Abstract
Aims/hypothesis
Overweight and obesity may significantly worsen glycaemic and metabolic control in type 2 diabetes. However, little is known about the effects of overweight and obesity on the brains of people with type 2 diabetes. Here, we investigate whether the presence of overweight or obesity influences the brain and cognitive functions during early stage type 2 diabetes.
Methods
This study attempted to uncouple the effects of overweight/obesity from those of type 2 diabetes on brain structures and cognition. Overweight/obese participants with type 2 diabetes had more severe and progressive abnormalities in their brain structures and cognition during early stage type 2 diabetes compared with participants with normal weight. Relationships between each of these measures and disease duration were also examined.
Results
Global mean cortical thickness was lower in the overweight/obese type 2 diabetes group than in the normal-weight type 2 diabetes group (z = −2.96, p for group effect = 0.003). A negative correlation was observed between disease duration and global mean white matter integrity (z = 2.42, p for interaction = 0.02) in the overweight/obese type 2 diabetes group, but not in the normal-weight type 2 diabetes group. Overweight/obese individuals with type 2 diabetes showed a decrease in psychomotor speed performance related to disease duration (z = −2.12, p for interaction = 0.03), while normal-weight participants did not.
Conclusions/interpretation
The current study attempted to uncouple the effects of overweight/obesity from those of type 2 diabetes on brain structures and cognition. Overweight/obese participants with type 2 diabetes had more severe and progressive abnormalities in brain structures and cognition during early stage type 2 diabetes compared with normal-weight participants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic type 2 diabetes increases the risk of various complications in multiple organs, including the brain [1]. Complications in the brain due to type 2 diabetes may accelerate cognitive dysfunction and may even increase the risk of dementia [2,3,4,5]. Type 2 diabetes-related cerebral atrophy and white matter lesions have been considered brain correlates linking type 2 diabetes and cognitive dysfunction [6]. Although the exact mechanism underlying type 2 diabetes-related brain alterations is not fully understood, several metabolic dysfunctions including insulin resistance, poor glycaemic control and inflammation have been suggested to be involved [5, 6].
In general, obesity may precede the onset of type 2 diabetes and is associated with an increased risk of developing type 2 diabetes [7, 8]. Furthermore, obesity has been linked to metabolic dysfunction and may further exacerbate type 2 diabetes-related metabolic abnormalities [9, 10]. Obesity-related metabolic dysfunction is also independently associated with brain alterations [11,12,13] and may impair cognitive function, which further increases the risk of dementia [14]. However, little is known about how the concurrent presence of overweight/obesity and type 2 diabetes may affect the brain. As the type 2 diabetes phenotype was recently suggested to differ between normal-weight and obese people [15, 16], it is important to study type 2 diabetic individuals according to their weight status.
As the increasing prevalence of overweight/obesity may contribute to the global epidemic of type 2 diabetes [17], it is important to understand the potential combined effects of overweight/obesity and type 2 diabetes on brain structure and cognitive function.
The primary aim of this study was to examine whether the presence of overweight/obesity may influence type 2 diabetes-related brain structural changes and cognitive dysfunction. Since brain structural changes may begin a long time before the clinical manifestation of cognitive impairment [18, 19], our study participants consisted of participants who had been diagnosed with type 2 diabetes within the past 5 years and had no chronic diabetic complications. Thus, they were considered to be in the early stages of type 2 diabetes.
Methods
Participants
Participants included 50 overweight/obese individuals with physician-diagnosed type 2 diabetes and aged between 30 and 60 years (disease duration <5 years). Standard BMI cut-offs were used to define overweight (25–29.9 kg/m2) and obesity (≥30 kg/m2) [20, 21]. Fifty normal-weight (BMI <25 kg/m2) individuals with type 2 diabetes matched for age, sex and disease duration and 50 normal-weight control individuals (without type 2 diabetes) matched for age and sex were also included in the study. The latter were defined as having a fasting plasma glucose level of <5.55 mmol/l and a 2 h postprandial plasma glucose level of <7.77 mmol/l [22]. HbA1c levels of all normal-weight control individuals were <5.7% (39 mmol/mol) [23].
Individuals with type 2 diabetes who had chronic diabetic complications including clinical diabetic nephropathy, proliferative diabetic retinopathy, painful or symptomatic diabetic neuropathy, or major cerebrovascular or cardiovascular events were excluded. Individuals with major medical, neurological or psychiatric disorders and those with contraindications to MRI were also excluded from the study.
All type 2 diabetic patients were undergoing lifestyle modification therapy and/or taking oral glucose-lowering agents to achieve adequate glycaemic control. None had received stable insulin therapy or experienced prior hypoglycaemic episodes requiring medical assistance [24].
Fifty overweight/obese type 2 diabetic participants (BMI range, 25.2–34.1 kg/m2; hereafter referred to as the overweight/obese type 2 diabetes group), 50 normal-weight type 2 diabetic participants (BMI range, 17.9–24.9 kg/m2; hereafter referred to as the normal-weight type 2 diabetes group) and 50 normal-weight control participants (BMI range, 19.0–24.9 kg/m2; hereafter referred to as the normal-weight control group) completed assessments that included metabolic, brain and cognitive measures. Of these 150 individuals, 23 in the overweight/obese type 2 diabetes group, 28 in the normal-weight type 2 diabetes group and 39 in the normal-weight control group underwent another assessment 1 year after the initial visit (Table 1). A total of 240 sets of brain images and cognitive performance data were included in the final analyses. Electronic supplementary material (ESM) Fig. 1 shows BMI changes in the overweight/obese and normal-weight type 2 diabetes groups plotted against disease duration.
The study protocol was approved by the institutional review board of the College of Medicine, Catholic University of Korea. All individuals provided written informed consent prior to participation.
Clinical and metabolic assessments
The characteristics, medical history and medication status of all participants were assessed at the initial visit and 1 year follow-up. BP, glycaemic control, renal function and lipid profiles were also evaluated. HbA1c, HOMA-IR and high-sensitivity C-reactive protein (hs-CRP) were evaluated as metabolic markers for glycaemic control, insulin resistance and subclinical systemic inflammation, respectively.
Brain imaging
Brain MRI data were acquired using a 1.5 Tesla whole-body imaging system (Signa HDx, GE Healthcare, Milwaukee, WI, USA) at the initial visit and 1 year follow-up.
Mean thickness across the entire cortex (global mean thickness) was the primary outcome measure of type 2 diabetes-related grey matter alterations. In addition, vertex-wise analyses were performed to examine group differences in global mean thickness between the total type 2 diabetes group (comprising the overweight/obese and normal-weight type 2 diabetes groups) and the normal-weight control group.
The mean fractional anisotropy (FA) value across the whole brain white matter skeleton (global mean FA) was the primary outcome measure for type 2 diabetes-related white matter alterations. In addition, voxel-wise analyses were performed to examine differences in FA values between the total type 2 diabetes group and the normal-weight control group.
Detailed information on acquisition variables and imaging preprocessing is presented in the following ESM Methods sections: MRI acquisition and preprocessing, Cortical thickness measurement and FA measurement.
Cognitive assessments
Memory, psychomotor speed and executive function were assessed using a series of neuropsychological tests because these cognitive functions are known to be affected in people with type 2 diabetes [6, 25, 26].
The Rey–Osterrieth complex figure test and California Verbal Learning Test were used to assess memory function. Psychomotor speed was measured using the digit symbol substitution test, the Stroop test – C form and the grooved pegboard test. Executive function was examined using trail making test B, the Stroop test – interference and the Wisconsin card sorting test.
Composite scores in the memory, psychomotor speed and executive function tests were chosen as the outcome measures to examine between-group differences in cognitive changes during early stage type 2 diabetes. Neuropsychological tests are described in ESM Table 1.
Statistical analysis
Group differences in brain and cognitive measures between the total type 2 diabetes group and the normal-weight control group were examined using a linear mixed-effects model with group (type 2 diabetes vs control group) as a fixed-effect term and within-individual dependence as a random-effect term. Age and sex were included as covariates. Intracranial volume (ICV) and educational level were included as additional covariates for estimating group differences in global mean thickness and cognitive measures, respectively.
A linear mixed-effects model was used to estimate whether disease duration has different effects on metabolic, brain and cognitive measures between the two subgroups of type 2 diabetic participants. This model included weight status (overweight/obese vs normal-weight) as a linear term for disease duration (time since diagnosis of type 2 diabetes) and their interactions as fixed effects and as within-individual random effects. Models including disease duration and interaction as additional higher-order terms were also tested and then a likelihood ratio test was performed to compare models for selecting the best-fitting model. Age and sex were included in the models as covariates. ICV and education level were included as additional covariates for estimating global mean thickness and cognitive measures, respectively. Terms included in each final best-fitting model are shown in ESM Table 2.
We also investigated region-specific interaction effects between disease duration and weight status on brain measures using a linear mixed-effects model. Mean cortical thickness and FA values for regions of interest (ROIs; defined as significant grey and white matter clusters for the total type 2 diabetes group effects; Tables 2 and 3, ESM Table 3), were also estimated.
Raw values for global mean thickness, global mean FA values and composite scores for cognitive functions were standardised into z scores using the means and SDs for the normal-weight control group.
The sample size of 50 control and 100 type 2 diabetic participants was large enough to detect an effect size of 0.6 for differences in each measurement (SD = 15%) between groups, with a power of 0.90 and an α level of 0.05.
An α level of 0.05 in two-tailed tests was considered as statistically significant. Data were analysed using Stata version 11 (Stata Corp, College Station, TX, USA).
Results
Study participants
Baseline demographic and clinical characteristics of study participants are presented in Table 1. At the initial assessment, the overweight/obese and normal-weight type 2 diabetes groups had similar demographic characteristics, metabolic data and medication use, except for fasting C-peptide levels (t = −2.07, p = 0.04).
Disease duration-related alterations in metabolic measures in the overweight/obese and normal-weight type 2 diabetes groups
Metabolic measures including HbA1c, HOMA-IR and hs-CRP for the two type 2 diabetes subgroups are presented in Fig. 1.
Metabolic measures in the early stage of type 2 diabetes (a, c, e). Relationship between disease duration and HbA1c (b), HOMA-IR (d) and hs-CRP (f). Time point for the nadir HbA1c level corresponds to 24.1 months (black triangle). Estimated values were smoothed using locally weighted scatterplot smoothing (LOESS), a locally polynomial regression method [44]. Error bars represent 95% CIs. To convert values for HbA1c in mmol/mol into %, multiply 0.0915 and add 2.15. Grey bars and dots, normal-weight NW type 2 diabetes; black bars and dots, overweight/obese O type 2 diabetes. T2DM, type 2 diabetes mellitus
A U-shaped relationship was observed between disease duration and HbA1c levels in type 2 diabetic individuals. HbA1c levels were lower in the first 2 years after type 2 diabetes diagnosis. After the 2 year mark since diagnosis, HbA1c levels were elevated in both the overweight/obese and normal-weight type 2 diabetes groups, although they were higher in the former. However, there was no interaction effect of disease duration and weight status on HbA1c levels (z = 0.65, p for interaction = 0.51; Fig. 1b).
HOMA-IR, which reflects insulin resistance, correlated negatively with disease duration in both type 2 diabetes groups, and there was no between-group difference in the relationship between HOMA-IR and disease duration (z = −0.61, p for interaction = 0.54; Fig. 1d).
A positive relationship between hs-CRP level and disease duration was observed in the overweight/obese type 2 diabetes group, while a negative relationship was observed in the normal-weight type 2 diabetes group (z = 2.57, p for interaction = 0.01; Fig. 1f).
Differences in brain measures between the type 2 diabetes and control groups
There were differences in the global mean thickness (z = −2.72, p for group effect = 0.006) and the global mean FA (z = −3.73, p for group effect <0.001) between the total type 2 diabetes group and the normal-weight control group. Vertex-wise cortical thickness analysis showed that grey matter was significantly thinner in clusters in the temporal, prefrontoparietal, motor and occipital cortices in the total type 2 diabetes group than in the normal-weight control group (at a corrected p value of <0.05; Table 2 and ESM Fig. 2a). Voxel-wise FA analysis showed that FA values were significantly lower in clusters in the temporal, prefrontoparietal, motor and occipital regions in the total type 2 diabetes group than in the normal-weight control group (at a corrected p value of <0.05; Table 3, ESM Fig. 2b). In addition, tract-specific alterations were examined using the Johns Hopkins University diffusion tensor imaging-based white matter atlas [27]. FA values from the major fibre tracts were extracted and compared between groups (ESM Fig. 3).
Disease duration-related alterations in brain measures in the overweight/obese and normal-weight type 2 diabetes groups
Brain measures including global mean thickness and global mean FA of the two type 2 diabetes subgroups are presented in Fig. 2. Global mean thickness was significantly lower in the overweight/obese type 2 diabetes group than in the normal-weight type 2 diabetes group (z = −2.96, p for group effect = 0.003; Fig. 2a), and there was no between-group difference in the relationship between disease duration and global mean thickness (z = 0.65, p for interaction = 0.52; Fig. 2b). There was a significant interaction between disease duration and weight status on global mean FA (z = 2.42, p for interaction = 0.02), where a gradual decrease in global mean FA was found in the overweight/obese but not in the normal-weight type 2 diabetes groups (Fig. 2d).
Brain measures in early stage type 2 diabetes. Negative standardised z scores represent values below the means of the normal-weight control group in SD units. Standardised z scores and relationships between disease duration and brain measures of global mean thickness (a, b) and global mean FA values (c, d). Estimated values were smoothed using locally weighted scatterplot smoothing (LOESS) [44]. Error bars represent 95% CIs. Grey bars and dots, normal-weight type 2 diabetes; black bars and dots, overweight/obese type 2 diabetes. **p < 0.01
To ensure that the results were robust, repeated analyses were performed using baseline data while excluding 1 year follow-up data. The general linear model was applied to estimate the interaction effects between disease duration and weight status on brain measures. The same covariates as those used in the original linear mixed-effects model, except for within-individual random effects, were included in the model. Similar results were obtained for these repeated analyses (ESM Results).
Brain region-specific interaction between disease duration and weight status
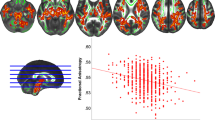
The overweight/obese type 2 diabetes group had a thinner cortex in the temporal and motor ROIs compared with the normal-weight type 2 diabetes group (z = −4.36, p for group effect <0.001 and z = −2.91, p for group effect = 0.004, respectively; Fig. 3a, c). Mean FA values in the prefrontoparietal ROI were lower in the overweight/obese type 2 diabetes group than in the normal-weight type 2 diabetes group (z = −2.06, p for group effect = 0.04; Fig. 3b). The relationships between disease duration and mean FA for the temporal and occipital ROIs in the overweight/obese type 2 diabetes group differed from those in the normal-weight type 2 diabetes group (z = 2.22, p for interaction effect = 0.03 and z = −2.07, p for interaction effect = 0.04, respectively; Fig. 3a, d).
Region-specific brain measures of ROIs in early stage type 2 diabetes. Pink regions on the standard cortical surface (upper) and the glass brain (lower), a render with transparent cortical structures, indicate ROIs of the study (ESM Table 3). Grey dots, normal-weight type 2 diabetes; black dots, overweight/obese type 2 diabetes. For relationships between disease duration and brain measures, estimates were smoothed using locally weighted scatterplot smoothing (LOESS) [44]. CC, corpus callosum; DLPFC, dorsolateral prefrontal cortex; IPC, inferior parietal cortex; ITC, inferior temporal cortex; L, left; OFC, orbitofrontal cortex; R, right; STC, superior temporal cortex
Differences in cognitive measures between the type 2 diabetes and control groups
There were differences in memory and psychomotor speed between the total type 2 diabetes group and the normal-weight control group (z = −3.11, p for group effect = 0.002 and z = −5.58, p for group effect <0.001, respectively). Differences in executive function between the total type 2 diabetes group and the normal-weight control group were not statistically significant (z = −1.78, p for group effect = 0.08).
Disease duration-related alterations in cognitive measures in the overweight/obese and normal-weight type 2 diabetes groups
Cognitive measures, including memory, psychomotor speed and executive function, for the two type 2 diabetes subgroups are presented in Fig. 4. There was a significant interaction effect of disease duration and weight status on psychomotor speed performance (z = −2.12, p for interaction = 0.03; Fig. 4d). A progressive decline in psychomotor speed performance was found in the overweight/obese type 2 diabetes group. There was no significant interaction effect on memory (z = −1.53, p for interaction = 0.13; Fig. 4b) or executive function (z = −0.25, p for interaction = 0.80; Fig. 4f).
Cognitive measures in early stage type 2 diabetes. Negative standardised z scores represent values below the means of the normal-weight control group in SD units. Standardised z scores and relationships between disease duration and cognitive measures of memory (a, b), psychomotor speed (c, d) and executive function (e, f). Estimated values were smoothed using locally weighted scatterplot smoothing (LOESS) [44]. Error bars represent 95% CIs. Grey bars and dots, normal-weight type 2 diabetes; black bars and dots, overweight/obese type 2 diabetes
Relationships between insulin resistance and brain measures
As HbA1c levels fluctuated in a U-shaped pattern, first decreasing in the first 2 years after diagnosis and then gradually increasing, HOMA-IR may be a more accurate marker than HbA1c levels for estimating brain and cognitive alterations that occur in early stages of the disease. Therefore, partial correlation analysis adjusting for age and sex was performed for insulin resistance and brain measures in type 2 diabetic patients whose disease duration was <2 years (90 data points). During the first 2 years after diagnosis, insulin resistance (as measured by HOMA-IR) was negatively associated with global mean thickness (r p = −0.24, p = 0.02) but not with global mean FA (r p = 0.06, p = 0.58).
Discussion
In the current study, we report for the first time that cortical thinning and disrupted white matter integrity are more pronounced in early stage type 2 diabetes. Furthermore, we found that disease duration-related alterations in cortical thickness and white matter integrity were more prominent in overweight/obese type 2 diabetic individuals than in those with normal weight. A similar trend was also found in psychomotor speed performance. These findings suggest that weight status may play additive roles in type 2 diabetes-related brain and cognitive alterations.
We selected grey and white matter ROIs in the temporal, prefrontoparietal, motor and occipital regions that showed type 2 diabetes-related alterations in cortical thickness or white matter integrity. We explored whether the concomitant presence of overweight or obesity influences grey and white matter of the brain in a region-specific manner. Interestingly, region-specific interaction effects were primarily observed in the temporal region, indicating that the temporal lobe is vulnerable to the combined effects of overweight/obesity and type 2 diabetes. An exploratory analysis also suggested that the reduction in FA values in white matter tracts including the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus and cingulum, which are mainly located in or pass through the temporal regions, were more pronounced in overweight/obese compared with normal-weight type 2 diabetic individuals.
Overweight/obesity and type 2 diabetes independently increase the risk of dementia [2, 28,29,30], and previous studies have shown that temporal lobe atrophy is one of the earliest neuroanatomical changes in Alzheimer’s dementia [18, 31]. Therefore, the temporal deficits specifically observed in the overweight/obese type 2 diabetes group may represent neuroanatomical substrates underlying the links among overweight/obesity, type 2 diabetes and the risk of dementia.
As pronounced deficits in the brain and in cognitive functions of people with type 2 diabetes may be potentiated by obesity, these patients can be divided into distinct prognostic subtypes according to their weight status [15, 16]. Our findings also highlight the need for early intervention aimed to reduce risk factors for overweight or obesity in type 2 diabetic individuals to preserve their brain structure and cognitive function. Furthermore, the contributory factors are unknown, our findings suggest that insulin resistance without obesity has distinct effects on the brain compared with conventional type 2 diabetes.
However, it is noteworthy that the current study did not recruit overweight/obese individuals without type 2 diabetes. Therefore, we could not determine the potential effects of overweight/obesity that are completely independent of type 2 diabetes on metabolic, brain and cognitive measures. Further studies using a more elaborate framework are recommended to better describe the independent and combined (additive, synergistic or interactive) effects of type 2 diabetes and obesity/overweight on the brain. To achieve this, we suggest using a balanced cohort comprising the following groups: normal-weight control individuals, obese individuals without type 2 diabetes, normal-weight type 2 diabetic patients and obese type 2 diabetic patients.
Interestingly, HbA1c levels (reflecting symptom severity) seem to decrease shortly after the diagnosis of type 2 diabetes. Furthermore, preliminary correlation analyses revealed that insulin resistance may be a significant determinant of cortical atrophy in early stage type 2 diabetes. Although the underlying mechanisms have not yet been clearly identified, impaired insulin signalling and consequent effects on synaptic plasticity may link insulin resistance and cortical atrophy [32]. Insulin receptors are widely distributed in the brain and are highly abundant in the hippocampus and specific cortical areas [33, 34]. Given that the current consensus recommendation for treating type 2 diabetes aims to maintain near-normal blood glucose and HbA1c levels, these findings suggest that it may be more important to manage insulin resistance (the underlying cause of the disease) during early stage type 2 diabetes than previously thought.
Although the final model included 1 year follow-up data, the current results may represent a cross-sectional estimation of trajectories in relation to the disease duration, rather than individual longitudinal changes. We used a linear mixed-effect model that accounted for within-individual random effects by assuming that changes occurring during the disease course are comparable among individuals [19]. This estimation was corroborated by a repeated analysis including only baseline data, which produced similar findings. Future longitudinal studies are necessary to estimate the longitudinal trajectory of brain measures in early stage type 2 diabetes.
The actual onset of type 2 diabetes often begins several years before its clinical diagnosis [35]. Thus, longitudinal follow-up of individuals with impaired glucose tolerance may be necessary to confirm the effects of overweight/obesity on the brain in individuals with type 2 diabetes. In this study, type 2 diabetic participants had not experienced previous hypoglycaemic episodes, so the potential effects of hypoglycaemia could not be determined [36].
Given that a series of studies suggest that glucose-lowering medications may have effects on the brain [37], the current findings should be interpreted with caution regarding the use of glucose-lowering medications. Specifically, glucagon-like peptide-1 receptor agonist, dipeptidyl peptidase 4 inhibitors, metformin and thiazolidinedione are known to have favourable effects on the brain [37]. Further longitudinal studies using larger cohorts are needed to discriminate and trace the potential interaction between glucose-lowering medication use and weight status on the brain in type 2 diabetic patients.
Our results suggest that a relationship exists between disease duration and metabolic measures in each diabetic group (classified according to BMI). However, as there was no information on fasting insulin and C-peptide levels in the normal-weight control group, differences in metabolic measures between the type 2 diabetic and control groups could not be determined in the present study.
The current WHO BMI cut-offs between overweight and obesity do not differ according to ethnic origin [17]. In Koreans, the overall risk of death was reported to be lowest for individuals with a BMI of 23.0–24.9 kg/m2 [38]. However, it is noteworthy that people of Asian ethnicity tend to be more vulnerable to slight increases in BMI even within the overweight range and are at a higher risk of type 2 diabetes than those of other ethnicities [39, 40]. Pre-existing functional deficits in beta cells, along with a tendency toward insulin resistance even in lean individuals, may contribute to the specific vulnerability of Asian people [17, 41]. Potential ethnic differences in brain vulnerability to type 2 diabetes and/or overweight/obesity may therefore need to be considered when interpreting the results of these studies.
This study showed that the concurrent presence of overweight/obesity was associated with cortical atrophy, disrupted white matter integrity and cognitive dysfunction in early stage type 2 diabetes. An increased awareness of overweight/obesity-related risk is necessary to prevent and manage type 2 diabetes-related brain atrophy and cognitive dysfunction from early stage type 2 diabetes onward.
Abbreviations
- FA:
-
Fractional anisotropy
- Hs-CRP:
-
High-sensitivity C-reactive protein
- ICV:
-
Intracranial volume
- ROI:
-
Region of interest
References
Klein JP, Waxman SG (2003) The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol 2:548–554
Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74
Biessels GJ, Deary IJ, Ryan CM (2008) Cognition and diabetes: a lifespan perspective. Lancet Neurol 7:184–190
Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ (2008) Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol 585:97–108
Strachan MW, Reynolds RM, Marioni RE, Price JF (2011) Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 7:108–114
McCrimmon RJ, Ryan CM, Frier BM (2012) Diabetes and cognitive dysfunction. Lancet 379:2291–2299
Neeland IJ, Turer AT, Ayers CR et al (2012) Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308:1150–1159
Biggs ML, Mukamal KJ, Luchsinger JA et al (2010) Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 303:2504–2512
Serrano Rios M (1998) Relationship between obesity and the increased risk of major complications in non-insulin-dependent diabetes mellitus. Eur J Clin Investig 28:14–17
Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L (2001) The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia 44:1148–1154
Brooks SJ, Benedict C, Burgos J et al (2013) Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obes 37:230–236
Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A (2012) Neuroimaging and obesity: current knowledge and future directions. Obes Rev 13:43–56
Debette S, Wolf C, Lambert JC et al (2014) Abdominal obesity and lower gray matter volume: a Mendelian randomization study. Neurobiol Aging 35:378–386
Pedditizi E, Peters R, Beckett N (2016) The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 45:14–21
Eckel N, Muhlenbruch K, Meidtner K, Boeing H, Stefan N, Schulze MB (2015) Characterization of metabolically unhealthy normal-weight individuals: risk factors and their associations with type 2 diabetes. Metabolism 64:862–871
Yaghootkar H, Scott RA, White CC et al (2014) Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 63:4369–4377
Yoon KH, Lee JH, Kim JW et al (2006) Epidemic obesity and type 2 diabetes in Asia. Lancet 368:1681–1688
Bateman RJ, Xiong C, Benzinger TL et al (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367:795–804
Benzinger TL, Blazey T, Jack CR et al (2013) Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A 110:E4502–E4509
Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (US), National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive Kidney Diseases (US) (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health, National Heart, Lung, and Blood Institute. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2003/. Accessed 8 Oct 2016
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
American Diabetes Association (2009) Standards of medical care in diabetes—2009. Diabetes Care 32:S13–S61
American Diabetes Association (2013) Standards of medical care in diabetes—2013. Diabetes Care 36:S11–S66
Punthakee Z, Miller ME, Launer LJ et al (2012) Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 35:787–793
van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ (2009) Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition. Biochim Biophys Acta 1792:470–481
Kodl CT, Seaquist ER (2008) Cognitive dysfunction and diabetes mellitus. Endocr Rev 29:494–511
Wakana S, Caprihan A, Panzenboeck MM et al (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36:630–644
Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I (2003) An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med 163:1524–1528
Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP Jr, Yaffe K (2005) Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330:1360
Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2011) Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 76:1568–1574
Scheltens P, Fox N, Barkhof F, De Carli C (2002) Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol 1:13–21
McNay EC, Recknagel AK (2011) Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 96:432–442
Bondy CA, Cheng CM (2004) Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol 490:25–31
Schulingkamp RJ, Pagano TC, Hung D, Raffa RB (2000) Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24:855–872
Harris MI, Klein R, Welborn TA, Knuiman MW (1992) Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 15:815–819
Frier BM (2014) Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 10:711–722
Patrone C, Eriksson O, Lindholm D (2014) Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol 2:256–262
Jee SH, Sull JW, Park J (2006) Body-mass index and mortality in Korean men and women. N Engl J Med 355:779–787
Yang W, Lu J, Weng J et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362:1090–1101
Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383:1084–1094
Ramachandran A, Ma RC, Snehalatha C (2010) Diabetes in Asia. Lancet 375:408–418
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383:1068–1083
Cleveland WS, Devlin SJ (1988) Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 83:596–610
Acknowledgements
The authors thank all volunteers for their participation in this study and thank S A. Chang, J. Kim, D.-J. Lim and J. M. Lee, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, for their valuable comments and assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A121080), the National Research Foundation of Korea (no. 2015M3C7A1028373) and the ICT R&D programme of the Institute for Information & Communications Technology Promotion (no. B0132-17-1001).
Duality of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SY, JK and IKL designed the study; and SY, HC and IKL collected and analysed/interpreted the data, wrote and reviewed the manuscript, and approved the final draft of the manuscript. JK, D-WL, GHK, YSH, SM, SP, SunL, SujL, SB and DCS substantially contributed to the conception and design, analysis and interpretation of data, and reviewed and approved the final draft of the manuscript. SY and IKL are the guarantors of this study.
Electronic supplementary material
ESM 1
(PDF 389 kb)
Rights and permissions
About this article
Cite this article
Yoon, S., Cho, H., Kim, J. et al. Brain changes in overweight/obese and normal-weight adults with type 2 diabetes mellitus. Diabetologia 60, 1207–1217 (2017). https://doi.org/10.1007/s00125-017-4266-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-017-4266-7