Abstract
The geno-, and eco-toxicity of nonlyphenol (NP) and bisphenol A (BPA) were investigated in Daphnia magna, and Chironomus riparius. BPA may exert a genotoxicity on both species, whereas NP-induced DNA damage occurred only in C. riparius. In NP-exposed D. magna, increased mortality, without effect on DNA integrity was observed, an example of a false-negative result from the biomarkers perspective. False-positive results from the genotoxicity were observed in BPA-exposed D. magna and in NP-exposed C. riparius. Considering the importance of genotoxic biomarkers in ecotoxicity monitoring, DNA damage in these species could provide useful information.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Among the available genotoxicity indicator tests, the Comet assay has recently attracted much attention. The Comet assay, also called the single-cell gel electrophoresis (SCGE) assay, primarily measures DNA strand breakage in single cells. DNA strand breaks are potential pre-mutagenic lesions and are sensitive markers of genotoxic damage. The Comet assay has been shown to respond quickly and accurately, and its findings are easy to measure. Thus, since the protocol was published by Singh et al. (1988), it has been increasingly used in different fields of study: clinical applications, human monitoring, radiation biology, and genetic toxicology. Application of Comet assay, using many different species, has been conducted in ecotoxicology (Clement et al. 2004; Cotelle and Ferard 1999; Palmqvist et al. 2003). Evaluation of genetic toxicity using Comet assay was performed on numerous wildlife organisms, including plants (Koppen and Verschaeve 1996; Navarrete et al. 1997), worms (Rajaguru et al. 2003), mollusks (Clement et al. 2004), fish (Mitchelmore and Chipman 1998; Schnurstein and Braunbeck 2001), amphibians (Ralph and Petras 1997) and mammalians (Tice et al. 2000). Only few genotoxic studies have been conducted on the aquatic invertebrates, such as, daphnid or chironomid (den Besten and Tuk 2000).
In this study, chemical-induced DNA damages were investigated by measuring DNA strand breaks in two biomonitoring species, the freshwater crustacean, Daphnia magna, and the larva of the aquatic midge, Chironomus riparius, in order to identify genotoxic biomarkers for risk assessment. They hold an important position in the aquatic food chain and are sensitive to many pollutants, easy to culture and have a short life cycle, and thus they are considered as suitable species for aquatic biomonitoring (Giesy et al. 1988; Cranston 1995; Choi et al. 2000; Atienzar et al. 2001). Taken into account of the importance of D. magna and C. riparius in the aquatic ecosystem, information concerning genotoxicity on these species can be valuable for freshwater monitoring and environmental risk assessment. As chemical stressors, two most representative endocrine disrupting chemicals (EDCs), nonlyphenol (NP), which is used in the polymer industry (EU 2002) and bisphenol A (BPA), which is an intermediary in the production of polycarbonate and epoxyresins (EU 2003), were selected. Despite the importance of EDC in aquatic ecosystems, few studies have been conducted on the genotoxic effect of these compounds on the aquatic ecosystem components. DNA damage was measured upon sublethal exposure condition in NP and BPA exposed D. magna and C. riparius using Comet assay and its involvement in response to oxidative stress was also investigated by measuring typical oxidative stress indicators, such as, lipid peroxidation and catalase activity. Conventional ecotoxicity tests, using growth and survival as toxic endpoints, were conducted, in order to validate ecotoxicological relevance of genotoxic biomarkers in these species as potential biomarker for environmental contamination.
Materials and Methods
Using an original strain provided by the Korea Institute of Toxicology (Daejeon, Korea), we obtained D. magna and C. riparius larvae from adults reared in our laboratory. D. magna were individually placed in glass beakers containing a culture medium, aerated M4 media (OECD 202 2004), for 2 days. Cultured daphnids were fed daily on the green alga Chlorella sp. at concentrations of 1 × 106–109 cells/mL; the larvae of C. riparius, which were fed with fish flake food (Tetramin, Tetrawerke, Melle, Germany), were reared in a 2 L glass chamber containing dechlorinated tap water and acid-washed and aerated sand. Culture of D. magna and C. riparius were maintained at 20 ± 1°C, 16 h light and 8 h dark cycle photoperiod regime.
We conducted the experiment at a constant temperature of 20 ± 1°C under light conditions of 16–8 h of light and darkness using 7-day-old Daphnia and the fourth instar larvae of Chironomus. For the chemical treatment, based on the results of the acute toxicity test (Lee and Choi 2006, 2007; Park and Choi 2007), three concentrations corresponding to 1/1,000, 1/100 and 1/10 of the 24-h L(E)C50 were selected for sublethal exposure conditions. Daphnia magna were exposed to 0.3, 3, and 30 μg/L for NP and BPA, whereas, C. riparius were exposed to 1, 10 and 100 μg/L for NP and 5, 50 and 500 μg/L for BPA. For each experiment, we added 0.1 mL of the test solution into the experimental beakers before introducing the larvae. Acetone was used as solvent. Three concentrations of each test chemical, solvent control (acetone) was prepared for each experiment. Three replicates were prepared for each concentration.
Twenty juveniles of Daphnia and 10 larvae of Chironomus were collected 24 h after treatment from the control and experimental tanks and were pooled for a Comet assay, as described previously (Park and Choi 2007). Briefly, a suspension of cells is mixed with low melting point agarose and spread onto a microscope glass slide. Following lysis of cells with detergent at high salt concentration, DNA unwinding and electrophoresis is carried out at a pH 13 above. Before analysis, the slides were stained with ethidium bromide, then analyzed at 400× magnification using a fluorescence microscope (Nikon, Kanagawa, Japan). DNA damage was expressed as the olive tail moment using an image analysis computerized method (Komet 5.5, Kinetic Imaging Limited, Nottingham, UK). Twenty juveniles of Daphnia and 10 larvae of Chironomus were collected 24 h after treatment from the control and experimental tanks and pooled for enzyme activity measurements. Catalase (CAT) activity and malonyldialdehyde (MDA) measurement were conducted, as described previously (Lee et al. 2008). Survival and growth were investigated using 20 Daphnia and 10 larvae of Chironomus, as described previously (Lee et al. 2008).
The data passed the normality test and the equal variance test. Statistical differences between the control and the treated larvae were examined using variation analysis with Dunnett’s multiple comparison test. A parametric Pearson test was conducted to study correlations among the parameters. All statistic tests were performed using SPSS® 12.0 KO (SPSS Incorporated, Chicago, IL, USA).
Results and Discussion
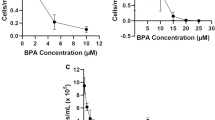
Genotoxicity testing in vivo is performed for hazard identification and is part of the risk assessment process. Results from in vivo DNA damage detection assay, such as, Comet assay, contribute to hazard identification and to dose-response assessment. In this study, genetic toxicity of NP and BPA was investigated in aquatic sentinel species, D. magna and C. riparius by investigating DNA strand breaks using Comet assay (Table 1). Exposure concentration-dependant increases in Olive tail moment were observed in both NP and BPA exposed Daphnia and Chironomus. For D. magna, statistically significant increase in Olive tail moments was observed only at 3 and 30 μg/L of BPA exposure, whereas, for C. riparius, Olive tail moment increased significantly at all concentrations of both chemicals tested. BPA may exert a genotoxic effect on D. magna and C. riparius, given that DNA strand breaks increased in both species exposed to this compound, whereas NP-induced DNA damage occurred only in C. riparius. In aquatic environment, most of genotoxic tests using Comet assays have been performed in vitro system from aquatic species, mostly using fish-driven cell lines (Cotelle and Ferard 1999; Nehls and Segner 2005). In this study, however, D. magna and C. riparius were exposed to each chemical in vivo and DNA damage was assessed in cells subsequently isolated from them. In vivo genotoxic biomarker obtained in aquatic sentinel species, as in our study, could be a powerful tool in environmental monitoring. Indeed, according to Ohe et al. (2004) and Chen and White (2004), DNA damage in wildlife species measured by Comet assay could provide a sensitive and rapid genotoxic biomarker in environmental monitoring.
Aquatic organisms can provide model systems for investigation of how genotoxicants damage cellular components, how cells respond, and how repair mechanisms ameliorate this damage (Di Giulio et al. 1989; Livingstone et al. 1994). Moreover, aquatic organisms are more sensitive to exposure and toxicity compared to terrestrial organisms including mammals and in this respect they may provide experimental data for evaluation of subtle effects of genotoxicity, oxidative stress, and other adverse effects of pollutants (Lackner 1998). Involvement of DNA damage in response to oxidative stress was investigated by measuring typical oxidative stress indicators, such as, lipid peroxidation and catalase activity, in NP and BPA exposed D. magna and C. riparius (Table 1). Statistically significant increase in MDA was observed in NP-exposed Daphnia and Chironomus, whereas, CAT activity rather decreased in both chemicals exposed animals. The result suggests that oxidative stress related response may be involved in NP and BPA toxicity, however, the exact physiological meaning of increased lipid peroxidation and decreased catalase activity is difficult to explain. To fully understand the involvement of oxidative stress in NP- and BPA-toxicity, experimental evidence provided in this study was not sufficient. Broad range of oxidative stress-related parameters and their physiological meanings are needed to be investigated. Until recently few studies have addressed the production of reactive oxygen species (ROS) for in vivo experiments, either in the presence or in the absence of toxic chemicals, in aquatic organisms, because of technical difficulties of appropriate measurements. But evidence was provided by indirect measurements of free radical formation (spin trapped) in digestive gland cell mixture (mussels) and DNA strand breaks (comet assay; Livingstone et al. 1997; Mitchelmore et al. 1998). Also, despite the numerous studies (laboratory and field) on the antioxidant defenses found widely in aquatic organisms, our knowledge of the regulation of antioxidant systems in aquatic organisms in relation to either endogenous or exogenous (pollutants) sources of ROS is limited (Livingstone 2001). The resulting oxidative damage to lipids, DNA, and proteins and the adverse effects on the antioxidant, enzymatic and nonenzymatic, defense mechanisms of aerobic organisms have been used in recent years as biomarkers for monitoring environmental pollution. The current knowledge that such processes of oxidative damage occur in aquatic organisms gave the impetus to extend environmental and ecotoxicological studies to aquatic organisms as sentinels of environmental contamination by toxic chemicals. All these studies indicate that oxidative biomarkers in combination with other types of biomarkers, such as genotoxic biomarker, in aquatic organisms can be useful in large-scale environmental monitoring programs (Almeida et al. 2003; Monserrat et al. 2003).
Biomarkers can be used to assess changes at individual and/or population levels. However, it has been widely recognized that the implementation of biomarkers, including genotoxic biomarkers in environmental monitoring is hampered by the lack of knowledge of how biomarker responses are related to population dynamics of the species in which the biomarker is applied (den Besten 1998; Hyne and Maher 2003). Indeed, although pollutants may influence the genetic constitution of populations by causing direct damage to DNA molecules within the individual cell nucleus, the ecological relevance of changes in single cells within some tissues of some individual organisms is extremely difficult to assess (Depledge 1998). Nonetheless, sensitive detection of DNA damage in wildlife species is necessary, as pollutant-induced DNA damage might influence the genetic constitution of populations. Therefore, in this study, to provide insight into the relative sensitivity and higher biological level consequences of DNA damage observed in Table 1, conventional ecotoxicity tests, using growth and survival as toxic endpoints, were conducted (Table 2). Studied concentrations of NP and BPA exposure do not seem to affect the general physiological status of C.riparius and D.magna, as any statistically significant change was not observed on body fresh and dry weights in NP- and BPA-exposed D. magna and C.riparius. The survival rate was evaluated at the end of the growth experiments in D. magna and C. riparius. In both species no clear trend among treatments was apparent, except at the highest level of NP exposure for both Daphnia and Chironomus, (30 and 100 μg/L, respectively), where statistically significant decrease in survival rate was observed.
The experiments with NP-exposed Daphnia show that 96 h effects on survival, while no 24 h effect on DNA integrity were found. Moreover, NP exposure seems to provoke long-term (21-day) reproduction failure in D. magna (Ha and Choi 2007). This may be an example of a false-negative result from the biomarkers’ perspective. It is clear that this type of error can occur; however, this result could be interpreted that mechanism other than genetic alteration might be involved in NP-induced mortality and reproduction failure in Daphnia. Indeed, it would not be ruled out that membrane damage may be related with higher level consequences, as increase in lipid peroxidation was observed in NP-exposed D. magna (Table 1). As for C. riparius, increased mortality by NP exposure was also observed (Table 2), and our previous study revealed 100 μg/L of NP exposure induced long-term development impairment (Lee and Choi 2006). The fact that DNA damage occurred concomitantly with decrease in organism and population level toxicity indicators (survival, development) suggests DNA alteration by this compound might provoke higher level consequences. Impairment of survival and development might be considered as a consequence of a serious progression of the sub-organisms level toxicities, such as increased DNA and lipid damage and decreased antioxidant activity in Chironomus. However, our data are not sufficient to provide a clear explanation for this phenomenon. If more sub-cellular parameters had been tested with longer exposure period, involvement of observed DNA damage in physiological pathway could probably be better evaluated and explained. On the other hand, effects on DNA integrity in BPA-exposed D. magna and C. riparius were not related to a degree of impairment of growth or survival of both organisms. Moreover, our previous studies revealed that BPA exposure did not seem to lead alteration on reproduction (Ha and Choi 2007) or on development (Lee and Choi 2007) for D. magna and C. riparius, respectively. False-positive results from genotoxic biomarker obtained in BPA-exposed D. magna and C. riparius make it more difficult to use DNA damage as an early warning biomarker.
The relationships between genotoxic biomarker responses and physiological/individual/population effects are complicated because of compensatory mechanisms that regulate physiological/individual fitness and population dynamics in a natural system. Some biomarkers do not appear to have a direct relationship to a higher level of biological organization. In this case, the use of biomarker will not give a reliable prediction of toxic effects upon organisms and is, therefore, only ever likely to indicate exposure to chemicals. In using such biomarkers of exposure, it is difficult to predict effects at the population level from biomarker changes measured in a sample of individuals (Depledge and Fossi 1994; Hyne and Maher 2003). However, as the mere presence of genotoxic compounds, which are potentially carcinogenic, is a major concern in human and ecosystem health, sensitive and rapid detection of genotoxic property in aquatic system itself is considered important, although it does not necessarily include alteration at a higher level of biological organization. Considering the potential of D. magna and C. riparius as bioindicator species, and the importance of genotoxic biomarkers in ecotoxicity monitoring, measurement of DNA damage in these species could provide useful information for freshwater monitoring and risk assessment.
References
Almeida EA, Bainy ACD, Loureiro APM, Medeiros MHG, Di Mascio P (2003) DNA and lipid damage in the brown mussel Perna perna from a contaminated site. Bull Environ Contam Toxicol 71:270–275. doi:10.1007/s00128-003-0160-8
Atienzar FA, Cheung VV, Jha AN, Depledge MH (2001) Fitness parameters and DNA effects are sensitive indicatiors of copper-induced toxicity in Daphnia magna. Toxicol Sci 59:241–250. doi:10.1093/toxsci/59.2.241
Chen G, White PA (2004) The mutagenic hazards of aquatic sediments: a review. Mutat Res 567:151–225. doi:10.1016/j.mrrev.2004.08.005
Choi J, Roche H, Caquet T (2000) Effects of physical (hypoxia, hyperoxia) and chemical (potassium dichromate, fenitrothion) stress on antioxidant enzyme activities in Chironomus riparius Mg. (Diptera, Chironomidae) larvae: potential biomarkers. Environ Toxicol Chem 19:495–500. doi:10.1897/1551-5028(2000)019<0495:EOPHHA>2.3.CO;2
Clement B, Devaux A, Perrodin Y, Danjean M, Ghidini-Fatus M (2004) Assessment of sediment ecotoxicity and genotoxicity in freshwater laboratory microcosms. Ecotoxicology 13:323–333. doi:10.1023/B:ECTX.0000033090.54897.94
Cotelle S, Ferard JF (1999) Comet assay in genetic ecotoxicology: a review. Environ Mol Mutagen 34:246–255. doi:10.1002/(SICI)1098-2280(1999)34:4<246::AID-EM4>3.0.CO;2-V
Cranston PS (1995) The chironomidae – the biology and ecology of non-bitting midges. Chapman & Hall, London
den Besten PJ (1998) Concepts for the implementation of biomarkers in environmental monitoring. Mar Environ Res 46:253–256. doi:10.1016/S0141-1136(97)00049-4
den Besten PJ, Tuk CW (2000) Relation between responses in the neutral red retention test and the comet assay and life history parameters of Daphnia magna. Mar Environ Res 50:513–516. doi:10.1016/S0141-1136(00)00129-X
Depledge MH (1998) The ecotoxicological significance of genotoxicity in marine invertebrates. Mutat Res 13:109–122. doi:10.1016/S0027-5107(97)00270-4
Depledge MH, Fossi MC (1994) The role of biomarkers in environmental assessment (2) Invertebrates. Ecotoxicology 3:161–172. doi:10.1007/BF00117081
Di Giulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of oxidative stress. Environ Toxicol Chem 8:1103–1123. doi:10.1897/1552-8618(1989)8[1103:BRIAAA]2.0.CO;2
EU (2002) European Union risk assessment report, EC: 284-325-5 and 246-672-0, Second priority list 10, European Union, Brussel
EU (2003) European Union risk assessment report, EC:201-245-8, Third priority list 37, European Union, Brussel
Giesy JR, Graney RL, Newsted JL, Rosiu CL, Benda A, Kreis RG Jr et al (1988) Comparison of three sediment bioassay methods using Detroit river sediments. Environ Toxicol Chem 7:483–498. doi:10.1897/1552-8618(1988)7[483:COTSBM]2.0.CO;2
Ha MH, Choi J (2007) Effects of environmental contaminants on hemoglobin gene expression in Daphnia magna : a potential biomarker for freshwater quality monitoring. Arch Environ Contam Toxicol. doi:10.1007/s00244-007-9079-0
Hyne RV, Maher WA (2003) Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotoxicol Environ Saf 54:366–374. doi:10.1016/S0147-6513(02)00119-7
Koppen G, Verschaeve L (1996) The alkaline comet test on plant cells: a new genotoxicity test for DNA strand breaks in Vicia faba root cells. Mutat Res 360:193–200
Lackner R (1998) Oxidative stress in fish by environmental pollutants. In: Braunbeck T, Hinton DE, Streit B (eds) Fish ecotoxicology. Birkhauser Verlag, Besel, pp 203–224
Lee SB, Choi J (2006) Multilevel evaluation of nonylphenol toxicity in fourth-instar larvae of Chironomus riparius (Diptera, Chironomidae). Environ Toxicol Chem 25:3006–3014. doi:10.1897/05-601R1.1
Lee SB, Choi J (2007) Effects of bisphenol A and ethynyl estradiol exposure on enzyme activities, growth and development in the fourth instar larvae of Chironomus riparius (Diptera, Chironomidae). Ecotoxicol Environ Saf 68:84–90. doi:10.1016/j.ecoenv.2006.07.003
Lee SW, Park K, Hong J, Choi J (2008) Ecotoxicological evaluation of octachlorostyrene in fourth instar larvae of Chironomus riparius (Diptera, Chironomidae). Environ Toxicol Chem 27(5):1118–1127. doi:10.1897/07-219.1
Livingstone DR (2001) Contaminant-stimulated reactive species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666. doi:10.1016/S0025-326X(01)00060-1
Livingstone DR, Förlin L, George S (1994) Molecular biomarkers and toxic consequences of impact by organic pollution in aquatic organisms. In: Sutcliffe DW (ed) Water quality and stress indicators in marine and freshwater systems: linking levels of organization. Freshwater Biological Association, Ambleside, pp 154–171
Livingstone DR, Nasci C, Solé M, Da Ros L, O’Hara SCM, Peters LD, Fossato V, Wootton AN, Goldfarb PS (1997) Apparent induction of a cytochrome P450 with immunochemical similarities to CYP1A in digestive gland of the common mussel (Mytilus galloprovincialis L) with exposure to 2,2′,3,4,4′,5′-hexachlorobiphenyl and Arochlor 1254. Aquat Toxicol 38:205–224. doi:10.1016/S0166-445X(96)00847-8
Mitchelmore CL, Chipman JK (1998) DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutat Res 399:135–147. doi:10.1016/S0027-5107(97)00252-2
Mitchelmore CL, Birmelin C, Livingstone DR, Chipman JK (1998) Detection of DNA strand breaks in isolated mussel (Mytilus edulis L.) digestive gland cells using the ‘comet assay’. Ecotoxicol Environ Saf 41:51–58. doi:10.1006/eesa.1998.1666
Monserrat JM, Geracitano LA, Pinho GLL, Vinagre TM, Faleiros M, Alciati JC, Bianchini A (2003) Determination of lipid peroxides in invertebrates tissues using the Fe(III) xylenol orange complex formation. Arch Environ Contam Toxicol 45:177–183. doi:10.1007/s00244-003-0073-x
Navarrete MH, Carrera P, Demiguel M, Delatorre C (1997) A fast comet assay variant for solid tissue cells: the assessment of DNA damage in higher plants. Mutat Res 389:271–277
Nehls S, Segner H (2005) Comet assay with the fish cell line rainbow trout gonad-2 for in vitro genotoxicity testing of xenobiotics and surface waters. Environ Toxicol Chem 24:2078–2087. doi:10.1897/04-301R.1
OECD (2004) OECD guidelines for testing of chemicals. Guideline 202 Daphnia sp., acute immobilisation test. Adopted:13 April 2004
Ohe T, Watanabe T, Wakabayashi K (2004) Mutagens in surface waters: a review. Mutat Res 567:109–149. doi:10.1016/j.mrrev.2004.08.003
Palmqvist A, Selck H, Rasmussen LJ, Forbes VE (2003) Biotransformation and genotoxicity of fluoranthene in the deposit-feeding polychaete Capitella sp. I. Environ Toxicol Chem 22:2977–2985. doi:10.1897/02-474
Park SY, Choi J (2007) Cytotoxicity, genotoxicity and ecotoxicity assay using human cell and environmental species for the screening of the risk from pollutant exposure. Environ Int 33(6):817–822. doi:10.1016/j.envint.2007.03.014
Rajaguru P, Suba S, Palanivel M, Kalaiselvi K (2003) Genotoxicity of a polluted river system measured using the alkaline Comet assay on fish and earthworm tissues. Environ Mol Mutagen 41:85–91. doi:10.1002/em.10134
Ralph S, Petras M (1997) Genotoxicity monitoring of small bodies of water using two species of Tadpoles and the alkaline single cell gel (Comet) assay. Environ Mol Mutagen 29:418–430. doi:10.1002/(SICI)1098-2280(1997)29:4<418::AID-EM11>3.0.CO;2-H
Schnurstein A, Braunbeck T (2001) Tail moment versus tail length-application of an in vitro version of the comet assay in biomonitoring for genotoxicity in native surface waters using primary hepatocytes and gill cells from zebrafish (Danio rerio). Ecotoxicol Environ Saf 49:187–196. doi:10.1006/eesa.2001.2050
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191. doi:10.1016/0014-4827(88)90265-0
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell/gel comet assay: guideline for in vitro and in vivo genetic testing. Environ Mol Mutage 35:206–221. doi:10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J
Acknowledgments
This work was supported by the Korean Ministry of Environment as “The Eco-technopia 21 Project”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, SY., Choi, J. Genotoxic Effects of Nonylphenol and Bisphenol A Exposure in Aquatic Biomonitoring Species: Freshwater Crustacean, Daphnia magna, and Aquatic Midge, Chironomus riparius . Bull Environ Contam Toxicol 83, 463–468 (2009). https://doi.org/10.1007/s00128-009-9745-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9745-1