Abstract
Heavy metals, which are among the important pollutants that threaten ecosystems, have been a particularly intriguing subject of accumulation studies. The present study aimed to reveal the water and sediment quality, pollution status, and their usability for living organisms in 10 stations for the first time in İnaltı cave, which has two underground ponds. Concentrations of 9 heavy metals (Cu, Pb, Zn, Ni, Mn, Fe, Cd, Cr, Al) and 1 metalloid (As) were determined in samples taken. These results were compared to the limit values in Sediment Quality Guides (SQGs) and analyzed further using different sediment evaluation methods. SQG values revealed that the amounts of Cd and Ni are of concern. Examining the concentrations of metals in the water, the ranking by concentration was found to be Al > Cr > Pb > Cu > As > Mn and the elements were considered not to pose any environmental risk. The enrichment of detected Cd metal in the sediment is remarkable. In addition, ANOVA, Pearson’s correlation analysis, principal component analysis (PCA), and hierarchical clustering analysis were carried out in order to make the obtained data easier to understand and interpret. While designing the most appropriate action plans for water management, more clear and understandable information can be obtained by using these methods and interpreting the raw data. In the cave, individuals belonging to the Niphargus genus, a member of the Malacostraca class, Niphargidae Family, were identified in the sediment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the environmental pollution directly threatening the ecosystem quality has become an important subject for researchers. Many ecosystems in the world have degraded due to the effects of environmental pollution. Because of the effects of pollution, some of the species in those ecosystems either went extinct or are in danger of extinction. Damage to or extinction of any species in an ecosystem affects other organisms in the life cycle. Caves, which are among the non-renewable natural resources, are one of the important ecosystems affected by pollutants (Du Preez et al., 2016). Geological formations in our country provide suitable conditions for cave ecosystems, and there are approximately 40,000 caves in our country (Nazik et al., 1996). Due to their nature, their low temperature, high humidity, nutrient scarcity, light absence, and season-independent stable ecological conditions make those caves unique ecosystems (Gèze, 1965; Mejía-Ortíz et al., 2021).
Acting as an ecological filter between surface and underground, caves are categorized by making use of continuity of darkness, scarcity of nutrient sources, and shortness of seasonal fluctuations (Culver & Pipan, 2009). Rather than considering these ecosystems as empty spaces below the ground, it is very important to know that they are habitats, where many organisms live (Rachmawati et al., 2021). These ecosystems, which are seen as the final border of the world by some people, constitute a part of the underground ecosystem. Moreover, they might host single and endemic species (Culver & Pipan, 2009).
Since heavy metals, which are one of the pollutants that are among the main problems of the current era, can accumulate through the food chain without degradation, it is very important to determine their concentrations, follow them, and reveal the accumulation rates for the health of ecosystems (Ferrante et al., 2018). Although some metals (Cu, Fe, Zn, Co, etc.) are necessary for organisms, they would have a toxic effect on organisms when they exceed a specific threshold level (Mansour et al., 2016). Caves became one of the ecosystems experiencing the pressure of heavy metal pollution in recent years. For this reason, many researchers investigated the current metal accumulation at the levels of water, sediment, and organic tissue (Cuculić et al., 2011; Ferrante et al., 2018; Printarakul & Meeinkuirt, 2022).
Amphipoda ordo is represented by approximately 6000 species worldwide. It is divided into 4 sub-ordos as Caprellidea, Hyperiidea, Ingolfiellidea, and Gammaridea. More than 80% of Amphipod species are included in the Gammaridea sub-ordo. The dominant body structure observed in Amphipoda ordo is the one compressed by the sides (Gammaridea). Besides that, they are characterized using the differentiation that they have three pairs of uropods and the first two pairs play role in nutrition. These species live in almost all habitats. The ones living in rapidly flowing waters live in the sand and under the small stones on the ground (Gledhill et al., 1993). Those living on the weeds in seas and freshwaters are generally herbivores, whereas those living in mud and sand feed on bacteria living on surface particles. Others are detritivores or scavengers feeding on plant-animal carcasses (Aydemir, 2007).
Analyzing metal contamination in sediment is crucial for predicting the status of contaminated aquatic environments. Different sediment quality standards have been developed for ecological risk assessments (Ergen, 2020; U.S.E.P.A., 1996). Sediment Quality Guides (SQGs), which were preferred in this study, have also been preferred by many researchers due to their short duration and low cost (Kükrer et al., 2015; Tunca et al., 2018; Yong et al., 2020; Fikirdeşici-Ergen et al., 2021). SQGs are a set of mathematical formulations developed and used to assess the risks posed by heavy metals (Bat & Şahin, 2018; Birch, 2018). It provides important outputs in revealing the potential of heavy metals to cause harm (Kwok et al., 2014). Determining and interpreting the amount of heavy metals in sediment is very important for the health of ecosystem and SQGs have been developed in order to meet exactly this need (Yong et al., 2020). This method incorporating a series of mathematical formulations is used in analyzing the risks posed by heavy metals and foreseeing the potential risks (Birch, 2018).
Among the multivariate statistical techniques (MSTs) allowing the interpretation of very complex water and sediment quality datasets, cluster analysis (CA), principal component analysis (PCA), and factor analysis (FA) are widely used in analyzing the water and sediment quality. These methods are also used in determining the water and sediment pollution sources, revealing the temporal and spatial changes, and interpreting them (Aydın-Uncumusaoğlu, 2018; Mutlu, 2019; Aydın-Uncumusaoglu & Mutlu, 2021).
The present study is the first biological study carried out on this cave system, which has a high tourism potential, in Ayancık district; new results and a new species were introduced to the literature from the İnaltı Cave and the water and sediment quality of the case was reported by using the statistical methods.
Methods and materials
Study area
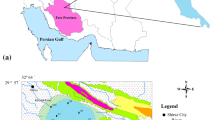
İnaltı Cave was registered as a Natural Park on 08.03.2020, and it has a surface area of 22.8 ha; it is located in İnaltı Village in Ayancık district of Sinop province (Northern Türkiye) (Fig. 1). Having the coordinates of 41°43′50.60″N, 34°34′7.63″E, the cave has an altitude of 1070 m. The case is not rich in speleothems, and the main point of interest is its height, which exceeds beyond 20 m at some locations, and the wide and high shelf area in the entrance. The average length of the cave is 658 m. Of 358 m part of the cave, only 125 m part was found to be suitable for tourism. The case courses in S-form in the east–west direction. The entrance opens to a smooth gallery that has a length of 125 m, ceiling height of 15 m, and width of 10–12 m. This gallery is the driest and widest part of the cave. In the cave, there are stalactites, stalagmites, pillars, cover and wall dripstones, and dripstone pools. On the other hand, there also are cauliflower- and popcorn-shaped dripstones in the near-ground parts of the cave and right above the waterline. High-grade limestone, which is dissoluble, plays an important role in the formation of caves and pits in this region (S.K.T.M., 2022).
Sample collection and analysis methodology
Since the cave entrance and sampling process require specific experience and technical equipment, the field study was carried out in cooperation with MAD-Association for Cave Research. Land study was carried out on 8 September 2019, before the rainy season began and the in-cave water level increased.
Water temperature (T, °C), pH, dissolved oxygen (DO mg/L), and electrical conductivity (EC) values were measured using a Hanna HI 9829 field-type multiparameter device.
Within the scope of this study, water and sediment samples were taken from 10 points (Fig. 2). Samplings were conducted using plastic materials, and only the surface water and sediment were used. Samples were kept in polyethylene containers until delivered to the laboratory.
Map of İnaltı Cave and sampling locations (Nazik et al., 1996)
Digestion-analysis of sediment samples and analysis of water samples was carried out by Bureau Veritas Mineral Laboratories Canada (ACME LAB.) by using AQ270 and SO200 methods, respectively. Sediment results were assessed using the sediment quality guide (SQG) (Table 1).
Anthropogenic effect analysis methods
Data provided by Turekian and Wedepohl were used as reference values (Turekian & Wedepohl, 1961). The results were interpreted using the scale in the study carried out by Fikirdeşici-Ergen et al. (2021) (Table 1).
Contamination factor (\({C}_{f}^{i}\)) (Hakanson, 1980)
\({C}^{i}\) = metal amount
\({C}_{n}^{i}\) = reference value
Contamination degree (Cd) (Hakanson, 1980)
\({C}_{f}^{i}\) = contamination factor
Modified contamination degree (mCd) (Abrahim & Parker, 2008)
\({C}_{f}^{i}\) = contamination factor n = number of metals examined
Pollution load index (PLI) (Tomlinson et al., 1980)
\({C}_{f1}\) = contamination factor n = number of metals examined
Enrichment factor (EF) (Hasan et al., 2013)
\({C}_{n}\) = metal amount
\({C}_{\mathrm{ref}}\) = metal amount in reference medium
\({B}_{n}\) = amount of reference element in sample
\({B}_{\mathrm{ref}}\) = amount of reference element in reference medium
Al was preferred as reference element.
Geo-accumulation index (Igeo) (Müller, 1969)
\({C}_{n}\) = metal amount
\({B}_{n}\) = amount of the metal in reference medium
= natural oscillation coefficient
Mean effect range-median quotient (m-ERM-Q) (Long et al., 2000) and Mean Probable effect level quotient (m-PEL-Q) (Carr et al., 1996)
\({C}_{i}\) = metal amount
ERM = effect range median of the metal,
PEL = mean probable effect level of the metal, n = number of metals examined.
Benthic macrofauna sample collection
In stations with water, it was aimed to discomfort the macroinvertebrates by muddying the water and mixing the sediment on the bottom by using a manual scoop, and samples were collected using the scoop. Moreover, in order to access more taxa in the cave, the samples taken from the mud on the rocks and stone in the water were poured into a tray and then transferred to 250–500 ml plastic jars by using a small shovel. They were kept by adding 96% ethyl alcohol into the jars.
Then, the samples taken to the laboratory were filtered using a three-sieve system with 0.5 mm, 1 mm, and 2 mm mesh sizes and rinsed with fresh water. The organisms on the sieves were collected and examined using a stereomicroscope. Then, the organisms were put into glass tubes containing 70% alcohol. The samples obtained from Gammaridea sub-ordo were analyzed in the laboratory by using NIKON SMZ 445 stereomicroscope, and species classification was performed using Ruffo’s (1953) classification atlas.
Statistical analysis methodology
Surface water, sediment, and biological analysis results were analyzed using IBM SPSS 25 statistical package program. Using one-way ANOVA analysis of parameters, both descriptive statistical analysis and significance tests (0.01 and 0.05) were performed in order to determine differences between stations. Pearson’s correlation index (PCI) was used in analyzing the relationships between sediment data and species found in the sediment (Alberto et al., 2001).
Multivariate hierarchical cluster analysis (HCA), a combination of techniques for classifying large data sets into similarities or clusters based on differences, was used to spatially represent the water and sediment quality of İnalti Cave (Cvejanov & Škrbić, 2017). HCA also utilized Ward’s method as a similarity measure (Barakat et al., 2016).
Today, multivariate statistical techniques (MSTs) such as cluster analysis (CA), principal component analysis (PCA), factor analysis (FA), and discriminant analysis (DA) are widely used to evaluate and interpret very complex and large data sets (Aydın-Uncumusaoğlu & Mutlu, 2022; Shrestha & Kazama, 2007; Singh et al., 2004).These methods also facilitate the identification and interpretation of natural or anthropogenic factors controlling temporal and spatial variations in water and sediment pollution sources and quality. Furthermore, these methods can provide rapid solutions to pollution problems and have been recognized as invaluable tools for reliable and rapid assessment and management of these natural resources to help improve water and sediment quality (Aydın-Uncumusaoglu & Mutlu, 2022). Principal component analysis (PCA/FA) and Pearson’s correlation analysis were used in analyzing the relationships between Niphargus sp., which was the biological parameter, and other parameters in the sediment in which it lives and to reveal the pollution sources. Prior to principal component analysis (PCA/FA), Kaiser–Meyer–Olkin (KMO) and Bartlett’s fit tests were performed. Since this value was 0.652 and Bartlett’s test (P = 0) for this cave, PCA test was found significant. In order to avoid misclassification due to large differences in datasets, analyzes were performed using standardized experimental image data with Z-scale transformation. Principal components were validated with eigenvalues (eigenvalues > 1) summarizing the total variance in the data set, that is, by the number of major components passing through 1 before a clear break of the scree plot (Jahin et al., 2020). The obtained PCA factor loadings were classified as “strong,” “moderate,” or “weak” corresponding to absolute loading values above 0.75, in the range of 0.75–0.50 or in the range of 0.50–0.30, respectively, as indicated in the study by Liu et al., 2003.
Results and discussion
Water and sediment quality assessment
Both physicochemical and biological parameters were studied in water and sediment samples collected from 10 stations in the cave. Statistically significant differences were found between the stations in terms of annual mean values of all parameters (P < 0.05).
Mean values of water quality parameters were found to be as follows, and the mean values were found respectively: pH: 7.80 ± 0.1, DO: 5.35 ± 0.22, EC: 685 ± 0.26 ms/cm, water temperature (WT): 7.43 ± 0.1 °C. The levels of heavy metals detected in the water were as follows: Al = 16.2 ug/L, As = 0.52 μg/L, Cd = 0.04 μg/L, Cr = 9.66 μg/L, Cu = 1.27 μg/L, Fe = 9.55 μg/L, Mn = 0.067 μg/L, Ni = 0.19 μg/L, Pb = 4.22 μg/L, and Zn = 0.49 μg/l (Table 2). The cave is in the high-quality water class according to the World Health Organization (WHO) and our country’s surface water quality management regulation criteria, and no parameter was found to be beyond the limit values (SWQR, 2016; WHO, 2011). No statistically significant difference was found between 10 stations in terms of the water quality (ANOVA, P > 0.05).
Mean concentrations of heavy metals found in the sediment were as follows: Al = 3.96 μg/L, As = 10.9 μg/L, Cd = 0.92 μg/L, Cr = 49.8 μg/L, Cu = 57.66 μg/L, Fe = 1.79 μg/L, Mn = 289.4 μg/L, Ni = 40.45 μg/L, Pb = 13.1 μg/L, and Zn = 123.4 μg/L (Table 2). Considering the sediment, Cd results were found to be above the crust, whereas Ni results were found to be close (S.W.Q.R., 2016; W.H.O., 2011). Cadmium is a metal, which has toxic effects on organisms and ecosystem even when at low doses. It has a high capacity to accumulate within an organism’s system thanks to its water-solubility and load, ionic radius, and chemical behavior that are identical to calcium (Fikirdeşici et al., 2012; Thornton, 1986). Uncontrolled use of Cd-containing fertilizers is among the most important anthropogenic sources. Moreover, combustion emissions, mining, and atmospheric accumulation play an important role in Cd concentration. Its natural release into nature occurs through the abrasion of cadmium from the rock structure (Kubier et al., 2019). Nickel is an element, which exists in the ecosystem from both natural and anthropogenic sources (Cempel & Nikel, 2006). Phosphatic fertilizers, mud combustion, mining activities, and combustion of diesel fuel, coal, and fuel oil are among the anthropogenic factors playing role in the release of Ni into nature. Moreover, plant cover also triggers the nickel release (Von, 1997; Kabata-Pendias & Pendias, 1992).
Sediment quality assessment
It was determined that the study area was mid-level polluted according to contamination degree (8.63), while very low level of pollution was found according to modified contamination degree (0.78). Examining the contamination factors of metals separately influencing this contamination degree, Pb, Ni, Mn, As, and Cr were found to have low contamination factors, whereas Cu and Zn were found to have mid-level results and Cd to have a high contamination factor. Using the pollution load index, no pollution was found in the study area (PLI < 1). Considering from the aspect of the enrichment factor, it was determined that Cd (62.02; > 50) had an excessively high enrichment level. Cu and Zn metals were determined to have a very high level and enrichment and Pb, Ni, As, and Cr to have a high-level enrichment. Geo-accumulation index values of all the metals, except for Cd, were found to be lower than 0. In other words, no contamination could be found in relation to other metals, but geo-accumulation value of Cd was found to be 1.03. Given the geo-accumulation index results, Cd contamination in the sediment of the study area was found to be at medium level. Given the mean effect range-median quotient (m-ERM-Q) results, the toxicity probability of the sediment was 21%. Given the mean probable effect level quotient (m-PEL-Q) results, it was determined that the sediment was affected at medium level (Table 3).
Examining the statuses of elements which have potential to exhibit intense toxic effects in the study area, it was determined that the elements having the highest level of risk were Cd and Ni. The same result was also achieved in different studies (Remeikaitė-Nikienė et al., 2018; Tunca et al., 2018). Du Preez et al. (2016), in a study carried out in South Africa, found the concentration of Cd element to be very high. Cd in the carbonate rocks dissolves because of the rain; some of them accumulate in sediment and some can directly reach water masses (Gabarrón et al., 2018). Thus, Cd can reach organic tissues by accumulating in sediment.
Biological assessment
To investigate the organic life in deep mud in the cave, the samples taken from water and mud by using a small hand scoop were rinsed with sieves in the laboratory to eliminate the mud, and then, they were examined using a microscope. As a result, 40 juvenile individuals and 24 mature individuals from only the Niphargidae family could be detected (Fig. 3). The order Amphipoda currently contains 10,226 described species most of which are free-living and benthic (Horton et al., 2019). The accumulation of heavy metals in sediments usually results in high concentrations of these metals in the sediment. As a consequence, these metals are considered a toxic risk in benthic systems.
Hierarchical cluster analysis (HCA) was used in order to determine temporal and spatial differences/similarities and define the groups. HCA analysis was conducted using seasonal and station averages of the parameters. During the analysis, the Ward method and Euclidian distance showing the relationship between distances and correlation the best were used. Given the HCA results based on the mean values of stations, the similarities and differences of stations were clustered as seen in Fig. 5.
Accordingly, the waters in the cave can be examined under three groups (Fig. 4). Cluster A consists of stations 3, 6, 5, 2, and 8; Cluster B consists of stations 4, 7, 1, and 10; and cluster C consists of station 9. There is no clear spatial distinction. Considering the similarities or differences for sediment samples, among the two clusters, cluster A consists of the first 6 stations. Cluster B includes the other stations, and the figure shows that the two groups clearly distinguish spatially in the sediment (Fig. 4).
Since the living organism was reached in the sediment, principal component analysis and Pearson’s correlation analysis were conducted in order to reveal the relationships between the organism and its ecological conditions (Mutlu & Aydın-Uncumusaoğlu, 2022; Aydın-Uncumusaoglu & Mutlu, 2022).
At the end of the PCA test, it was determined that 5 principal components could represent the sediment data of the cave (Table 4). Those principal components explain 90.22% of the total variance in the dataset, and the eigenvalues were found to be > 1 (Fig. 5).
The first principal component explaining 38.31% of the total variance has a strong positive load with Cu, ORP, DO, pH, EC, As, and Cd TH (r > 0.75) parameters. Niphargus sp. has a negative load with the first two principal components. Amphipods have a relatively high Cu requirement as oxygen-transport occurs by the copper-based, respiratory protein hemocyanin (Veltman et al., 2008). Detoxification of Cu is a characteristic seen in decapod crustaceans, but not observed in the other Crustacea orders (Guven et al., 1999). With a percentage of 14.17%, the second principal component has a strong negative load only with Zn and Ni (r > 0.75). Niphargus sp. had a strong positive load with the third principal component of the cave sediment. The fourth principal component was found to have a strong positive load with Al. The last (5th) principal component was represented with a strong positive load with WT, which is a physical parameter (Fig. 5).
Pearson’s correlation analysis was conducted in order to reveal the relationship of Niphargus sp. with other parameters in the sediment and, given the results achieved, this species has a strong positive relationship only with Cd (Table 5). Among the parameters analyzed in the sediment, very strong positive relationships were found between Cd, Cr, Cu, Al, Fe, and Ni.
Biological indicators and monitoring species are used as a tool to observe how the natural environment changes in response to pollution and other environmental degradation. The use of living organisms to monitor pollution is preferred over traditional water chemical analyses as it provides accurate information about the bioavailability of metals in the environment (Guerra-Garcia et al., 2009; Marin, 2017; Turemis et al., 2018). Bioindicators are organisms that show an ecological effect or change merely by their presence or absence. In this context, amphipod species can also be used as a bioindicator of contamination.
Forty percent of Türkiye’s tectonic units consists of rocks that are suitable for cave formation. Located in the Alpen-Himalayan mountain belt, it is thought that there are more than 40,000 caves in Türkiye. However, since the speleological and bio speleological studies were not sufficient, although 2850 caves could be detected in Türkiye, only 1450 of them could be examined to be registered in Türkiye’s Cave Inventory (Nazik, 2008). The studies in biospeleology started only in the early twentieth century, this discipline is still in its infancy in Türkiye (Kunt et al., 2010). Considering the organisms, caves are interesting habitats from an ecological aspect and caves constitute ecosystems on their own. Since they are semi-isolated environments and there are dark zones, some of which get no light at all, the competitive pressure on organisms living there has decreased and the organisms have evolved adapting to the absence of light (Ozansoy & Mengi, 2006).
In a previous study, Plénet (1999) emphasized that Niphargus species (hypogean) were affected by Cd exposure less than epigean species. However, Cd enrichment in sediment suggests that Cd might become toxic to those organisms too in the future. Amphipods can uptake trace elements through both absorption and ingestion (Barka et al., 2010; Ivanciuc et al., 2006). According to a study by Borga et al. (2006), there may be significant differences in the biological characteristics and interactions of amphipod species with elements. Therefore, it can be concluded that different amphipod species in the same region may differ from each other, especially in terms of element concentrations. Thus, it is important to classify and identify amphipods at the species level. This way, the determination of the characteristics of different species and the understanding of the effects of natural environmental element cycling and biological interactions on species become easier.
Conclusion
The results showed that except for Cd and Ni, there was no elemental accumulation that can be dangerous. For Cd and Ni, the comparisons made using SQGs suggest that those two elements should be given specific attention. These two elements constitute 50% of the toxic effect in the sediment in the study area. A biological indicator species can serve as an indicator of an ecological effect simply by its presence or absence in the environment. As a result of the principal components analysis on the sediment data, the fact that Niphargus sp. was found to have a strong positive load with the first two principal components proves that the existence of living organisms in this cave was already at very low dominance. The exposure of amphipods to heavy metals can disrupt their movement, feeding, and reproductive processes, leading to increased mortality rates and negative effects. It is clear that the organisms detected in the cave would be affected especially by Cd exposure. The data revealed that it is very important to investigate metal accumulation in organic tissues in future studies. Moreover, since organisms might convey the stress even if the exposure ended, this subject is suitable for epigenetic studies.
Data availability
Not applicable.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland. New Zealand. Environmental Monitoring and Assessment, 136(1–3), 227–238.
Alberto, W. D., del Pilar, D. M., Valeria, A. M., Fabiana, P.S., Cecilia, H.A., & de Los Ángeles, B. M. (2001). Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. a case study: Suquı́a River Basin (Córdoba–Argentina). Water Research, 35(12), 2881–2894.
Aydemir, E. (2007). Crustacea (Arthropoda) fauna of rocky substrates along the Sinop Peninsula coast and their bioecological characteristics, Ondokuz Mayıs University, Institute of Natural and Applied Sciences.Undergraduateno:185864.199p.https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp
Aydın-Uncumusaoğlu, A. (2018). Statistical assessment of water quality parameters for pollution source identification in Bektaş Pond (Sinop, Turkey ). Global NEST Journal, 20(1), 151–160.
Aydın-Uncumusaoğlu, A., & Mutlu, E. (2021). Water quality assessment in Karabogaz Stream Basin (Turkey) from a multi-statistical perspective. Polish Journal of Environmental Studies, 30(5), 4747–4759. https://doi.org/10.15244/pjoes/132909
Aydın-Uncumusaoğlu, A., & Mutlu, E. (2022). Water quality index and multivariate statistical approach in assessing the quality of irrigation water of caykoy pond. Fresenıus Envıronmental Bulletın, 3, 3447–3459.
Bat, L., & Şahin, F. (2018). Assessment of heavy metal pollution and potential ecological risk in sediments of Sinop shores of the Black Sea, International Marine & Freshwater Sciences Symposium / MarFresh 2018.
Barakat, A., El Baghdadi, M., Rais, J., Aghezzaf, B., & Slassi, M. (2016). Assessment of spatial and seasonal water quality variation of Oum Er Rbia River (Morocco) using multivariate statistical techniques. International Soil and Water Conservation Research, 4(May), 0–1. https://doi.org/10.1016/j.iswcr.2016.11.002
Barka, S., Pavilion, J., & Amiard-Triquet, C. (2010). Metal distributions in Tigriopus brevicornis (Crustacea, Copepoda) exposed to copper, zinc, nickel, cadmium, silver, and mercury, and implication for subsequent transfer in the food web. Environmental Toxicology, 25(4), 350–360. https://doi.org/10.1002/tox.20505
Birch, G. F. (2018). A review of chemical-based sediment quality assessment methodologies for the marine environment. Marine Pollution Bulletin, 133, 218–232.
Borga, K., Campbell, L., Gabrielsen, G., Norstrom, R., Muir, D., & Fisk, A. (2006). Regional and species specific bioaccumulation of major and trace elements in Arctic seabirds. Environmental Toxicology and Chemistry, 25(11), 2927–2936. https://doi.org/10.1897/05-574r1.1
Carr, R. S., Long, E. R., & Windom, H. L. (1996). Sediment quality assessment studies of Tampa Bay. Florida. Environmental Toxicology and Chemistry, 15(7), 1218–1231.
Cempel, M., & Nikel, G. (2006). Nickel: A review of its sources and Environmental Toxicology. Polish Journal of Environmental Studies, 15(3), 375–382.
Cuculić, V., Cukrov, N., Kwokal, Ž, & Mlakar, M. (2011). Distribution of trace metals in anchialine caves of Adriatic Sea, Croatia. Estuarine, Coastal and Shelf Science, 95(1), 253–263.
Culver, D. C., & Pipan, T (2009). The biology of caves and other subterranean habitats. Oxford: Oxford University Press; 256 p. https://doi.org/10.1093/oso/9780198820765.001.0001
Cvejanov, J. D., & Škrbić, B. D. (2017). Application of principal component and hierarchical cluster analyses in the classification of Serbian bottled waters and a comparison with waters from some other European countries. Journal of the Serbian Chemical Society, 82, 711–721.
Directive 2008/105/ECD. Environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC. European Parliament and the Council of the European Union. Allinson, G., Zhang, P., Bui, A., Allinson, M., Rose, G., Marshall, S., et al. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52020IP0377&from=EN
Du Preez, G., Wepener, V., & Dennis, I. (2016). Metal enrichment and contamination in a karst cave associated with anthropogenic activities in the Witwatersrand Basin. South Africa. Environmental Earth Sciences, 75(8), 1–13.
Ergen, ŞF. (2020). Sediment-friendly formulas: A review on the sediment quality guidelines. Communications Faculty of Sciences University of Ankara Series C Biology, 29(2), 202–212.
Ferrante, M., Spena, M. T., Hernout, B. V., Grasso, A., Messina, A., Grasso, R., & Copat, C. (2018). Trace elements bioaccumulation in liver and fur of Myotis from two caves of the eastern side of Sicily (Italy): A comparison between a control and a polluted area. Environmental Pollution, 240, 273–285.
Fikirdeşici, Ş., Altindağ, A., & Özdemir, E. (2012). Investigation of acute toxicity of cadmium-arsenic mixtures to Daphnia magna with toxic units approach. Turkish Journal of Zoology, 36(4), 543–550. https://doi.org/10.3906/zoo-1006-36
Fikirdeşici-Ergen, Ş., Tekatlı, Ç., Gürbüzer, P., Üçüncü-Tunca, E., Türe, H., Biltekin, D., & Tunca, E. (2021). Elemental accumulation in the surficial sediment of Kesikköprü, Çubuk II and Asartepe Dam Lakes (Ankara) and potential sediment toxicity. Chemistry and Ecology, 37(6), 552–572.
Gabarrón, M., Faz, A., & Costa, J. A. (2018). Use of multivariable and redundancy analysis to assess the behavior of metals and arsenic in urban soil and road dust affected by metallic mining as a base for risk assessment. Journal of Environmental Management, 206, 192–201.
Gèze, B. (1965). La Speleologie Scientifique. Paris: Editions du Seuil. Published by SEUIL, 1965. https://www.abebooks.com/signed/SPELEOLOGIE-SCIENTIFIQUE-COLLECTION-RAYON-SCIENCE-N%C2%B022/22419520242/bd
Gledhill, T., Sutclife, D. W. & Williams, W. D. (1993). British freshwater Crustacea Malacostraca: A key with ecological notes, Series Editor: Eliot, JM., Freshwater biological association, Scientific Publication No. 52, 173. https://www.nhbs.com/british-freshwater-crustacea-malacostraca-book
Guerra-Garcia, J., Baeza-Rojano, E., Cabezas, M., Diaz-Pavon, J., Pacios, I., & Garcia-Gomez, J. (2009). The amphipods Caprella penantis and Hyale schmidtii as biomonitors of trace metal contamination in intertidal ecosystems of Algeciras Bay. Southern Spain. Marine Pollution Bulletin, 58(5), 783–786. https://doi.org/10.1016/j.marpolbul.2009.02.018
Guven, K., Ozbay, C., Unlu, E., & Satar, A. (1999). Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.) (Amphipoda). Turkish Journal of Biology, 23(4), 513–521.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A Sedimentological Approach. Water Research., 14, 975–1001.
Hasan, A. B., Kabir, S., & Reza, A. H. M. S. (2013). Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary-Kumira), Chittagong. Bangladesh. Journal of Geochemical Exploration, 125, 130–137.
Horton, T., Lowry, J., De Broyer, C., Bellan-Santini, D., Coleman, C., Corbari, L., Costello, M., Daneliya, M., Dauvin, J. - C., Fišer, C., Gasca, R., Grabowski, M., Guerra-García, J., Hendrycks, E., Hughes, L., Jaume, D., Jazdzewski, K., Kim, Y. - H., King, R., Krapp-Schickel, T., LeCroy, S., Lörz, A., Mamos, T., Senna, A., Serejo, C., Sket, B., Souza-Filho, J., Tandberg, A., Thomas, J., Thurston, M., Vader, W., Väinölä, R., Vonk, R., White, K., & Zeidler, W. (2019). World Amphipoda. Database. Amphipoda. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=1135on2019–04–15
Ivanciuc, T., Ivanciuc, O., & Klein, D. (2006). Modeling the bioconcentration factors and bioaccumulation factors of polychlorinated biphenyls with posetic quantitative superstructure/activity relationships (QSSAR). Molecular Diversity, 10(2), 133–145. https://doi.org/10.1007/s11030-005-9003-3
Jahin, H. S., Abuzaid, A. S., & Abdellatif, A. D (2020). Using multivariate analysis to develop irrigation water quality index for surface water in Kafr El-Sheikh Governorate. Egypt. Environmental Technology and Innovation. 17. 100532. https://doi.org/10.1016/j.eti.2019.100532
Kabata-Pendias, A., & Pendias, H. (1992). Trace elements in soils and plants. CRC Press, Boca Raton, 365. https://doi.org/10.1201/9781420039900
Kubier, A., Wilkin, R. T., & Pichler, T. (2019). Cadmium in soils and groundwater: A review. Applied Geochemistry, 108, 104388.
Kunt, B. K., Yağmur, A. E, Özkütük, S., & Anlaş, S. (2010). Checklist of the cave dwelling invertebrates (Animalia) of Turkey. Biological Diversity and Conservation, 3(2):26–41. http://www.biodicon.com/YayinlananMakaleler/7.04.pdf
Kükrer, S., Erginal, A. E., Şeker, S., & Karabıyıkoğlu, M. (2015). Distribution and environmental risk evaluation of heavy metal in core sediments from Lake Çıldır (NE Turkey). Environmental Monitoring and Assessment, 187, 1–14.
Kwok, K. W., Batley, G. E., Wenning, R. J., Zhu, L., Vangheluwe, M., & Lee, S. (2014). Sediment quality guidelines: Challenges and opportunities for improving sediment management. Environmental Science and Pollution Research, 21, 17–27.
Liu, C. W., Lin, K. H., & Kuo, Y. M. (2003). Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Science of the Total Environment., 313(1–3), 77–89. https://doi.org/10.1016/S0048-9697(02)00683-6
Long, E. R. (1990). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program (Vol. 52). US Department of Commerce, National Oceanic and Atmospheric Administration, National Ocean Service.
Long, E. R., MacDonald, D. D., & Severn, C. G. (2000). Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environmental Toxicology and Chemistry, 19(10), 2598–2601.
Mansour, S. A., Soliman, S. S., & Soliman, K. M. (2016). Monitoring of heavy metals in the environment using bats as bioindicators: First Study in Egypt. ISSN, 61–78, 1213–6123.
Marin, I. (2017). The invaders as natural biondicators using the example invasive amphipod Gmelinoides fasciatus (Stebbing, 1899) in the Moscow River Basin: The suggestion of G. lacustris/G. fasciatus bioindication pair. Ukrainian Journal of Ecology, 7(4), 457–461.https://doi.org/10.15421/2017_142
Mejía-Ortíz, L., Christman, M. C., Pipan, T., & Culver, D. C. (2021). What’s the relative humidity in tropical caves? PLoS One, 16(9).
Mutlu, E. (2019). Evaluation of spatio-temporal variations in water quality of Zerveli stream (northern Turkey) based on water quality index and multivariate statistical analyses. Environmental Monitoring and Assessment. 191(6). https://doi.org/10.1007/s10661-019-7473-5
Mutlu, E., & Aydın-Uncumusaoglu, A. (2022). Assessment of spatial and temporal water pollution patterns in Aydos River (Turkey) by using water quality index and multivariate statistical methods. Desalınatıon and Water Treatment, 246, 196–211. https://doi.org/10.5004/dwt.2022.28030
Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118p.
Nazik, L., Özel, E., Mengi, H., & Aksoy, B. (1996). İnaltı Mağarası (Ayancık-Sinop) ön araştırma raporu. MTA Raporu, Derleme No: 9898, Ankara.
Nazik, L, (2008) Mağaraların Araştırılması, Koruma ve Kullanım İlkeleri, Ankara: MTA Genel Müdürlüğü, Yerbilimleri ve Kültür Serisi‐2.
Ozansoy, C., & Mengi, H. (2006). Mağarabilimi ve Mağaracılık. Ankara: Tübitak Yayınları. ISBN:9789754033878
Plénet, S. (1999). Metal accumulation by an epigean and a hypogean freshwater amphipod: Considerations for water quality assessment. Water Environment Research, 71(7), 1298–1309.
Printarakul, N., & Meeinkuirt, W. (2022). The bryophyte community as bioindicator of heavy metals in a waterfall outflow. Scientific Reports, 12(1), 1–12.
Rachmawati, S., Matin, H. H. A., Suhardono, S., Setyono, P., Kusumaningrum, L., Rinawati, S., & Sholiqin, M. (2021). Biodiversity of Rambut Cave Sale Central Java, IOP Conf. Series: Earth and Environmental Science.https://doi.org/10.1088/1755-1315/896/1/012006
Remeikaitė-Nikienė, N., Garnaga-Budrė, G., & Lujanienė, G. (2018). Distribution of metals and extent of contamination in sediments from the south-eastern Baltic Sea (Lithuanian zone). Oceanologia, 60(2), 193–206.
Ruffo, S. (1953). Studi sui Crostacei Anfipodi XXXVIII. Nuovi Niphargus (Amphipoda Gamaridae) della Venezia Tridentina. Trentini Di Scienze Naturali, 30, 115–127.
Singh, K. P., Malik, A., Mohan, D., Sinha, S., (2004). Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India) - a case study. Water Research, 38(18), 3980e3992. https://doi.org/10.1016/j.watres.2004.06.011
Shrestha, S., & Kazama, F. (2007). Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin. Japan. Environmental Modelling and Software, 22(4), 464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Field, L. J. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22(3), 624–638.
S. K. T. M. (2022). https://bolge10.tarimorman.gov.tr/Menu/51/Sinop-Inalti-Magarasi-Tabiat-ParkiErişim:23/11/2022
S.W.Q.R. (2016) Turkey’s Ministry of Forestry and Water Affairs Surface Water Quality Regulations. Retrieved March 8, 2021, from http://www.resmigazete.gov.tr/eskiler/2016/08/20160810–9.htm
Thornton, I. (1986). Geochemistry of cadmium In: Mislin H, Ravera O (Eds.), Cadmium in the environment. Birkhaeuser, Basel, Boston, Stuttgart, 7–12.
Tomlinson, D. L., Wilson, J. G., & Harris, C. R. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters., 33(1–4), 566–575.
Tunca, E., Aydın, M., & Şahin, Ü. A. (2018). An ecological risk investigation of marine sediment from the northern Mediterranean coasts (Aegean Sea) using multiple methods of pollution determination. Environmental Science and Pollution Research, 25(8), 7487–7503.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Geological Society of America Bulletin, 72, 175–192.
Turemis, M., Silletti, S., Pezzotti, G., Sanchis, J., Farre, M., & Giardi, M. (2018). Optical biosensor based on the microalga-paramecium symbiosis for improved marine monitoring. Sensors and Actuators B: Chemical, 270, 424–432. https://doi.org/10.1016/j.snb.2018.04.111
U.S.E.P.A. (1996) Ecotox thresholds. United States Environmental Protection Agency, Washington, DC EPA540/F-95/038, 12.
Veltman, K., Huijbregts, M., Van Kolck, M., Wang, W. X., & Hendriks, A. (2008). Metal bioaccumulation in aquatic species: Quantification of uptake and elimination rate constants using physicochemical properties of metals and physiological characteristics of species. Environmental Science & Technology, 42(3), 852–858. https://doi.org/10.1021/es071331f
Von Burg, R. (1997). Toxicology update: Nickel and some Nickel compounds. Journal of Applied Toxicology, 17, 425–431.
W.H.O. (2011). Guidelines for Drinking-Water Quality, 4 ed. World Health Organization. https://apps.who.int/iris/handle/10665/44584
Yong, N., Jiang, X., & Wang, K. (2020). Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu. China. Science of the Total Environment, 700.
Acknowledgements
We would like to thank the MAD-Cave Research Association and its members Mustafa Özkiliç and Burcu Aydin for their support in safely entering to the cave and collecting the samples.
Funding
This work was financially supported by the Sinop University (no. MMF-1901–18-34, TÜRKİYE).
Author information
Authors and Affiliations
Contributions
Eylem Aydemir Çil and Arzu Aydin Uncumusaoğlu provided conceptualization and first draft preparation; Şeyda Fikirdeşici Ergen and Pınar Gürbüzer reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors carefully read and approved the study.
Consent for publication
All authors are agreed to publish the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.”
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çil, E.A., Uncumusaoğlu, A.A., Ergen, Ş.F. et al. Evaluation of water and sediment quality of İnaltı Cave (Northern Türkiye) by using multivariate statistical methods. Environ Monit Assess 195, 667 (2023). https://doi.org/10.1007/s10661-023-11262-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11262-1