Abstract
Spatial variations in estuarine intertidal sediment have been often related to such environmental variables as salinity, sediment types, heavy metals and base cations. However, there have been few attempts to investigate the difference condition between high and low tides relationships and to predict their likely responses in an estuarine environment. This paper investigates the linkages between environmental variables and tides of estuarine intertidal sediment in order to provide a basis for describing the effect of tides in the Mengkabong lagoon, Sabah. Multivariate statistical technique, principal components analysis (PCA) was employed to better interpret information about the sediment and its controlling factors in the intertidal zone. The calculation of Geoaccumulation Index (I geo) suggests the Mengkabong mangrove sediments are having background concentrations for Al, Cu, Fe, and Zn and unpolluted for Pb. Extra efforts should therefore pay attention to understand the mechanisms and quantification of different pathways of exchange within and between intertidal zones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mengkabong lagoon formerly earmarked as the Mengkabong Forest Reserve. The Mengkabong mangrove forest in Tuaran District has experienced a 15% decrease from 1991 to 2000. In 1991 the mangroves covered 12.6 km2 while in 2000 it was 10.7 km2. Most of the mangroves have been lost due to the spread of rural development such as housing, aquaculture projects and surrounded by an industrial zone, Kota Kinabalu Industrial Park (KKIP) (Environmental Indicator Report 2003). Therefore, it was decided conduct a study of Mengkabong lagoon mangrove sediment and its pollution status.
The purpose of this study was: (1) to identify the controlling factors by using principal components analysis (PCA) at high and low tides and (2) to gauge the degree of anthropogenic influence on heavy metals concentration in mangrove sediment using Geoaccumulation Index (I geo).
Materials and Methods
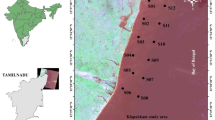
This study was conducted in Mengkabong mangrove forest, West Coast of Sabah which is 40 km away from Kota Kinabalu. The total of study area spreads over from latitude 06°06′N to 06°11′N and longitude 116°08′E to 116°13′E (Fig. 1). The Mengkabong mangrove forest consists of two shallow spurs, with the southern spur forming the administrative boundary between Tuaran and Kota Kinabalu districts. This spur ends in Salut Bay which is entirely surrounded by an industrial zone, Kota Kinabalu Industrial Park (KKIP). The southern spur of the estuary has been significantly degraded already and there is little left to protect (Environmental Impact Assessment 1992; Town and Regional Planning Department of Sabah 2003). Mangrove surface sediments were sampled randomly and taken in triplicates with an auger at 33 stations from March 2006 to November 2006 (Fig. 1) at low and high tides. The sampling was done based to the accessibility to the mangrove forest. Mangrove surface sediments were chosen for this study as this layer controls the exchange of metals between sediments and water (El Nemr et al. 2006). The surface sediments were kept cool in an icebox during transportation to the laboratory. The physicochemical measurements of the surface sediment were made as soon as possible in the lab. The pH, electrical conductivity and salinity electrodes were calibrated before the measurements were taken.
For other analyses, the air-dried surface sediments were digested using aqua regia. Approximately 2 g of each sample was digested with 15 mL of aqua-regia (1:3 HCl:HNO3) in a Teflon bomb for 2 h at 120°C. After cooling, the digested samples were filtered and kept in plastic bottles before the analysis. Organic matter was determined using Loss on Ignition (LOI) method (Radojevic and Bashkin 1999; Heiri et al. 2001) while granulometric analysis was conducted using pipette method elaborated by Radojevic and Bashkin (1999). For base cations (Na, K, Ca and Mg), measurement of exchangeable cations using ammonium acetate was used. Heavy metals and base cations were analyzed using AAS (APHA 1995) with air/acetylene (Cu, Fe, Pb, Zn, Na, K, Ca and Mg) and nitrous oxide-acetylene (Al) at specific wavelengths (Atomic Absorption Spectrometer Perkin Elmer 4100).
Results and Discussion
PCA was applied to discover and interpret relationships between variables at high and low tides. The results showed a different trend at high tide and at low tide. Tables 1 and 2 display the factor loadings with a Varimax rotation as well as the eigenvalues, percentile of variance and cumulative percentage at high and low tides. In reference to the eigenvalues, six factors at high tide and seven factors at low tide were extracted as they have eigenvalues greater than 1.
The Geoaccumulation Index (I geo) introduced by Muller (1979) was used to assess metal pollution in sediments (Eq. 1). I geo is expressed as follows: where Cn = measured concentration of heavy metal in the mangrove sediment, Bn =geochemical background value in average shale (Turekian and Wedepohl 1961) of element n, 1.5 is the background matrix correction in factor due to lithogenic effects. The calculated values for the Mengkabong mangrove sediment are given in Table 3 and remain in either class 0 or class 1, which indicates that the investigated mangrove sediments in Mengkabong mangrove sediment are unpolluted.
At high tide (Table 1), factor one in PCA results contain salinity, electrical conductivity and clay fraction shows that the contributions of seawater during high tide in Mengkabong. Seawater contains 3.5% of salinity of which 90% is fully ionized ions. The fully ionized ions and high salinity at high tide explained the high load of salinity and electrical conductivity at high tide (Church 1989). While factor three consists of Cu and K with total variance of 11. According to Preda and Cox (2000), tidal flooding can bring additional ions such as K, Mg and Na into system. It enables ion exchange to occur, such as between K and Cu. Grande et al. (2003) observed a negative correlation with marine indicators such as K. The new condition induced by tidal clash causes precipitation of metals such as Cu. These associations are mainly related to anthropogenic inputs and reflect the complexing nature of clay.
Pb most probably arises from indirect sources for instance atmospheric deposition (Liu et al. 2003). Based on PCA (factor four, factor five and factor six) showed the relationship between Al, Ca, Fe and Zn. pH value in estuarine sediments is one of the important factor that regulates the concentration of dissolved metals in water and sediment (Hsue and Chen 2000; Grande et al. 2003). Seawater plays an important role in buffering the pH change (Hsue and Chen 2000). The process of tidal clash occurs in the Mengkabong mangrove forest. The influx of seawater from the high tide resulted in major inputs of selected cations which were then adsorbed by the sediment (clay, silt and sand). Rubio et al. (2000) explained that the association between Mg and sand is strongly controlled by biogenic carbonates and plays an important role as a dilutant material of the heavy metals in the samples. Biogenic carbonates are the dominant source of Ca, an abundant component in shallow marine sands (Morad 1998). Adding up, Zhou et al. (2004) states that Ca is an important components of marine biota and plays vital a role in the marine biogeochemical cycle.
At low tide (Table 2), salinity and electrical conductivity have low loadings compared at high tide demonstrates the lower contributions of seawater at low tide compared to high tide. Table 2 shows the factor one accounted for 24% of total variance and is mainly characterized by high levels of silt, Al, Zn and EC. Besides, there is also an association of Zn of factor one in PCA with Fe (factor six in PCA), K and salinity (factor four in PCA). Heavy metals occur naturally in silt and clay-bearing minerals of terrestrial and marine geological deposits. The natural occurrence of heavy metals complicates the assessment of potentially contaminated estuarine sediments. The measureable concentrations of metals do not automatically infer anthropogenic enrichment in the surface sediment; furthermore heavy metal enrichment assessment must be conducted in detail (Zhou et al. 2004). While association of K is explained as a function of ionic strength, a monovalent cation with low replacing power compared with divalent cations such as Fe, Zn etc (Hussien and Rabenhurst 2001). These associations are consistent with PCA results with the loading factor of factor two, factor three, factor five and factor seven showed associations between organic matter and pH, clay fraction, sand, Cu, Pb, Ca and Mg. In coastal environments such as in mangroves, the associations between organic matter, granulometric fractions, heavy metals and base cations are become as functions of ionic strength of sediment solution and surface cation complexation (Hussien and Rabenhurst 2001). The concept of cation exchange capacity at low tide implies that ions will be exchanged between wetlands, colloid surface and the surrounding water. Sediment organic matter has higher ion exchange capacity than sediment colloids and plays an important role in cation exchange capacity (Matagi et al. 1998). The replacing power of the cation exchange complex depends on its valence, ionic strength, and its diameter in hydrated form and its concentration in water. This clarifies the loadings of marine indicator divalent cations (Mg, Ca) together with organic matter and Pb2+ due to the ionic strength (Hussien and Rabenhurst 2001).
The I geo index proposed by Muller (1979) showed that all the heavy metals are in Class 0 and Class 1 (Table 3) at high and low tide. Karbassi et al. (2006) elobrated that I geo values can be used effectively and more meaningful in explaining the sediment quality. This suggests that the mangrove sediment of Mengkabong is unpolluted. The input of metals into sediment that are located seawards to be low in the total concentration of most of the elements and this could be due to the mixing of enriched particulate material with relatively clean marine sediments (Soto-Jimenez and Paez-Osuna 2001).
References
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. Washington, USA
Church AH (1989) The ionic of the sea. The Phytologist 68:239–247
El Nemr A, Khaled A, Sikaily AE (2006) Distribution and statistical analysis of leachable and total heavy metals in the sediments of the Suez Gulf. Environ Monit Assess 118:89–112. doi:10.1007/s10661-006-0985-9
Environmental Impact Assessment (1992) Proposed mangrove paradise resort complex on LA 91040377 Tuaran, Sabah. Perunding Sekitar, Sabah
Environmental Indicator Report (2003) The Environment Protection Department (EPD) Sabah: Syarikat Bumi Yakin, Sabah
Grande JA, Borrego J, Morales JA, Torre ML (2003) A description of how metal pollution occurs in the Tinto-Odiel Rias (Huelva-Spain) through the application of cluster analysis. Mar Pollut Bull 46:475–480. doi:10.1016/S0025-326X(02)00452-6
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110. doi:10.1023/A:1008119611481
Hsue ZY, Chen ZS (2000) Monitoring the changes of redox potential, ph and electrical conductivity of the mangrove soils in Northern Taiwan. Proc Nat Sci Counc 24:143–150
Hussein AH, Rabenhorst MC (2001) Tidal inundation of transgressive coastal areas: pedogenesis of salinization and alkalinization. Soil Sci Soc Am J 65:536–544
Karbassi AR, Bayati I, Moatta F (2006) Origin and chemical partitioning of heavy metals in riverbed sediments. Int J Environ Sci Technol 3:35–42
Liu WX, Li XD, Shen ZG, Wang DC, Wai OWH, Li YS (2003) Multivariate statistical study of heavy metal enrichment in sediments of the Pearl river estuary. Environ Pollut 121:377–388. doi:10.1016/S0269-7491(02)00234-8
Matagi SV, Swai D, Mugabe R (1998) A review of heavy metals mechanism in wetlands. African J Trop Hydrobiol Fish 8:23–35
Morad S (1998) Carbonate cementation in sandstones: distribution patterns and geochemical evolution. Blackwell Science Limited, London
Muller G (1979) Schwermetalle in den sediments des Rheins-Veranderungen seitt 1971. Umschan 79:778–783
Preda M, Cox ME (2000) Sediment-water interaction, acidity and other water quality parameters in a subtropical setting, Pimpama river, Southeast Queensland. Environ Geo 39:319–329. doi:10.1007/s002540050011
Radojevic M, Bashkin VN (1999) Practical environmental analysis. Royal Society of Chemistry, Cambridge
Rubio B, Nombelia MA, Vilas F (2000) Geochemistry of major and trace elements in ediments of the Ria De Vigo (NW Spain): an assessment of metal pollution. Marine Pollut Bull 40:968–980. doi:10.1016/S0025-326X(00)00039-4
Soto-Jimenez MF, Paez-Osuna F (2001) Distribution and normalization of heavy metal concentrations in mangrove and lagoon sediments from Mazatlan Harbor (SE Gulf California). Estuar Coast Shelf Sci 53:259–274. doi:10.1006/ecss.2000.0814
Town and Regional Planning Department (TRPD) (2003). Project Sabah, 2003. Environmental Local Planning (ELP), Kota Kinabalu, Sabah
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192. doi:10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2
Zhou H, Peng X, Pan J (2004) Distribution, source and enrichment of some chemical elements in sediments of the Pearl river estuary, China. Conti Shelf Res 24:1857–1875. doi:10.1016/j.csr.2004.06.012
Acknowledgements
We would like to thank Mr. Asram and Mr. Neldin Jeoffrey for assisting with the field sampling. The author gratefully acknowledges her Universiti Malaysia Sabah Scholarship (YTL Foundation).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Praveena, S.M., Ahmed, A., Radojevic, M. et al. Multivariate and Geoaccumulation Index Evaluation in Mangrove Surface Sediment of Mengkabong Lagoon, Sabah. Bull Environ Contam Toxicol 81, 52–56 (2008). https://doi.org/10.1007/s00128-008-9460-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9460-3