growth assays were carried out using seeds from two species of grass (Festuca rubra L. and Lolium perenne L.) and lettuce (Lactuca sativa L.). The two grass species included in the study are commonly used in lawns both on golf courses and in recreational areas. Lettuce represents an important agricultural species. Firstly, seeds were washed in a 10% bleach solution for 20 min to prevent fungal growth during the incubation, and then rinsed with ultrapure water. The seeds were place in plastic Petri dishes containing absorbent paper disks. Ten seeds were added to each Petri dish and the paper disks moistened with either 2 mL of the cyanobacteria extract solution or 2 mL of water (for control samples). Petri dishes were then sealed with Parafilm® to prevent evaporation. Five replicate Petri dishes were used for each of the Microcystis treatments and the control. A total of eight Microcystis strains were included in the study (Table 1). Petri dishes containing lettuce seeds were placed in the dark, which is a requirement for the germination of this species. Petri dishes containing grass seeds were positioned under white fluorescent light at an intensity of approximately 10 μm m−2 s−2 and photoperiod of 14 h, at a temperature of 24 ± 1°C. The number of germinated seeds in each Petri dish was recorded after 5 days. Germination was considered to have occurred when the root had a length of at least 2 mm. Root length was recorded for each specimen.
Detection and quantification of MCs in the pure extracts was carried out using the ELISA assay (EnviroGard® Microcystin Plate Kit), with a detection limit of 0.1 ng mL−1. For each of the Microcystis strains, 5 mg of freeze-dried material was sonicated in 2 mL of ultrapure water, and then frozen and thawed twice to facilitate the release of MCs into solution. Solutions where then filtered (0.45 μm) and dilluted if necessary.
The results obtained for root length and germination in each of the treatments were compared statistically using ANOVA and the Tukey test for comparison of means (Statistica version 6.0). Correlations between MC concentration and root inhibition used only data from those strains that had the MC profile characterized (all but IZ38) with Statistica version 6.0.
Results and Discussion
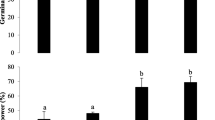
Exposure of L. sativa L. seeds to extracts of MC-producing and non-MC-producing strains of M. aeruginosa for 5 days did not have any observable effects on germination (F = 1.741, df = 8, p = 0.118). The results obtained for the root length of L. sativa L. following exposure to extracts of M. aeruginosa are shown in Fig. 1. The M. aeruginosa treatments resulted in reduced root growth compared with the control samples (F = 20.938, df = 8, p = 0.00). The Tukeys test for comparison of means also showed that there were significant differences between strains that produced MC and others that did not (Fig. 1a).
Root length of Lactuca sativa L. (a), Festuca rubra L. (b) and Lolium perenne L. (c) following a 5-days exposure to extracts of MC-producing and non-MC-producing strains of M. aeruginosa (open bars – Non-MC-producing strains). Line represents MC concentration. Bars with the same number are not significantly different
For the grass species F. rubra L., there was much lower variability in terms of differences in the inhibition of germination and root growth between the different strains of M. aeruginosa. No significant difference was found in the germination rates in the eight treatments (F = 1.212, df = 8, p = 0.316) compared with the control samples, suggesting an absence of acute toxic effects. In addition, there were no significant differences obtained for the root length measurements for MC-producing and non-MC-producing strains (F = 1.626365, df = 8, p = 0.115584) (Fig. 1b). For the other grass species, Lolium perenne L., no significant differences were found between MC-producing and non-MC-producing strains with respect to the effects on germination (F = 0.672, df = 8, p = 0.713). Neither was root growth inhibited by any of the treatments (F = 1.048, df = 8, p = 0.399) (Fig. 1c).
A clear correlation was found between the root growth inhibition of L. sativa L. and MC concentration (r 2 = 0.87) for the strains that produced identified variants of MC (Table 2). However, even the two strains of M. aeruginosa lacking detectable concentrations of MC caused a reduction in root growth of between 15–30% compared with the control samples. M. aeruginosa strain IZ38 which contained a relatively low toxin content (Table 1) had a significant effect on root growth, resulting in more than 40% reduction in root length compared with the control samples. Exposure of L. sativa L. to strains of M. aeruginosa resulted in an average root length reduction of 25.7% relative to the control treatment. For Lolium perenne L. the average reduction was only 3.4%. F. rubra L. actually showed an increase in root growth of 2.5% relative to the control treatment following exposure to M. aeruginosa extracts and a negative correlation with toxin concentration of −0.48 (Table 2).
Previous works have shown that MC can affect seed germination in some terrestrial plants. Kurki–Helasmo and Meriluoto (1998) showed that germination of mustard seeds (Sinapsis alba L.) is affected by exposure to MC-RR, with an IC50 of 800 μg L−1. Kós et al. (1995) obtained an IC50 of 3,000 μg L−1 in assays using S. alba L. and unidentified MC variants. McElhiney et al. (2001) showed that the toxicity of MCs to S. alba L. may be dependent on the hydrophilicity of the toxins. MC-RR and -LR showed more pronounced toxic effects with IC50 values of 1,600 and 1,900 μg L−1, respectively, whereas for the more lipophylic MC-LF, IC50 values were three times higher. In this study, we found that ecologically relevant concentrations of MC (5.9–56.4 μg L−1), much lower than those used in many other studies, did not affect seed germination of L. sativa L., L. perenne L. or F. rubra L.
The inhibition of the root development is a useful indicator of toxicity because it can have a direct effect on the plant survival and growth. The results obtained using extracts of M. aeruginosa cultivated in the laboratory showed that studies carried out using purified microcystins might underestimate the toxicity of natural blooms. Our results with L. sativa L. showed that values close to IC50 were obtained with a MC concentration of 50–60 μg L−1. This value is 10–30 times more toxic than the values presented by other authors to S. alba L. using purified MCs. The concentration of MC-LR used in this our study (5.9–56.4 μg L−1) commonly occur during cyanobacterial blooms in the natural environment. Although high concentrations such those found in Australia (1,800 μg L−1) (Jones and Orr 1994) or Japan (1,300 μg L−1) (Ueno et al. 1996), may occur, lower concentrations such as those reported for Portugal (37 μg L−1) (Ueno et al. 1996) or the UK (131 μg L−1) (Codd et al. 1995) are more common. Accumulation of MC in the agricultural fields or lawns is unlikely because MC degrades rapidly with UV irradiation (Rositano and Nicholson 1994).
Microcystins may affect growth of terrestrial plant species but other substances may also be implicated in reduced growth rate when these plants are watered with cyanobacteria contaminated water.
References
Carmichael WW (1992) Cyanobacteria secondary metabolites–the cyanotoxins. J Appl Bact 72:445–459
Codd GA, Edwards C, Beattie KA, Lawton LA, Campbell DL, Bell SG (1995) Toxins from blue-green algae. The Pringsheim lecture. In: Wiessner W, Schnepf E, Starr RC (eds) Algae, Environment and Human Affairs. Biopress, Bristol, pp 1–17
Codd GA, Metcalf JS, Beattie KA (1999) Retention of Microcystis aeruginosa and microcystins by salad lettuce (Lactuca sativa) after spray irrigation with water containing cyanobacteria. Toxicon 37:1181–1185. doi:10.1016/S0041-0101(98)00244-X
Hamvas MM, Mathe C, Molnar E, Vasa G, Grigorsky I, Borbely G (2003) Microcystin–LR alters the growth, anthanocyanin content and single-stranded DNase enzyme activities in Sinapsis alba L. seedlings. Aquat Toxicol 61:1–9. doi:10.1016/S0166-445X(01)00273-9
Jones GJ, Orr PTY (1994) Release and degradation of microcystins following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876. doi:10.1016/0043-1354(94)90093-0
Kós P, Gorzó G, Surányi G, Borbély G (1995) A simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapsis alba L). Analyt Biochem 225:49–53. doi:10.1006/abio.1995.1106
Kuiper-Goodman T, Falconer I, Fitzgerald J (1999) Human health aspects. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water. E & FN Spon, London, pp 113–153
Kurki–Helasmo K, Meriluoto J (1998) Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapsis alba L.) seedlings. Toxicon 36:1921–1926. doi:10.1016/S0041-0101(98)00114-7
LeBlanc S, Pick FR, Rodriguez RA (2005) Allelopathic effects of the toxic cyanobacterium Microcystis aeruginosa on Duckweed, Lemna gibba L. Environ Toxicol 20(1):67–73. doi:10.1002/tox.20079
MacKintosh C, Beattie KA, Klumpp S, Cohen P, Cood GA (1990) Cyanobacterial microcysyin–LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 264:187–192. doi:10.1016/0014-5793(90)80245-E
McElhiney J, Lawson LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420. doi:10.1016/S0041-0101(01)00100-3
Mitrovic SM, Pflugmacher S, James KJ, Furey A (2004) Anatoxin-a elicits an increase in peroxidase and glutathione S-transferase activity in aquatic plants. Aquat Toxicol 68:185–192. doi:10.1016/j.aquatox.2004.03.017
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins with reference to microcystin–LR, in aquatic ecosystems. Environ Toxicol 17:407–413. doi:10.1002/tox.10071
Romanowska-Duda Z, Tarczynska M (2002) The influence of microcystin-LR and hepatotoxic cyanobacterial extract on the water plant Spirodela oligorrhiza. Environ Toxicol 17(5):434–440. doi:10.1002/tox.10076
Rositano J, Nicholson BC (1994) Water treatment techniques for removal of cyanobacterial toxins from water. Australian Centre for Water Quality Research, Salisbury, p 55
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water. E & FN Spon, London, pp 41–111
Ueno Y, Nagata S, Tsusumi T, Hasegawa A, Yoshida F, Sutajjit M, Mebs D, Vasconcelos V (1996) Survey of microcystins in environmental water by a highly sensitive immunoassay based on monoclonal antibody. Nat Toxins 4:271–276
Vasconcelos VM, Sivonen K, Evans WR, Carmichael WW, Namikoshi M (1995) Isolation and characterization of microcystins (heptapeptide hepatotoxins) from Portuguese strains of Microcystis aeruginosa Kutz emed Elekin. Arch Hydrobiol 134:295–305
Weiss J, Liebert HP, Braune W (2000) Influence of microcystins-RR on growth and photosynthetic capacity of the duckweed Lemna minor L. J Appl Bot 74:100–105
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, S., Saker, M.L., Vale, M. et al. Comparison of Sensitivity of Grasses (Lolium perenne L. and Festuca rubra L.) and Lettuce (Lactuca sativa L.) Exposed to Water Contaminated with Microcystins. Bull Environ Contam Toxicol 83, 81–84 (2009). https://doi.org/10.1007/s00128-009-9763-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9763-z