Abstract
Persistent organic pollutants (POPs) are ubiquitous and coexisted in the aquatic environment. Individual and combined toxic effects of benzo[a]pyrene (BaP) and 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) on embryogenesis, and larval survival of the Pacific oyster were investigated. The EC50 values of BaP, BDE-47 and their mixture on embryogenesis were 18.4, 203.3 and 72.0 µg/L respectively, while the LC50 values for 96 h larval mortality were 26.8, 244.5 and 108.9 µg/L respectively. The Marking-Dawson additive toxicity indices were −0.02 and −0.19, indicating an additive effect with a trend to antagonism. In addition, DNA strand breaks were also observed in oyster embryos after exposure. Our study suggests that BaP and BDE-47 exposure can cause developmental abnormalities, DNA damage and larval mortality. Furthermore, the toxicity of the mixture is slightly lower than individual pollutant. These data will be helpful to predict the toxicity of organic pollutants, and provide criteria for marine water quality standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
BaP is a model compound that features on the list of 16 priority PAHs of the US Environmental Protection Agency (EPA). Due to its potent carcinogenic and mutagenic properties, BaP receives much concern and is often used in genotoxicity and carcinogenicity studies (Binelli et al. 2008). It has been documented that BaP can disrupt energy production and aromatic compound catabolic processes, and affect gonad development and immune response in marine bivalves (Tian et al. 2013; Deng et al. 2014; Jiang et al. 2016). In Bohai Sea (China), the average measured concentration of BaP in sediments is 12.3 ng/g (Li et al. 2015). BDE-47 is also a concerned contaminant and potential to be bioaccumulated in aquatic animals, such as invertebrates, fishes and birds (Elliott et al. 2005). The average measured concentration of BDE-47 in sediments of Bobai Sea is 81.0 ng/g dw (Pan et al. 2010). Available toxicological evidence shows that BDE-47 exposure can cause developmental retardation and reduced fecundity in marine copepod (Han et al. 2015). In fact, BaP and BDE-47 often coexist at high levels in the environment, and the combined toxic effects of these two chemicals can be additive, synergistic, antagonistic or no interactive. However, there is no literature about the co-exposure of BaP and BDE-47 on early life stages of invertebrate.
The Pacific oyster, Crassostrea gigas, has been proposed as a sentinel organism to assess the toxicities of many pollutants (His et al. 1999; Paredes et al. 2013). Previous studies have shown that the early life stages of the Pacific oyster were highly impacted by heavy metals, pesticides, herbicides and PAHs (Wessel et al. 2007; Akcha et al. 2012; Mai et al. 2012). However, the literature about individual and combined toxicities of BaP and BDE-47 on early life stages of the Pacific oyster is unreported. In this study, we investigated the embryotoxicity, genotoxicity and larval mortality of BaP and BDE-47 and their mixture on C. gigas.
Materials and Methods
The oysters were purchased from a local aquaculture farm in Yantai (Shandong, China). Mature male and female oysters were stripped to get the gonad. Spermatozoa and oocytes from ten individuals were sieved separately through a 50 and 100 µm meshes, respectively. Then the eggs were fertilized with sperms in a ratio of 1:10 in filtered seawater. Fertilization success was verified under microscope and the embryos were transferred to glass beakers for embryotoxicity and genotoxicity assays.
BaP (>98%) and BDE-47 (>98%) were purchased from AccuStandard, Inc (New Haven, CT, USA). Stock solutions (1000 mg/L) were prepared in dimethyl sulfoxide (DMSO, Sinopharm Chemical Reagent, China). For the exposure experiments, the stock solutions were diluted to reach the final tested concentrations and the maximal final concentration of DMSO in sea water was 320 μg/L. The maximal concentration of DMSO was found to be nontoxic to oyster embryos and larvae in our preliminary tests. Three DMSO solvent controls (320 μg/L) and three replicates per concentration were prepared. The actual concentrations of BaP and BDE-47 in the experimental solutions were quantified by GC-MS. The liquid–liquid extraction, identification, quantification and quality control of these pollutants were conducted according to protocols provided by USEPA (1997) and Wurl et al. (2006). Briefly, 20 mL of water sample was extracted three times with 20 mL of dichloromethane in a separating funnel. Then the extracts were concentrated by rotary evaporation and dried using a gentle stream of nitrogen, reconstituted with 0.1 mL of n-hexane and then analyzed on GC-MS (Agilent GC 6890N coupled with 5973N MSD). A HP-5MS (30 m × 0.25 mm i.d., 0.25 mm film thickness) capillary column was used for the determination of BaP and BDE-47. To determine BaP concentrations, samples were injected at 260 °C in splitless mode and the oven program was 50°C for 3 min, ramped at 2°C/min–200°C, 5°C/min–250°C for 2 min, and further ramped at 2°C/min–290°C and held for 1 min. To measure BDE-47 concentrations, samples were injected at 230°C in splitless mode and the oven program was 110°C for 1.5 min, ramped at 3°C/min–280°C and held for 5 min, and the ions m/z 79 and 81 were monitored for BDE-47. The recoveries for spiked samples were 68.3%–116.1% (average value 87.0%, n = 3) for BaP and 70%–122.3% (average value 97.3%, n = 3) for BDE-47, respectively. Because the objective compounds were not detected in procedural blanks, the method detection limit (MDL) was set to the (instrumental detection limit) IDL. The MDLs were 140–200 ng/L for BaP and 80–150 ng/L for BDE47 respectively. The nominal and measured concentrations of pollutants in this study were summarized in Table 1.
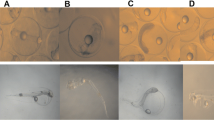
For embryotoxicity assay, approximately 500 embryos were incubated in beakers containing 200 mL of filtered seawater for 24 h (at 24°C in the dark), which enabled the embryos to develop to D-shaped larvae. There were three replicates for each exposure group and control. After the exposure, the larvae of each beaker were fixed using 8% formalin (0.5 mL/beaker), and about three hundred larvae of each beaker were observed under a microscope (Olympus, BX51, Japan) to determinate the percentage of abnormal D-shaped larvae according to the criteria described by His et al. (1997) (Fig. 1). The median effective concentration (EC50) defined as the pollutant concentration that resulted in a 50% reduction in normal D-shaped larvae number. The calculation of EC50 was normalized to the mean percentage of larval abnormality in the control group using Abbot’s formula, P = (Pe − Pc/100 − Pc) × 100, where Pc and Pe are control and experimental percentage response, respectively. The EC50s and the lowest observed effective concentrations (LOECs) were calculated by the probit method (Newman 1995) with SPSS 16.0 statistical software.
For genotoxicity assay, embryos were incubated in 250 mL beakers at 24°C for 16 h in the dark. This incubation time enables the embryos to reach unshelled larvae that can be enzymatically digested for comet assay. Three replicates were performed per treatment, and each replicate contained about a total of 20 × 104 larvae. Cell isolation was performed by the method described by Wessel et al. (2007). The comet assay was performed on isolated cells following the method proposed by Akcha et al. (2003), and the DNA damage was expressed as the percentage of total DNA that has migrated from the head (Tail DNA%).
The D-shaped larvae were obtained and re-suspended in 250 mL glass beakers (approximately 2 × 103 larvae/beaker), each containing 200 mL of different concentrations of pollutant solution (Table 1). There were three replicates for the control and exposure groups. The D-shaped larvae were fed with Isochrysis spp. at a concentration of 1–10 × 104 cells/mL three times per day, and the seawater was fully changed daily. The larval mortality was assessed under a microscope after 96 h exposure. The LC50 was defined as the pollutant concentration that resulted in half larval mortality compared to the control group.
To identify the type of interaction in binary mixtures of BaP and BDE-47, the additive toxicity index (ATI) of Marking and Dawson (1975) and its 95% CI were calculated. The effective contributions of two chemical (A and B) in a mixture are represented by the formula: S = A m /A i + B m /B i , where A and B are chemicals, m and i are the toxicities (EC50’s/LC50’s) of the individual chemicals and the mixtures, respectively. S is the sum of the biological activity, and additive effects are demonstrated when S = 1. Additive, synergism and antagonism effects are indicated by zero, positive, and negative values of this index, respectively. The toxicity unit (TU) for combined pollutants was calculated using the formula, TU = concentration/EC50 (or LC50).
Regression linear analysis was used to assess relationships between DNA damage and the percentage of abnormal D-shape larvae. The percentage data were transformed (arcsine of the square root) before ANOVA, and presented in figures as non-transformed percentages. Homogeneity of variance (Levene’s test) was checked and the data was analyzed by ANOVA using SPSS 16.0. Tukey’s test was used to compare the results between the control group and treated groups. p value <0.05 was considered statistically significant.
Results and Discussion
The determined concentrations of BaP and BDE-47 were 19.2%–70.7% and 10.2%–45.8% of the nominal concentrations, respectively (Table 1), which were much lower than the corresponding nominal concentrations. This might be due to the fact that the partitioning of BaP and BDE-47 to biota decreased their concentrations in water considering their high octanol–water partition coefficients, which was about 5.4 for BaP (Dabestani and Ivanov 1999) and 6.81 for BDE-47 (Braekevelt et al. 2003) respectively. In addition, the adsorption of pollutant onto the surfaces of both the organisms and exposure tank will also contribute towards the reduction of pollutant levels measured in seawater (Hannam et al. 2010). Significant differences (p < 0.05) were observed between the nominal and measured concentrations. Therefore, measured concentrations were used for the presentation and calculation of toxicity parameters. In addition, both the determined concentrations of Bap and BDE-47 in seawater of control group were below the detection limit.
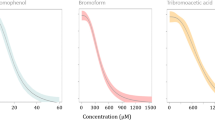
The toxic effect of BaP on embryogenesis is illustrated in Fig. 2a. BaP could significantly affect the embryogenesis of the oyster at a concentration of 13.8 µg/L. The level of DNA damage was also significantly increased when the BaP exposure concentration increased. For BDE-47, a significant increase of abnormal D-shaped larvae was observed at a concentration of 72.5 µg/L (Fig. 2c). The percentage of tail DNA increased significantly with the increase of concentration of BDE-47. In addition, a strong positive correlation was observed between the DNA damage level and the percentage of abnormal D-shaped larvae after BaP (Fig. 2b) and BDE-47 (Fig. 2d) exposure. The EC50 and LOEC values for BaP and BDE-47 were shown in Table 2.
a, c Percentages (mean ± SD, n = 3) of abnormal D-shaped larvae and tail DNA following oyster embryos exposed to different concentrations of BaP and BDE-47. Asterisks indicate significant differences between exposed and control treatment (*p < 0.05, **p < 0.01, ***p < 0.001). b, d Relationship between tail DNA and D-shaped larvae abnormalities in oyster (p = 0.0025)
Our results also suggested that the embryo development of the Pacific oyster was significantly affected by BaP exposure. It has been shown that the toxic effects of PAHs on aquatic organisms include carcinogenesis, oxidative stress, impairment of immune responses, endocrine effects, and altered embryo development (Hylland 2006). Lyons et al. (2002) and Wessel et al. (2007) have reported that BaP exposure could significantly increase the embryo abnormalities of C. gigas at a concentration of 2.5 and 0.05 µg/L, which were lower than our results. By now, only a few studies have focused on the toxicity of BDE-47 on embryonic stage of aquatic organisms (Chan and Chan 2012), and there has been no report assessing embryotoxicity of BDE-47 on marine invertebrates. Our study showed that BDE-47 had significant toxicity on early life stages of marine oysters, and its toxicity was less than BaP and Aroclor1254 (Wang et al. 2012).
BaP and BDE-47 also induced DNA damage in oyster embryos after 16 h exposure. The genotoxicity and carcinogenicity of BaP have been described in marine organisms (van der Oost et al. 2003), and an increase of DNA strand breaks level in oyster embryos was also reported by Wessel et al. (2007). In addition, it has been revealed that BDE-47 could induce ROS production and cause DNA damages in marine mussel (Jiang et al. 2017). Significant positive correlation between embryotoxicity and genotoxicity was demonstrated after individual BaP and BDE-47 exposure. Similarly, high correlation between embryotoxicity and genotoxicity was also reported in oyster embryos after exposure to BaP, 17-ethinylestradiol and endosulfan (Wessel et al. 2007) and pesticides (Mai et al. 2012).
In this study, 13.8 µg/L of BaP exposure induced a significant increase in larval mortality (Fig. 4a), while 72.5 µg/L of BDE-47 exposure significantly elevated the larval mortality (Fig. 4b). The LC50 and LOEC values for BaP and BDE-47 were shown in Table 2. Our results suggested that the embryos were more sensitive to these pollutants than larvae. The findings were consistent with those in other bivalves such as C. gigas (Beiras and Albentosa 2004) and Meretrix meretrix (Wang et al. 2012).
The percentage of abnormal D-shaped larvae and DNA damage level were significantly increased at the dose of 0.49 TU (Fig. 3a). There was a positive correlation between genotoxic and embryotoxic effects in oyster embryos after the mixture exposure, but it was not as strong as the single exposure to BaP and BDE-47 (Fig. 3b). The mortality of the larvae increased from 10.3% at 0.16 TU to 65.5% at 2.54 TU (Fig. 4c). The LC50 and LOEC values for embryotoxicity and larval mortality were shown in Table 2. The additive toxicity index (ATI) of these pollutants combination for embryotoxicity and larval mortality are −0.02 (−0.42, 0.72) and −0.19 (−0.95, 0.31), respectively (Table 3). The results presented a slight but significant negative interaction (antagonism) between these two pollutants.
a Percentages (mean ± SD, n = 3) of abnormal D-shaped larvae and tail DNA following oyster embryos exposed to different concentrations of the mixture of two pops (BaP + BDE-47). The X axis shows the toxicity units (TU, TU = concentration/EC50). Asterisks indicate significant differences between exposed and control treatment (*p < 0.05, **p < 0.01, ***p < 0.001). b Relationship between tail DNA and D-shaped larvae abnormalities in oyster (p = 0.0168)
The marine environment is subjected to a mixture of pollutants, which can interact with each other to produce adverse effects in marine organisms. In present study, the combined toxicity obtained by toxic bioassay was slightly lower than the toxicity predicted by the additive model, which would be considered as antagonistic according to the Marking-Dawson method. Our results are consistent with those of Zhao et al. (2013) who demonstrated that BDE-47 could significantly reduce BaP-induced toxic effects in Japanese medaka Oryzias latipes.
In conclusion, the results indicated that oyster embryos were highly susceptible to BaP and BDE-47 exposure. Moreover, combined BaP and BDE-47 exposure showed an antagonism effect on early life stages of C. gigas. It would be necessary to test the combined toxicity of organic pollutants on other marine invertebrates.
References
Akcha F, Hubert FV, Pfhol-Leszkowicz A (2003) Potential value of the comet assay and DNA adduct measurement in dab (Limanda limanda) for assessment of in situ exposure to genotoxic compounds. Mutation Res-Gen Tox En 534:21–32
Akcha F, Spagnol C, Rouxel J (2012) Genotoxicity of diuron and glyphosate in oyster spermatozoa and embryos. Aquat Toxicol 106–107:104–113
Beiras R, Albentosa M (2004) Inhibition of embryo development of the commercial bivalves Ruditapes decussatus and Mytilus galloprovincialis by trace metals; implications for the implementation of seawater quality criteria. Aquaculture 230:205–213
Binelli A, Riva C, Cogni D, Provini A (2008) Assessment of the genotoxic potential of benzo(a) pyrene and pp’-dichlorodiphenyldichloroethylene in Zebra mussel (Dreissena polymorpha). Mutat Res 649:135–145
Braekevelt E, Tittlemier SA, Tomy GT (2003) Direct measurement of octanol–water partition coefficients of some environmentally relevant brominated diphenyl ether congeners. Chemosphere 51:563–567
Chan WK, Chan KM (2012) Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo–larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat Toxicol 108:106–111
Dabestani RT, Ivanov IN (1999) A compilation of physical, spectroscopic, a photophysical properties of polycyclic aromatic hydrocarbons. Photochem Photobiol 70:10–34
Deng X, Pan L, Miao J, Cai Y, Hu F (2014) Digital gene expression analysis of reproductive toxicity of benzo[a]pyrene in male scallop Chlamys farreri. Ecotox Environ Safe 110:190–196
Elliott JE, Wilson LK, Wakeford B (2005) Polybrominated diphenyl ether trends in eggs of marine and freshwater birds from British Columbia, Canada, 1979–2002. Environ Sci Technol 39:5584–5590
Han J, Won EJ, Lee MC, Seo JS, Lee SJ, Lee JS (2015) Developmental retardation, reduced fecundity, and modulated expression of the defensome in the intertidal copepod Tigriopus japonicus exposed to BDE-47 and PFOS. Aquat Toxicol 165:136–143
Hannam ML, Bamber SD, Galloway TS, John Moody A, Jones MB (2010) Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 78(7):779–784
His E, Seaman MNL, Beiras R (1997) A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Res 31:351–355
His E, Beiras R, Seaman MN (1999) The assessment of marine pollution-bioassays with bivalve embryos and larvae. Adv Mar Biol 37:1–178
Hylland K (2006) Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J Toxicol Environ Health A 69:109–123
Jiang X, Qiu L, Zhao H, Song Q, Zhou H, Han Q (2016) Transcriptomic responses of Perna viridis embryo to benzo(a)pyrene exposure elucidated by RNA sequencing. Chemosphere 163:125–132
Jiang Y, Tang X, Sun T, Wang Y (2017) BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: an explanation based on ROS-mediated pathway. Aquat Toxicol 182:58–66
Li J, Dong H, Zhang D, Han B, Zhu C, Liu S (2015) Sources and ecological risk assessment of PAHs in surface sediments from Bohai Sea and northern part of the Yellow Sea, China. Mar Pollut Bull 96:485–490
Lyons BP, Pascoe CK, Mcfadzen IR (2002) Phototoxicity of pyrene and benzo[a]pyrene to embryo-larval stages of the pacific oyster Crassostrea gigas. Mar Environ Res 54:627–631
Mai H, Cachot J, Brune J, Geffard O, Belles A, Budzinski H, Morin B (2012) Embryotoxic and genotoxic effects of heavy metals and pesticides on early life stages of Pacific oyster (Crassostrea gigas). Mar Pollut Bull 64:2663–2670
Marking LL, Dawson VK (1975) Method for assessment of toxicity or efficacy of mixtures of chemicals. In: Investigations in fish control, no. 67. U.S. Department of Interior, U.S. Fish and Wildlife Service, Washington, DC
Newman MC (1995) Quantitative methods in aquatic ecotoxicology. Lewis Publishers, CRC Press, Boca Raton, p 426
Pan X, Tang J, Li J, Guo Z, Zhang G (2010) Levels and distributions of PBDEs and PCBs in sediments of the Bohai Sea, North China. J Environ Monit 12:1234–1241
Paredes E, Bellas J, Adams SL (2013) Comparative cryopreservation study of trochophore larvae from two species of bivalves: Pacific oyster (Crassostrea gigas) and Blue mussel (Mytilus galloprovincialis). Cryobiology 67:274–279
Tian S, Pan L, Sun X (2013) An investigation of endocrine disrupting effects and toxic mechanisms modulated by benzo[a]pyrene in female scallop Chlamys farreri. Aquat Toxicol 144–145:162–171
USEPA (1997) Simultaneous analysis of monocyclic aromatic hydrocarbons in water by microextraction. EPRI Report TR-Research Project
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wang Q, Yang H, Liu B, Wang X (2012) Toxic effects of benzo[a]pyrene (bap) and aroclor1254 on embryogenesis, larval growth, survival and metamorphosis of the bivalve Meretrix meretrix. Ecotoxicology 21:1617–1624
Wessel N, Rousseau S, Caisey X, Quiniou F, Akcha F (2007) Investigating the relationship between embryotoxic and genotoxic effects of benzo[a]pyrene, 17alpha-ethinylestradiol and endosulfan on Crassostrea gigas embryos. Aquat Toxicol 85:133–142
Wurl O, Lam PKS, Obbard JP (2006) Occurrence and distribution of polybrominated diphenyl ethers (PBDEs) in the dissolved and suspended phases of the sea-surface microlayer and seawater in Hong Kong, China. Chemosphere 65:1660–1666
Zhao Y, Luo K, Fan Z, Huang C, Hu J (2013) Modulation of benzo[a]pyrene-induced toxic effects in Japanese medaka (Oryzias latipes) by 2,2′,4,4′-tetrabromodiphenyl ether. Environ Sci Technol 47:13068–13076
Acknowledgements
This research was supported by grants from the Key Research Program of the Chinese Academy of Sciences (Grant No. KZZD-EW-14), the National Natural Science Foundation of China (No. 41576122), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA11020702), the Youth Innovation Promotion Association of CAS (2016196).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xie, J., Yang, D., Sun, X. et al. Individual and Combined Toxicities of Benzo[a]pyrene and 2,2′,4,4′-Tetrabromodiphenyl Ether on Early Life Stages of the Pacific Oyster, Crassostrea gigas . Bull Environ Contam Toxicol 99, 582–588 (2017). https://doi.org/10.1007/s00128-017-2164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2164-9