Abstract
Orchards and vineyards account for significant copper (Cu) accumulation in the soil due to frequent Cu fungicide applications to control leaf diseases. Although grass species are distributed in these areas likely because of their physiological mechanisms to combat Cu toxicity-related stress, the aim of the present study is to identify grass species presenting biochemical-physiological responses that feature adaptive Cu toxicity tolerance mechanisms. Three grass species native to the Pampa and Atlantic Forest biomes (Paspalum notatum, P. plicatulum, and P. urvillei) and an exotic species (Cynodon dactylon) were tested. Plants were cultivated in pots filled with 4 kg of typic Hapludalf soil, under two Cu availability, control, and toxicity conditions (80 mg Cu kg soil−1). Photosynthetic parameters, relative growth rate, root dry matter, shoot dry matter, the activity of stress-fighting enzymes (superoxide dismutase and guaiacol peroxidase), root biometry, soluble organic carbon, soil pH, and electrical conductivity were evaluated. P. notatum and P. urvillei have physiological characteristics that allow high translocation factor and Cu accumulation in the root and shoot, and it allows their use in phytoremediation processes due to (1) greater activity of stress-fighting enzymes such as POD in the shoot; (2) to larger diameter roots, which allow greater Cu complexation in them — they are lesser sensitive to stress caused by Cu than the other species; and (3) greater soluble organic carbon exudation in the rhizosphere than species P. plicatulum and C. dactylon, which can complex Cu2+ and reduce the presence of forms toxic to plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungal diseases in leaves and fruits can be observed in agricultural and fruit species, mainly when they are cultivated in humid regions and under higher air temperatures. Monilinia fructicola (Wint.) honey and Cladosporium carpophilum in peach trees (Prunus persica), Venturia inaequalis and Colletotrichum gloeosporioides in apple trees (Malus domestica), and Plasmopara viticola in vines (Vitis sp.) are some examples of these species.
Diseases reduce fruit yield and depreciate their composition; they also affect the quality of by products and their commercial value. Orchards and vineyards in different regions worldwide are subjected to frequent copper fungicide applications for disease control purposes, on a yearly basis. However, copper (Cu) in fungicides can reach soil surface through its unidirectional application on plants; Cu flows from leaves during precipitation events or from senescent leaves that fall on the ground — these processes increase soil Cu levels over time.
Studies have indicated that acidic, sandy soils presenting low organic matter contents and that have been cultivated with 40-year-old vineyards may have more than 104.2 mg Cu kg−1 — natural contents are close to 1.2 mg Cu kg−1 (Girotto et al. 2014). Copper (Cu) accumulation intensity in vineyard soils changes depending on soil characteristics, application frequency, and cultivation period. Accordingly, soil Cu levels reported for different grape producing regions worldwide change — they can reach 250 mg Cu kg−1 in Australia (Pietrzak and McPhail 2004), 372 mg Cu kg−1 in Italy (Dell’Amico et al. 2008), and 583 mg Cu kg−1 in Spain (Fernández-Calviño et al. 2008). Korchagin et al. (2020) have shown that Cu concentration in the soil surface layer of a centenary vineyard in Southern Brazil was close to 2000 mg Cu kg−1, and this number represents high risk of toxicity to plants, as well as of this metal transfer to aquatic environments.
Grass species native to South America, such as Axonopus affinis, Paspalum notatum, and Paspalum plicatulum, grow naturally between orchards and vineyards’ rows under contamination conditions. Assumingly, they use different mechanisms to both reduce absorption and minimize absorbed Cu toxicity effects (De Conti et al. 2018). De Conti et al. (2018) and Silva et al. (2020) highlighted the importance of these native grass species for phytoremediation. They reported that genus Paspalum can absorb and accumulate more Cu, mainly in the root system, than other native species. In addition, they also found that, because of increased soil Cu levels, native grasses cultivation mostly accounted for changing soil solution’s chemical composition, for increasing organic acids’ concentration and, consequently, organic carbon soluble — this process enhances Cu+2 complexation by decreasing the toxicity potential. However, organic acids, mainly in the rhizosphere region, can adsorb H+, rise solution pH and, consequently, contribute to decrease Cu2+ activity (Oburger et al. 2009).
Cover plant species, such as some species natives to the Pampa Biome, can be sensitive to Cu toxicity. Such a toxicity leads to reactive oxygen species (ROS) formation in the roots and shoot; therefore, it can be a tool to detect stress level in plants due to Cu excess (Huang et al. 2019). The superoxide anionic radical (O2•−) produced by the monoelectronic reduction of oxygen, which is rapidly converted into hydrogen peroxide (H2O2), is one of the most representative ROS, given its spontaneous or enzymatic dismutation, which is triggered by the enzyme superoxide dismutase (SOD) (Gill and Tuteja 2010). H2O2 is lesser reactive than O2•−; therefore, SOD activity is a mechanism adopted to fight oxidative stress (Gill and Tuteja 2010).
Identifying native grass species presenting tolerance mechanisms against Cu toxicity can be a strategy to mitigate contamination in these areas, since they can be grown in contaminated vineyards and orchards. Native grass species are highly adapted to the soil and climate conditions of each region; therefore, their tolerance to high soil Cu concentrations can vary. Previous studies carried out in Australia have reported a wide variety of metal-tolerant tree and grass species (Grant et al. 2002; Lamb et al. 2010). Species belonging to genera Axonopus and Paspalum, in South America, have already been reported as having soil Cu toxicity tolerance mechanisms (De Conti et al. 2019) — the best adaptations to fight toxicity are related to increased root yield and to high shoot dry matter, photosynthetic rate, as well as to larger amounts of free radical fighting enzymes.
Identifying the biochemical and physiological responses to Cu toxicity of several grass species native to the Pampa and Atlantic Forest Biomes is essential to identify species presenting greater potential to phytoremediate soils contaminated with Cu. Our hypothesis indicates that native grass species with the potential to be used for phytoremediation have one, or more, biochemical mechanisms capable of allowing greater Cu accumulation in the shoot and consequently, lesser stress, which leads to satisfactory dry matter production. The aim of the current study was to identify native grass species presenting biochemical-physiological responses that highlight adaptive Cu toxicity tolerance mechanisms capable of mitigating Cu contamination in areas such as vineyards and orchards.
Materials and methods
Used soil and species’ selection
The study was carried out in a greenhouse, at Federal University of Santa Maria (UFSM), Santa Maria County, Rio Grande do Sul State, Southern Brazil. Mean temperature in the greenhouse throughout the study was 26 °C, and relative humidity was 50%. Three grass species native to the Pampa and Atlantic Forest biomes were used, namely, Paspalum notatum (fork grass), Paspalum plicatulum (mattress grass), and Paspalum urvillei (Roça grass), as well as an exotic species (Cynodon dactylon — star grass). The species were selected based on their ability to accumulate Cu — according to data from a previous study (Silva et al. 2020). Seedlings were collected from vineyards and orchards located in Santana do Livramento County, Rio Grande do Sul State, Southern Brazil (S30°46′, W55°22′).
Seedlings were multiplied for standardization before the experiments; In that protocol, the collected plant species were divided into tillers, separated, and washed. The roots of all plants were standardized to 5 cm in length, and three roots and shoot were standardized to 5 cm in length. After this procedure, were transplanted to plastic trays (15-L) filled with sand (substrate), and cultivated in greenhouse under 50% shading for 3 months, based on the protocol proposed by Marques (2020a). Sand trays were irrigated three times a day, for 15 min, with 10 l of nutrient solution by using an automatic pump and timer system with complete nutrient solution (mg L−1) containing 149.80 of NO3−, 24.80 of H2PO4−, 39.27 of SO42−, 41.31 of Mg2+, 288.72 of Ca2+, 234.60 of K+, 0.03 of Mo, 0.26 of B, 0.06 of Cu, 0.50 of Mn, 0.22 of Zn, and 4 of Fe. One month after the start of the multiplication procedure, in order to expand the plant bank and increase homogeneity among seedlings belonging to each species, again the species were divided into tillers, roots of all plants were standardized to 5 cm in length and three roots and shoot was standardized to 5 cm in length. This process was repeated once a month, for 3 months.
Treatments and experimental procedures
The soil used in the experiment was classified as typic Hapludalf (Soil Survey Staff-Soil Taxonomy, 2006). It was collected from the 0–20 cm layer in a site cultivated with native pastures located in Campanha Gaúcha region, Santana do Livramento, which is known by its low natural Cu levels. Soil physical chemical features are shown in Table 1. Soil samples were air dried, homogenized, and sieved in 2-mm mesh. Soil pH was adjusted by adding Ca carbonate (CaCO3) and Mg carbonate (MgCO3) at 2:1 ratio to 0.57 g kg−1 of soil. Subsequently, 40 mg P kg−1 and 100 mg K kg−1 were added to the soil with triple superphosphate and K chloride, respectively. Humidity was restored to 80% of field capacity (FC) and incubated for another 25 days.
The treatments counted on two Cu availability conditions: control (natural Cu concentration in the natural field) and Cu toxicity condition — 80 mg Cu kg soil−1 (soil Cu level in approximately 40-year-old vineyards) (Girotto et al. 2014). Copper addition was performed 30 days after concealer application through CuSO4.5H2O solution application (Reagent P.A., Vetec). Soil samples were incubated for 40 additional days in greenhouse under controlled temperature condition (25 ± 5 °C). Distilled water was added to the samples in order to replenish the evaporated water and maintain soil water retention capacity at 80%.
The study design consisted of a root concentration system developed to separate rhizospheric from non-rhizospheric soil in 77-micron polyester screen (Supplementary material 1). Seedlings were removed from the sand and weighed for initial fresh matter determination (FMi). They were implanted in the sampling units after they were weighed. Therefore, all plants were standardized: three roots and three fully expanded leaves. Pots filled with 4 kg of soil, with their respective treatments, distributed in bifactorial model (two soil Cu levels and four grass species) were the sampling units. The experiment followed a completely randomized design, with four repetitions.
Seven seedlings were initially implanted in the pots; only five plants were left in each pot after 7 days. The experiment started on November 3rd, 2019, and it was conducted for 75 days. In total, 15 mg N kg−1 of soil (in the form of urea) were applied at 25 and 50 days after grass seedling transplantation. Soil moisture was maintained at 70% of field capacity, based on daily vessel weighing.
Photosynthesis analysis
Net photosynthetic rate (A), CO2 stomatal conductance (Gs), water use efficiency (WUE), and instantaneous carboxylation efficiency by RUBISCO [ribulose-1,5-bisphosphate-carboxylase/oxygenase (ECR) were the evaluated parameters. These variables were assessed in a chamber, at CO2 concentration of 400 μmol mol−1, temperature ranging from 20 to 25° C, 50% ± 5% relative humidity, and photon flux density of 1000 μmol m−2 s−1. Therefore, the association between CO2 assimilation capacity and water loss to the environment was established due to tolerance to Cu toxicity stress.
Collection, relative growth rate determination, and biomass partitioning
Plants were removed from the experimental system on February 3rd, 2020 (after 75-day cultivation). Rhizospheric soil was separated from the roots and stored in refrigerator for soil solution extraction. Subsequently, plant roots were washed with water to remove adhered soil particles. Whole plants (i.e., roots + shoots) were weighed to find the final fresh matter (FMf). Plants’ relative growth rate (RGR) was calculated through Eq. 1, based on FMi and FMf values:
Subsequently, plants were washed in 1 mol L−1 HCl solution and in distilled water. They were separated into leaves, stem, and roots. The organs were dried in oven, at 65 °C, until reaching constant mass; they were used for later leaf, stem, and root dry matter determination (LDM, SDM, and RDM).
Physiological stress analysis
The last two fully expanded leaves were removed from five plants per sample unit at plant harvesting time (after 75-day cultivation). Leaves were frozen in liquid N2 and, subsequently, preserved in ultrafreezer, at − 80 °C, for superoxide dismutase (SOD) and guaiacol peroxidase (POD) determination.
SOD enzyme (enzyme units (U mg−1 protein) activity was estimated through the spectrophotometric method (Giannopolitis and Ries 1977). Potassium phosphate (50 mM, pH 7.8), methionine (13 mM), riboflavin (2 μM), nitroblue tetrazolium (75 μM), ethylene diaminetetraacid (0.1 mM), and enzyme extract (100 μL) solution was used for such a purpose. Formosanblue photochemically produced from nitroblue tetrazolium was monitored through absorbance increase by 560 nm. One SOD unit was defined as the amount of enzyme required to inhibit nitroblue-tetrazolium photoreduction by 50% (Beauchamp and Fridovich 1971).
Guaiacol was used as substrate to evaluate POD enzyme (U mg−1 protein) activity. Potassium phosphate (100 mM, pH 6.5), guaiacol (15 mM), and hydrogen peroxide (3 mM) solution were used. Enzyme activity was measured through guaiacol oxidation into tetraguaiacol; it recorded absorbance at 470 nm. Molar extinction coefficient of 26.6 mM−1 cm−1 was used to calculate enzyme activity.
Protein was determined based on the method by Bradford (1976) by using albumin, in all enzymatic assays.
Root biometrics
Roots were separated from the shoot, sieved in 1-mm mesh, washed in distilled water and stored in freezer at 4 °C, at plant harvesting time (after 75-day cultivation). Roots were thawed, suspended in 0.5 cm water slide on 30 × 40 cm2 clear acrylic tray, and scanned at 600 dpi in scanner EPSON Expression 11,000, equipped with additional TPU light to determine total root length, diameter, and volume (Marques et al. 2020a, b). WinRHIZO© Pro 2007 software (Regent Instruments, Quebec, Canada) was used to analyze the digitalized images. Subsequently, each root sample was dried for 72 h, at 65 °C, and weighed in order to obtain root dry matter (RDM).
Soil solution and soil and tissue Cu concentration
Rhizospheric soil samples were thawed and weighed to assess chemical changes in soil solution, which was extracted through the centrifuge method, as described by Somavilla et al. (2017). Then, soluble organic carbon (SOC), pH, and electrical conductivity (EC) were determined.
In total, 1 ml of solution was subjected to digestion for 4 h, at 60o C, in sulfochrome mixture of K2Cr2O7 1.25 mol L−1, with 80 mL of concentrated H2SO4, at sample:mix ratio of 1:1 for SOC analysis. Reading was performed based on the colorimetry method, at 580 nm. Factor of 1.35 was used in comparison to the glucose curve, since this procedure is correlated (r = 0.997) to the method described by Moore (1985). The pH was determined in pH meter. R-TEC-7-MP conductivity was determined in digital conductivity meter model TEC-4MP.
Shoot and root DM were ground in Wiley mill for Cu concentration determination. Then, 0.2 g of DM was digested in 3.0 ml of HNO3 + 1 ml of HClO4 (Tedesco et al. 1995). Samples’ digestion was carried out in digestion block heated to 130 °C, for 4 h. Copper (Cu) concentration in the extracts was analyzed in atomic absorption spectrophotometer (EAA; Varian SpectrAA-600, Australia). Translocation factor (TF) was determined based on Cu concentration in shoot and root DM. Plants’ ability to accumulate a certain metal in the shoot, in relation to its concentration in the roots, was calculated through Eq. (2) (Ali et al. 2013):
Cu accumulated in both the root (CuRac) and shoot (CuSac) was determined by multiplying Cu concentration in tissue by its DM.
Statistical analysis
Variables were subjected to analysis of variance in RStudio software—Easyanova package. Differences between means were compared through Tukey test at 5%, based on bifactorial model, species (4) × treatments (2), with four repetitions, in case treatment effects were significant at 5% probability level, in the F test.
Principal component analysis was performed in MULTIV software in order to assess the association between TF, CuRac and CuSac, and the assessed variables (Pillar 2001).
Results
Biomass growth and partition
There was interaction between factors in all variables. P. notatum presented the highest relative growth rate (RGR) among the assessed species under natural soil Cu availability conditions (Table 2). However, all species showed the same RGR pattern under toxicity conditions, and they did not statistically differ from each other. All species had their RGR reduced in Cu-contaminated soil.
All species recorded reduced root partition rate in total dry matter, and it resulted in increased leaf and stem participation (Fig. 1). Roots’ contribution to total dry matter in species C. dactylon, P. notatum, P. plicatulum, and P. urvillei decreased by 15, 18.2, 21.2, and 22.1%, respectively. Species P. notatum and P. plicatulum recorded greater leaf contribution to total dry matter due to toxicity condition; it increased leaf contribution to total dry matter by 15%.
Photosynthesis analysis
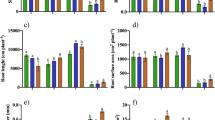
The net photosynthetic rate (A) recorded for C. dactylon, P. plicatulum, and P. urvillei was the highest under natural Soil Cu availability conditions (Fig. 2a). However, P. plicatulum and P. urvillei presented higher A under high Cu availability — it increased by 112% and 69% in comparison to the control condition, respectively. CO2 (Gs) stomatal conductance showed higher values in P. urvillei and P. notatum (Fig. 2b); however, P. plicatulum and P. urvillei presented the highest Gs values — approximately 113% higher than that of C. dactylon and P. notatum — when plants were cultivated under Cu toxicity conditions.
Photosynthetic parameters of grass species’ net photosynthetic rate (A), CO2 stomatal conductance (GS), internal CO2 (Ci) concentration, transpiration rate (TR), water use efficiency (WUE), and RUBISCO’s instantaneous carboxylation efficiency (ECR) based on soil Cu conditions. Same lowercase letter did not statistically differ (P < 0.05) in species subjected to the same treatment. Same capital letter did not statistically differ (P < 0.05) between treatments applied to the same species
P. notatum presented the lowest A values and the highest Ci values, either under natural soil Cu concentration conditions or at natural toxicity levels when the internal CO2 (Ci) concentration was taken into account. This finding results from the low A values, since lower CO2 consumption by the photosynthetic process tends to increase intercellular CO2 concentration — this process is evidenced by RUBISCO’s lower instantaneous carboxylation efficiency (ECR).
The species did not show differences in transpiration rate (TR) under natural Cu availability conditions (Fig. 2d). However, P. plicatulum and P. urvillei presented the highest TR values under Cu toxicity conditions. Water use efficiency (WUE) by the assessed species recorded different responses. On the one hand C. dactylon and P. notatum reduced WUE, but, on the other, P. urvillei and P. plicatulum maintained the same WUE, without reduction in it due to toxicity. RUBISCO’s instantaneous carboxylation efficiency for P. plicatulum did not decrease under toxicity conditions (80 mg of Cu kg soil−1); it accounted for the highest ECR values among the assessed species.
Root biometrics
C. dactylon recorded the highest root length values in the control group, among all the assessed species (Table 3). However, C. dactylon root length was close to that of P. urvillei under Cu toxicity conditions -this species recorded the lowest root length decrease, 78%, respectively, in comparison to the control.
C. dactylon recorded the greatest root surface area (Table 3) among the assessed species under natural Cu concentration conditions. However, P. urvillei was the species accounting for the highest root surface area values under toxic soil Cu concentrations. C. dactylon root mean diameter was always smaller, regardless of soil Cu concentration — this species always showed thinner roots than the other species, on average, as well as no root diameter changes. Species P. plicatulum, P. notatum, and P. urvillei produced larger roots under toxic conditions.
Root volume decreased due to Cu concentration increase in all species (Table 3). However, the volume of roots produced in each plant presented different patterns depending on soil Cu availability. C. dactylon recorded the highest root volume under natural Cu availability conditions (Control). However, P. urvillei was the species accounting for the highest root volume under Cu toxicity conditions.
Species C. dactylon showed the highest total length rate among fine roots — 32% of root lengths were shorter than 0.2 mm under the control treatment (Fig. 3a). This species showed 26.7% of root lengths in roots < 0.2 mm (in diameter), even under toxic conditions. Species P. urvillei recorded the smallest root length variation rate among the thinnest class of roots (between 0 and 0.2 mm): 18.7% in the control, and 17.9% under toxicity condition.
Root surface area rate (between 0 and 0.2 mm, in diameter) decreased due to Cu concentration increase in all species (Fig. 3b). However, P. urvillei recorded the highest rate of roots greater than 1 mm in diameter under Cu treatment. P. notatum and P. urvillei recorded a very close rate of roots > 1 in diameter; P. notatum accounted for the highest rate (50%) of roots > 1 mm, in diameter, in the control group.
Physiological stress analysis
Peroxidase activity (POD) under natural soil Cu concentration conditions was the highest in P. notatum. However, P. plicatulum showed POD activity increase in tissue at toxic soil Cu concentration (Table 4). P. urvillei was the only species that did not show POD activity modification between natural and Cu toxicity conditions. Differently, P. notatum is the species with high activity in natural conditions. On the other hand, the species C. dactylon and P. plicatulum showed increased POD activity under stress conditions.
The highest superoxide dismutase (SOD) activity values were recorded for species P. notatum and P. plicatulum, mainly under soil Cu toxicity conditions (Table 4). Species P. urvillei also showed high SOD activity under natural soil Cu concentration conditions. Overall, there was no SOD activity increase due to high soil Cu concentration in these species.
Soil chemical attributes
Soluble organic carbon (SOC) concentration under natural soil Cu conditions was higher in the three species belonging to genus Paspalum (Table 5). P. notatum presented the highest soil solution SOC values under soil Cu toxicity conditions. SOC concentrations decreased in all species under soil Cu toxicity conditions.
The aforementioned finding may have resulted from lower root production, which led to less soil rhizodeposition; it may have also resulted from lower carbon assimilation determined by a rhizospheric soil pH value decreased in all species due to Cu concentration increase. Mean soil pH value among species was 6.4 under natural soil Cu availability conditions; this number is 30% higher than that recorded for soil presenting high Cu concentration (mean pH at 4.9).
Solution electrical conductivity (EC) values increased in the soil presenting higher Cu concentration, mainly in species P. plicatulum and P. urvillei. P. urvillei was the species accounting for the highest electrical conductivity values under the highest soil Cu concentration.
Cu concentration in tissue
Shoot Cu concentration (CuS) was higher in all species under Cu toxicity condition (Table 6). Overall, species belonging to genus Paspalum, mainly P. notatum, presented higher CuS concentrations. Similarly, root Cu concentration (CuR) was high in all species, under Cu toxicity condition. Species P. plicatulum recorded the highest CuR values. P. notatum accounted for the highest translation factor (TF) value under Cu toxicity condition. This species was the species with the highest potential to absorb and accumulate Cu in tissue; this finding is indicative of its potential to be used in phytoremediation processes. P. notatum was even better than C. dactylon, which is an exotic species featured by inhabiting contaminated areas.
Cu accumulated in both the root (CuRac) and shoot (CuSac) was higher in all species under Cu toxicity condition, except for C. dactylon, which showed the same CuSac under both conditions (Table 6). P. notatum and P. urvillei showed the highest CuRac and CuSac values under copper toxicity condition. Physiological features likely contributing to higher TF are shown in Fig. 4. Principal component analysis (PCA) indicated that the Cu translocation factor (TF) was associated with plants recording higher CuR and CuS concentrations. Furthermore, plants accounting for higher SOD and POD activity recorded higher TF values. It likely happened due to their greater capacity to tolerate high soil Cu concentrations because of physiological stress reduction, which led to higher RDM and SDM production.
Principal component analysis (PCA) applied to translocation factor (FT), Cu accumulated in root (CuRac), Cu accumulated in the shoot (CuSac), root Cu concentration (CuR), shoot Cu concentration (CuS), soluble organic carbon (SOC), soil solution pH (pH), electrical conductivity (EC), peroxidase activity (POD), superoxide dismutase (SOD) activity, root length, root surface area, mean root diameter, root volume, relative growth rate (RGR), root dry matter production (RDM), shoot dry matter production (SDM), root dry matter rate (%R), leaf dry matter rate (%L), stem dry matter rate (%S), rubisco instantaneous carboxylation efficiency (ECR), water use efficiency (WUE), transpiration rate (TR), intercellular CO2 concentration (Ci), stomatal conductance (GS), and net assimilation rate (A) in native grass species based on concentration of 80 mg Cu kg soil.−1
Discussion
Morphological responses under toxicity conditions
High RGR in plants belonging to genus Paspalum, mainly in P. notatum, can be attributed to its great nutrient-use efficiency, such as P, which allows greater dry matter production per absorbed nutrient unit (Marques et al. 2020a, b). Furthermore, this genus has good shoot (Oliveira et al. 2018) and root area production capacity per dry matter unit (Marques et al. 2020a, b). This feature ensures leaf surface production for light capture, and roots for nutrient absorption. This feature also resulted in high dry matter production by P. urvillei.
Growth reduction, similar to what is observed in the root system of species accounting for the highest Cu accumulation in the roots, is not necessarily a negative fact. Heavy metals’ complexation/immobilization processes under the aforementioned conditions are remarkable on root surface (Barceló and Poschenrieder 2002); they cause complexed Cu2+ increase. This is a positive aspect to reduce potential heavy metals’ toxicity in plant species, during phytoremediation processes, mainly in production areas (Barceló and Poschenrieder 2002).
Complexation processes in- and outside the roots limit the translocation of the free Cu2+ fraction available to the shoot. Furthermore, when these plants are intercropped with fruit plants, fruit trees reduce their metal in soils contaminated with Cu. Thus, the improved development of roots from these grass species in intercropping system could be a cultivation strategy for Cu-contaminated soils (De Conti et al. 2018). Copper (Cu) accumulation in roots is a tolerance mechanism to prevent and/or reduce the translocation of excess Cu to the shoot, since it would cause significant damage to important plant physiological processes (De Conti et al. 2018).
Biochemical responses under toxicity conditions
Grass species belonging to genus Paspalum, mainly Paspalum plicatulum, showed high photosynthetic rate (Fig. 2a), and this finding is indicative of high CO2 assimilation capacity per leaf dry matter unit (Marques et al. 2020a, b). Based on photosynthetic parameters, P. urvillei was the least affected species or the species presenting the best response to Cu treatment. Therefore, it resulted in the smallest RGR reduction for this species (45%) in comparison to that recorded under control conditions.
De Conti et al. (2018) reported that the net photosynthetic rate (A) significantly decreased due to increased concentration of Cu added to soil cultivated with vines (monoculture). On the other hand, whenever vines were intercropped with species belonging to genus Paspalum, A decrease only happened at very high soil Cu concentrations (Conti et al. 2018). This outcome showed that these native species have mechanisms to fight toxicity and that they can benefit from physiological processes such as photosynthesis and intercropping systems (De Conti et al. 2018).
High POD and SOD activity in P. notatum and P. plicatulum in Cu-contaminated soils represented response to fight metal toxicity by these grass species. SOD activity — which is one of the most effective intracellular antioxidant enzymes observed in all aerobic organisms and subcellular compartments in these species — is an oxidative response to abiotic or biotic stress. This enzyme provides the first defense line against the toxic effects of high reactive oxygen species’ levels (Gill and Tuteja 2010). SOD removes O2•− and catalyzes dismutation — one O2•− was reduced to H2O2 and another was oxidized to O2. Accordingly, the enzyme removes O2•− and, therefore, reduces the risk of OH• formation inside the cell. However, SOD was not efficient in reducing stress caused by Cu, since it was not activated and there was growth decrease; thus, this enzyme is not featured as efficient mechanism in these species.
On the other hand, POD, from the group of oxidoreductases, can catalyze a large number of oxidative reactions in plants by using H2O2 as substrate, or, in some cases, by using oxygen as hydrogen acceptor. The produced H2O2 is efficiently removed by enzymes such as POD, and it reduces plant stress. We observed that POD action can be an important mechanism to fight stress, since it makes plants able to grow and tolerate these conditions. This process results in plants capable of accomplishing phytoremediation processes, such as P. plicatulum and C. dactylon, which showed increased POD activity due to increased soil Cu concentration.
On the other hand, the fact that P. urvillei did not show POD and SOD activity decrease between natural and Cu toxicity conditions points out that this species has defense mechanisms against oxidative stress and that such mechanisms allow high dry matter and RGR production, even in soils contaminated with Cu. These mechanisms can be related to complex enzymatic and non-enzymatic antioxidant defense systems that work together to protect plant cells from oxidative damage by eliminating ROS (Gill and Tuteja 2010).
Changes in soil attributes
Soluble organic carbon (SOC) exudation by native grass species certainly contributes to maintain/increase Cu2+ content complexed to carboxylic groups (-COO-) (Brunetto et al. 2014). Another mechanism that may be related to tolerance and needs to be studied is the possibility of exudation of organic acids in the rhizosphere that reduce Cu absorption. Plant roots release a very wide variety of organic compounds that change the rhizosphere’s chemical features. For example, changes in pH and the presence of organic binders can increase stable Cu2+ complexation in soil solution and, consequently, reduce its bioavailability. These processes favor plant growth, since Cu uptake by roots mostly happens in the free form (De Conti et al. 2018).
In addition to concentration, it should be noticed that plants can also change SOC composition through soluble organic compounds’ exudation from the root, such as organic acids and phenolic compounds, as described for malic acid in response to high Cu levels (Nian et al. 2002). This process decreases contaminants’ phytotoxic potential (Kim et al. 2010). These compounds’ metal complexation property and their release into the rhizosphere have also been described as an attempt by plants to ensure balanced absorption in cases of nutrient scarcity or to limit the availability of toxic elements (Jones 1998; Malta et al. 2016). For example, Cu interaction with SOC functional groups likely account for Cu2+ availability decrease in soils treated with biochar and organic compounds (Oustriere et al. 2016).
Interestingly, high soil Cu concentrations led to lower pH in comparison to the control. This phenomenon is strongly related to root concentration effect on the control and to its effect on rhizospheric soil. Custos et al. (2020) highlighted that this process is the effect of absorption by plants — simulations have suggested that the nutrient absorption process is mainly caused by nitrogen, which has strong influence over rhizosphere’s pH. Nitrate is approximately 5 to 400 times more absorbed than other ions in aerated soils without ammonium fertilization. Concentration reduction in the rhizosphere is compensated by root H+ absorption or by equivalent OH− exudation. Thus, rhizosphere pH of non-legume plants in non-flooded soils can oftentimes be more alkaline than that in soil layers farther from the roots (Custos et al. 2020).
Interactions of factors allowing tolerance to toxicity
Photosynthetic variables were not necessarily related to TF, but it is related to dry matter increase and production. Although features related to dry matter production are important, P. notatum, which is the species accounting for the highest TF in copper-treated plants, did not present higher values, mainly for A, GS, and TR. This pattern was not consistent in all species.
The highest stem rate (%S) — 57% — is an interesting feature that may have contributed to the highest CuSac recorded for P. urvillei. This finding may highlight the most adapted plants to this toxicity condition. Stem is a plant fraction that has good Cu retention capacity, but such a feature leads to less Cu translocation to the leaves, a fact that results in less damage to the photosynthetic apparatus, which, in its turn, leads to higher CO2 assimilation rates.
The highest values of A led to greater ECR, as in the P. plicatulum. However, higher TR results in lower carbon concentration (Ci), which can estimate higher ECR. Therefore, higher ECR does not necessarily result in more carbon converted to dry matter. Thus, although the species has greater ECR in the condition of toxicity, it produces the same dry matter as the other species in the condition of toxicity. This is because part of the fixed carbon can be converted to stress-reducing compounds, as exudates by the roots like organic acids, used to generate energy in cellular respiration for synthesis of stress-reducing compounds such as antioxidants. Thus, part of the carbon is not used for the production of dry matter and is directed to for the synthesis of stress-reducing compounds (Souri et al. 2019; Hagemeyer 2004).
Plants’ root system showed significant association between TF and root diameter. Overall, plants recording the highest TF were the ones accounting for the largest root diameter, such as P. notatum. Even without Cu toxicity conditions, these plants presented larger root diameters. This finding is evidenced by the fact that P. notatum is the species accounting for the highest root length and surface area rates in the thickest root classes cultivated in Cu-contaminated soils. On the other hand, these variables showed negative association with CuR and CuS, due to root length and volume reduction under Cu toxicity conditions.
Under toxicity conditions, De Conti et al. (2018) highlighted the growth and toxicity reduction capacity by species belonging to genus Paspalum, mainly due to intercropping, when it comes to Cu toxicity, since it reduces the negative impact of Cu toxicity (mainly, on leaf and stem dry matter). These results, along with those observed in the current study, point towards the need of integrating the cultivation of native plants, such as P. notatum, to Cu-contaminated soils’ phytoremediation, in order to ensure plants’ proper development, mainly of young fruit trees.
P. notatum consortium can reduce Cu2+ chemical form, if Cu2+ is considered the form preferentially absorbed by plants and microorganisms. The consequences of complexed Cu chemical species (Cu-SOC) increase in soil solution lies on reducing fruit plants’ bioavailability and potential toxicity. Part of this process is assumingly related to greater root exudation in this species, which releases greater SOC amounts into the rhizosphere (Table 5).
According to De Conti et al. (2018), young vines intercropping with P. plicatulum was efficient in promoting plant growth in soils with moderate and low Cu contamination levels by reducing Cu bioavailability. They also showed that Cu phytotoxic effects on root morphology in young vines were reduced by intercropping with these grass species at 40 mg Cu kg−1. This outcome indicates that native grass species maintenance, such as those belonging to genus Paspalum, in soils contaminated with Cu based on the effective phytoremediation strategy.
Conclusions
The growth patterns that allow the use of native grasses in phytoremediation are high TF, high CuSac, and high CuRac These patterns occur due to (1) increased activity of stress-fighting enzymes such as POD, (2) larger roots for greater Cu complexion, which makes roots less sensitive to stress caused by Cu, and (3) greater soluble organic carbon exudation in the rhizosphere, since it can complex Cu2+ and reduce the presence of toxic forms in plants. Native grass species do not only have a specific mechanism to reduce Cu toxicity, but a set of physiological features that allow greater dry matter production and higher CuSac and CuRac. P. notatum and P. urvillei have the greatest potential for phytoremediation in Cu-contaminated areas.
Data availability
The datasets generated and analyzed during the current study are not publicly available due do not belong to a public database but are available from the corresponding author on reasonable request.
References
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Barceló J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(248):255
Brunetto G, Miotto A, Ceretta CA, Schmitt DE, Heinzen J, Maraes MP, Canton L, Tiecher TL, Comin JJ, Girotto E (2014) Mobility of copper and zinc fractions in fungicide-amended vineyard sandy soils. Arch Agron 65:609–624
Custos J-M, Moyne C, Sterckeman T (2020) How root nutrient uptake affects rhizosphere pH: a modelling study. Geoderma 369:114–314
De Conti L, Bastos de Melo GW, Tarouco CP, Marques ACR, Nicoloso FT, Tassinari A, Tiecher TL, Cesco S, Mimmo T, Brunetto G (2018) Photosynthesis and growth of young grapevines intercropped with native grasses in soils contaminated with copper. Acta Hortic 1217:179–184
De Conti L, Ceretta CA, Melo GWB, Tiecher TL, Silva LOS, Garlet LP, Mimmo T, Cesco S, Brunetto G (2019) Intercropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 216:147–156
Dell’Amico E, Mazzocchi M, Cavalca M, Allievi L, Andreoni V (2008) Assessment of bacterial community structure in a long-term copper-polluted ex-vineyard soil. Microbiol Res 163:671–683
Fernández-Calviño D, Pateiro-Moure M, López-Periago E, Arias-Estévez M, Nóvoa-Muñoz JC (2008) Copper distribution and acid-base mobilization in vineyard soils and sediments from Galicia (NWSpain). Eur J Soil Sci 59:315–326
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol 48:909–930
Girotto E, Ceretta CA, Brunetto G, Miotto A, Tiecher TL, De Conti L, Lourenzi CR, Lorensini F, Gubiani PI, Silva LS, Nicoloso FT (2014) Copper availability assessment of Cu-contaminated vineyard soils using black oat cultivation and chemical extractants. Environ Monit Assess 186:9051–9063
Grant CD, Campbell CJ, Charnock NR (2002) Selection of species suitable for derelict mine site rehabilitation in New South Wales. Australia Water Air Soil Poll 139:215–235
Hagemeyer J (2004) Ecophysiology of plant growth under heavy metal stress. In: Prasad MNV (ed) Heavy Metal Stress in Plants, 2nd edn. Springer, Berlin, pp 201–222
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:75–92
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44
Kim KR, Owens G, Naidu R, Kwon SLK (2010) Influence of plant roots on rhizosphere soil solution composition of long-term contaminated soils. Geoderma 155:86–92
Korchagin J, Moterle DF, Escosteguy PAV, Bortoluzzi EC (2020) Distribution of copper and zinc fractions in a Regosol profile under centenary vineyard. Environ Earth Sci 79:439–452
Lamb DT, Ming H, Megharaj M, Naidu R (2010) Relative tolerance of a range of Australian native plant species and lettuce to copper, zinc, cadmium, and lead. Arch Environ Contam Toxicol 59:424–432
Malta PG, Silva SA, Ribeiro C, Campos NV, Azevedo AA (2016) Rudgea viburnoides (Rubiaceae) overcomes the low soil fertility of the Brazilian Cerrado and hyperaccumulates aluminum in cell walls and chloroplasts. Plant Soil 408:369–384
Marques ACR, Oliveira LB, Brunetto G, Tavares MST, Quadros FLF, Nicoloso FT (2020a) Interaction between growth strategies and phosphorus use efficiency in grasses from South America natural grasslands. Rev Ceres 67:062–069
Marques ACR, Oliveira LB, Schwalbert R, Del Frari BK, Brunetto B, Quadros FLF, Nabinger C, Nicoloso FT (2020b) A growth strategies as determinants of CO2 sequestration and response to nitrogen fertilisation in C4 grasses in South American natural grasslands. Crop Pasture Sci 71:776–784
Moore TR (1985) The spectrophotometric determination of dissolved organic carbon in peat waters. Soil Sci Soc Am J 49:1590–1592
Nian H, Yang ZM, Ahn SJ, Chen ZJ, Matsumoto H (2002) A comparative study on the aluminium- and copper-induced organic acid exudation from wheat roots. Physiol Plant 116:328–335
Oburger E, Kirk GJD, Wenzel WW, Puschenreiter M, Jones DL (2009) Interactive effects of organic acids in the rhizosphere. Eur J Soil Biol 41:449–457
Oliveira LB, Marques ACR, Quadros FLF, Farias JG, Piccin R, Brunetto G, Nicoloso FT (2018) Phosphorus allocation and phosphatase activity in grasses with different growth rates. Oecologia 186:633–643
Oustriere N, Marchand L, Galland W, Gabbon L, Lottier N, Motelica M, Mench M (2016) Influence of biochars, compost and iron grit, alone and in combination, on copper solubility and phytotoxicity in a Cu-contaminated soil from a wood preservation site. Sci. Total Environ. 567:816–825
Pietrzak U, McPhail DC (2004) Copper accumulation, distribution and fractionation in vineyard soils of Victoria. Australia Geoderma 122:151–166
Pillar VD (2001) Multivariate exploratory analysis, randomization testing and bootstrap resampling User’s Guide. Porto Alegre, Universidade Federal do Rio Grande do Sul. Disponível em: http://ecoqua.ecologia.ufrgs.br/arquivos/Software/Multiv/MultivManual.pdf. Accessed 19 June 2020
Silva ICB, Marques ACR, Quadros FLF, Sans GA, Soares VM, De Conti L, Ceretta CA, Ferreira PAA, Brunetto Toselli M, G, (2020) Spatial variation of herbaceous cover species community in Cu-contaminated vineyards in Pampa biome. Environ Sci Pollut Res 27:13348–13359
Somavilla A, Dessbesell A, Santos DR (2017) Centrifugation methodology to extract soil solution. Sci Agric 18:44–47
Souri Z, Cardoso AA, da-Silva CJ, de Oliveira LM, Dari B, Sihi D, Karimi N (2019) Heavy metals and photosynthesis: recent developments. In: Ahmad P, Ahanger MA, Alyemeni MN, Alam P (eds) Photosynthesis, Productivity and Environmental Stress, 1st edn. Wiley, New York, pp 107–134
Tedesco MJ, Gianello C, Biassani CA, Bohnen H, Volkweiss SJ (1995) Análise de solo, plantas e outros materiais. Boletim técnico 5, UFRGS: 174
Funding
This article was supported financially by the National Council for Scientific, Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Contributions
Collected the data: ACRM, JH, ET, AS, LAT, RS, and TPB; analyzed the data: ACRM, JH, GB; wrote the paper: ACRM, JH, ET, LD, GLD, AS, LAT, RS, TPB, FTN, and GB.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish the manuscript in this form.
Competing interests
The authors declare competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marques, A.C.R., Hindersmann, J., Trentin, E. et al. Physiological and biochemical characterization of copper-toxicity tolerance mechanism in grass species native to Pampa Biome and Atlantic Forest for use in phytoremediation. Environ Sci Pollut Res 30, 5076–5088 (2023). https://doi.org/10.1007/s11356-022-22570-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22570-3