Abstract
Aims/hypothesis
High intake of carbohydrates, particularly sucrose, in western societies is associated with the development of non-alcoholic fatty liver (NAFL) and diabetes mellitus. It is unclear whether this is related primarily to the carbohydrate quantity or to the hormonal responses, particularly glucose-dependent insulinotropic polypeptide (GIP), which is released in the proximal intestine. Therefore, we investigated the role of GIP by comparing two glucose–fructose dimers, sucrose and Palatinose (isomaltulose), resorbed proximally or distally.
Methods
The glycaemic and incretin responses to sucrose and Palatinose were studied by oral gavage and meal tests. We then analysed phenotypic and metabolic diet-induced changes in C57Bl/6J mice exposed to isoenergetic diets differing in carbohydrate type. Studies were repeated in GIP receptor knockout (Gipr −/−) mice and their wild-type littermates.
Results
Compared with sucrose, Palatinose intake resulted in slower glucose absorption and reduced postprandial insulin and GIP levels. After 22 weeks, Palatinose feeding prevented hepatic steatosis (48.5%) compared with sucrose and improved glucose tolerance, without differences in body composition and food intake. Ablation of GIP signalling in Gipr −/− mice completely prevented the deleterious metabolic effects of sucrose feeding. Furthermore, our microarray analysis indicated that sucrose increased 2.3-fold the hepatic expression of Socs2, which is involved in the growth hormone signalling pathway and participates in the development of NAFL.
Conclusions/interpretation
Our results suggest that the site of glucose absorption and the GIP response determine liver fat accumulation and insulin resistance. GIP may play a role in sucrose induced fatty liver by regulating the expression of Socs2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver (NAFL) affects up to 30% of adults and up to 10% of children in developed countries [1]. The disease is characterised by an excessive accumulation of lipids, mainly triacylglycerol (TG), in the liver [2]. NAFL is considered the hepatic feature of metabolic syndrome and is associated with numerous diseases, including insulin resistance (IR) and type 2 diabetes [3, 4]. The most common cause of NAFL can likely be attributed to an exaggerated intake of dietary energy, especially carbohydrates, inducing a strong insulin response. Therefore, dietary components capable of decreasing postprandial glucose and insulin levels are promising approaches to reduce the development of NAFL. Recently, we observed that acarbose, an α-glucosidase inhibitor, reduced liver fat by attenuating the release of glucose in the gut [5].
The glycaemic response to various carbohydrates is dependent on the rate of their digestion and absorption [6]. Studies indicate that the intake of rapidly digestible sugars such as sucrose, known as high glycaemic index (GI) sugars, as compared with Palatinose (isomaltulose), a slowly and completely resorbed sucrose analogue composed of α-1,6-linked glucose and fructose, has deleterious effects on postprandial glucose, insulin and TG levels, which are associated with the risk of obesity, IR and fatty liver (FL) [7, 8]. We reported previously that mice fed a high-GI diet showed a rapid onset and marked increase in body fat mass, liver fat and hepatic lipogenesis [9].
Delaying carbohydrate absorption in the gut reduces the secretion of the gut hormone glucose-dependent insulinotropic polypeptide (GIP) from intestinal K cells and attenuates glucose appearance in the blood [10]. GIP is an incretin that is synthetised and released from the duodenum and proximal jejunum in response to fat, carbohydrate and protein [11]. Increased postprandial GIP responses are associated with obesity and the severity of liver diseases [12, 13]. Moreover, GIP was recently linked to unfavourable effects of diet-induced FL in animal models [14]. However, previous studies were hampered by the development of obesity in high-GI-fed animals such that the consequences of obesity were not separable from the effects of high-GI foods and the release of GIP [6, 15, 16].
Muscle fatty acid uptake and oxidation are facilitated by fatty acid transporters, which play a role in metabolic flexibility by altering cellular and mitochondrial fat uptake and oxidation and, therefore, are a potential target of incretins.
Suppressor of cytokine signalling 2 (SOCS2) coordinates the complex interplay between inflammation, growth hormone (GH) action and nutritional factors, and integrates the glucose and lipid responses in the liver [17]. Recently, it was shown that Socs2 −/− mice are protected from diet-induced FL [18]. Therefore, nutrients capable of reducing GH and SOCS2 action could be used to prevent or treat NAFL and its related disorders.
In the present study, we investigated the effects of distinct dietary sugars with different absorption rates in the gut, namely Palatinose and sucrose, on stimulating GIP release and the progression to IR and FL. To determine the role of GIP in diet-induced FL and impaired glucose homeostasis, we performed a long-term diet intervention in GIP receptor knockout (Gipr −/−) mice and their wild-type (WT) littermates. Finally, molecular mechanisms were investigated by unbiased microarray analysis and confirmed by quantitative PCR.
Methods
Animals
Experimental protocols were approved by the local governmental animal ethical committee in the State of Brandenburg, Germany. Experiments were performed in 18-week-old male C57Bl/6J mice (Janvier Labs, Saint Berthevin, France), unless otherwise stated. Mice were housed in individual cages with free access to water and standard rodent chow, with a 12:12 h light–dark cycle and a temperature of 23 ± 2°C. Mice were allowed a 1-week acclimatisation period before starting the experiments. Gipr −/− mice on a C57Bl/6J strain background were generated as previously described [19]. In order to explant organs, overnight fasted mice were sedated using isoflurane (Baxter, Unterschleissheim, Germany) and killed by cervical dislocation. Organs were isolated rapidly, snap frozen in liquid nitrogen and kept at −80°C for RNA isolation.
Oral administration of sugars
Sugars were administered orally as described in the electronic supplementary material (ESM).
Feeding test
In a separate set of animals, mice were trained for 4 days to consume either a Palatinose- or a sucrose-containing diet as detailed previously [9]. Briefly, individually housed mice were given 500 mg of the experimental diet following an overnight fast. Blood samples from the tail vein were drawn at 0 (overnight fasted), 30, 60, 90 and 120 min of consuming the whole portion of test meals within 15 min.
Both interventional diets were isoenergetic and contained 40.5% (wt/wt) carbohydrate, 41.5% (wt/wt) fat and 18% (wt/wt) protein (Table 1).
Dietary intervention
Body weight (BW) matched mice were fed the above diets for 22 weeks. To elucidate the role of GIP, another long-term experiment was performed in BW-matched Gipr −/− and WT mice fed the aforementioned diets.
Body composition
Body fat and lean mass were measured before the experiment and at indicated times using nuclear magnetic resonance spectroscopy (Mini Spect MQ10 NMR Analyser Bruker, Karlsruhe, Germany). Throughout the diet interventions, BW was determined once per week.
Digestibility of diets
Food intake (FI) was measured weekly and expressed as grams per week. The metabolisable energy intake was calculated according to the weekly FI and 1-week faecal samples. After drying, the energy content of diet and faeces samples were determined as previously explained [14].
Hydrogen breath test as a biomarker of colonic fermentation
A hydrogen exhalation test was performed as previously described [20] in samples, which were collected in duplicates in the fed state.
Liver TG and glycogen quantification
These variables are described in detail in the ESM.
Indirect calorimetry
Daily total energy expenditure (TEE) was estimated as described previously [14]. Oxygen consumption and CO2 production were determined to calculate RQ by dividing \( \overset{\cdot }{V}{\mathrm{CO}}_2 \) by \( \overset{\cdot }{V}{\mathrm{O}}_2 \). TEE is expressed per metabolic body mass and, therefore, allows comparison between groups.
Plasma analysis
Retro-orbital blood samples were collected for evaluation of plasma glucose, TG, fructoseamine, alanine aminotransferase (ALT) and NEFA using a commercial kit (glucose: HKCP; TG: Fructo Cal; ALT CP: ABX Pentra, Montpellier, France). Each variable was measured by an autoanalyser (Cobas Mira S, Hoffmann-La Roche, Basel, Switzerland). Plasma insulin levels were measured by ELISA as described elsewhere [9]. GH was assessed using a commercial ELISA kit for rat/mouse GH (EMD Millipore, St Charles, MO, USA).
Plasma resistin and leptin levels were measured by a commercial mouse Milliplex kit based on multiplex technology (Millipore, Billerica, MA, USA). Adiponectin levels were measured using the MilliplexMAP mouse adiponectin single-plex panel (Millipore). All measurements were performed on a Luminex 200 system (Luminex, Austin, TX, USA) in 96-well format as previously explained [21].
Incretin measurement
Plasma GIP levels were quantified using a rat/mouse total GIP ELISA kit (EMD Millipore). Blood samples were collected in tubes containing heparin lithium (Sigma-Aldrich, St Louis, MO, USA). Levels of plasma glucagon like peptide-1 (GLP-1) were determined by the GLP-1 (active) ELISA kit (Shibayagi, Gunma, Japan). Recombinant GLP-1 (7-36) was used as the standard. Blood samples were collected in tubes containing EDTA-Na2 (Sigma-Aldrich) and aprotinin (Carl Roth, Karlsruhe, Germany). To avoid the degradation of GLP-1, a DPP-IV inhibitor (EMD Millipore) was added to the plasma samples. All samples were stored at −80°C until assay.
Glucose tolerance test
A glucose tolerance test (GTT) was performed by i.p. glucose (2 g/kg BW) injection after overnight fasting. Plasma samples for glucose and insulin measurements were collected before and at 10, 30, 60 and 120 min after glucose challenge.
RNA extraction and quantitative RT-PCR
Total RNA was purified and quantified from liver and gastrocnemius muscle tissue samples as described [9]. Quantitative RT-PCR was performed using ABI Prism 7900 HT Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The quantity of target and the housekeeping gene (Hprt) were calculated according to a standard curve. Primer sequences are listed in ESM Table 1.
Microarray analysis
The quantity and quality of liver RNA were assessed using the Agilent 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA, USA). Total RNA (300 ng) was amplified using the Illumina TotalPrep RNA Amplification kit (Ambion, Carlsbad, CA, USA). Amplified cRNA was hybridised to Mouse Ref-8 v2.0 Expression BeadChips (Illumina, San Diego, CA, USA). Staining and scanning were done according to the Illumina expression protocol. Transcriptome analyses were performed by the statistical programming environment R implemented in CARMAweb (https://carmaweb.genome.tugraz.at; version 1.5.13) [22]. Genewise testing for differential expression was done using the Limma t test (Linear Models for Microarray Data, www.bioconductor.org/packages/release/bioc/html/limma.html; version 3.4.0) and Benjamini–Hochberg multiple testing corrections. Pathway enrichment analyses were done with the Ingenuity pathway software (Qiagen, Hilden, Germany). Array data were submitted to the Gene Expression Omnibus (GSE54723).
Data analysis
Data were analysed using IBM SPSS statistics 20 (SPSS, Chicago, IL, USA). Comparisons between two groups were performed using unpaired Student’s t test. Multiple comparisons were tested by one-way ANOVA, followed by post hoc Tukey or Games–Howell tests according to the homogeneity of variances (Levene’s test). Statistically significant effects of genotype and diet were determined using two-way ANOVA. The AUC was calculated by the trapezoid rule. Statistical significance was defined as p < 0.05. Results are presented as mean ± SEM.
Results
Glucose homeostasis and incretin response to sucrose and Palatinose
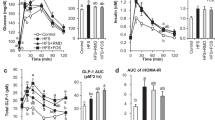
Oral challenge with the experimental sugars revealed that exposure to sucrose solutions induced a remarkable acute increase in glucose, insulin and GIP levels, without effects on GLP-1 secretion, which were avoided with Palatinose (ESM Fig. 1a–h).
To resemble normal eating we performed food training tests. Acute intake of diets (Table 1) with Palatinose or sucrose showed similar patterns in regulating fasting and postprandial glucose levels (Fig. 1a, b). Fasting insulin levels were significantly higher in sucrose-fed compared with Palatinose-fed mice (p < 0.01), indicating a compensatory effect of insulin upon sucrose feeding to maintain similar glucose levels. Feeding challenge resulted in increased insulin release in both groups, which was significantly greater at 30 min in sucrose-fed compared with Palatinose-fed animals (p < 0.05; Fig. 1c). InsulinAUC was significantly different between both groups (p < 0.01; Fig. 1d). Despite comparable fasting values, the GIP response was 12-fold higher after 30 min ingestion of sucrose compared with Palatinose (p < 0.001), and the response remained significantly elevated over 120 min of test (Fig. 1e). The dramatic differences are also evident in the GIPAUC (p < 0.001; Fig. 1f).
Palatinose and sucrose diets resulted in comparable body composition and energy intake
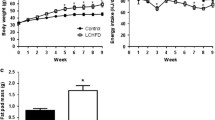
Ad libitum access to diets containing Palatinose and sucrose resulted in similar BW over the experimental period (Fig. 2a). Body composition analysis revealed that body fat and lean mass were comparable between the two groups (Fig. 2c, d). Weekly food consumption and even cumulative intake were similar in Palatinose- and sucrose-fed mice (Fig. 2b). Importantly, energy intake did not differ between the groups throughout the study (Table 2). Similar colonic fermentation rates, estimated at week 12, indicated complete resorption of both sugars (p = 0.08; Fig. 2e).
RQ and TEE at week 10 exhibited circadian rhythmicity in energy expenditure, with no significant differences in either sucrose- or Palatinose-fed groups (Fig. 2f, g). Therefore, the metabolic and phenotypic alterations described below are not related to obesity, FI or circadian rhythms.
Similar digestibility of the diets
For further analysis of digestibility of the diets, faecal excretion and FI were recorded at week 7. The energy content of the respective diets was comparable between groups (Palatinose, 20.14 kJ/g; sucrose, 20.2 kJ/g [Table 1]). Accordingly, faecal energy excretion was not significantly different between the groups. Digestibility of the diets was 89% with sucrose and 88.5% with Palatinose, indicating similar digested energy (Table 2).
Palatinose feeding reduced hepatic lipid accumulation and improved glucose metabolism
After a 22-week dietary intervention, liver TG levels were nearly 2-fold lower in Palatinose-fed vs sucrose-fed mice (p < 0.01; Fig. 3a). In agreement with this finding, plasma ALT levels were also lower in Palatinose-fed mice (p < 0.05; Fig. 3c). Liver glycogen content did not differ between Palatinose and sucrose challenge (p = 0.4; Fig. 3b). The dietary intervention did not result in significant changes in terms of plasma TG, NEFA, fructoseamine and insulin levels (Table 3). However, postprandial glucose levels were significantly higher in sucrose-fed mice (p < 0.01; Table 3).
Effects of long-term diet intervention on (a) liver TG, (b) liver glycogen and (c) plasma ALT and markers of glucose metabolism by i.p. GTT as shown on plasma and AUCs for (d, e) glucose and (f, g) insulin. White circles and bars: sucrose; black circles and bars: Palatinose. n = 8–10 per group. *p < 0.05, **p < 0.01; for part (f) p values are vs Palatinose
Additionally, we assessed glucose/insulin homeostasis following a 15-week dietary intervention. The findings revealed a significant impairment of glucose tolerance in sucrose-fed mice (Fig. 3d) as illustrated by the AUC results (p < 0.05; Fig. 3e). The significantly reduced levels of insulin in Palatinose-fed compared with sucrose-fed mice (p < 0.05; Fig. 3f, g), indicate improved glucose metabolism in the former.
Thus excluding the possibility that FI and BW differences are contributing factors to the reduced liver TG observed in the Palatinose-fed mice, we suspected that these findings might be due to attenuated GIP and insulin secretion.
Gipr−/− mice are protected from diet-induced FL and impaired glucose metabolism
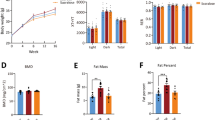
To further delineate the role of GIP in FL, we fed Gipr −/− and WT mice with diets containing sucrose or Palatinose. Remarkably, liver TG levels in Gipr −/− mice fed sucrose and Palatinose were twofold and threefold less than those of the WT sucrose-fed mice, respectively. The levels of liver TG in WT mice were approximately double those of Palatinose-fed mice (Fig. 4a). Hepatic TG levels were significantly influenced by diet (p = 0.003) and genotype (p = 0.015). BW gain, fat mass and TEE of Gipr −/− and WT mice are presented in ESM Fig. 2.
WT and Gipr −/− (knockout [KO]) mice were exposed to diets. (a) Sucrose diet in WT mice induced higher levels of hepatic TG compared with WT Palatinose-fed and Gipr −/− mice. (b) Adiponectin, leptin and resistin plasma levels in the overnight fasted animals. Glucose tolerance, evaluated by i.p. GTT, is presented for plasma and AUCs for (c, d) glucose and (e, f) insulin. White circles and bars: WT, sucrose; black circles and bars: WT, Palatinose. White triangles and hatched bars: KO, sucrose; black triangles and hatched bars: KO, Palatinose. n = 9 WT and 11 Gipr −/− groups. *p < 0.05, **p < 0.01
To determine additional variables contributing to GIP function and nutrient metabolism, we measured adiponectin, leptin and resistin levels in WT and Gipr −/− mice. Plasma adiponectin and leptin levels revealed a significant influence of genotype (p = 0.034 and p = 0.013, respectively), with no effect of diet either for adiponectin or leptin levels (both p > 0.05; Fig. 4b). Plasma resistin levels were not significantly different among the groups.
Glucose challenge showed that WT mice fed sucrose had a significantly higher total glucose response relative to Palatinose-fed mice, while plasma glucose levels were not different between Palatinose- and sucrose-fed Gipr −/− mice (Fig. 4c). There was no influence of diet (p = 0.058), genotype (p = 0.48) and interaction of diet x genotype (p = 0.064) in the glucoseAUC (Fig. 4d). Fasting insulin levels in WT mice were significantly higher than Gipr −/− mice (p < 0.01). After 10 min glucose load, there was a significant divergence in plasma insulin levels of WT mice fed sucrose compared with WT mice fed Palatinose and Gipr −/− mice (p = 0.01; Fig. 4e). In addition, insulinAUC displayed a significant interaction of genotype × diet (p = 0.03; Fig. 4f).
Dietary regulation of Socs2 expression and plasma GH
Molecular mechanisms for the observed effects in liver TG without differences in BW and energy metabolism were assessed by mRNA expression of transcription factors and genes involved in metabolic pathways leading to the development of NAFL, including de novo lipogenesis (Acca, Fas, Srebp1c and Chrebp [also known as Acaca, Fasn, Srebf1 and Mlxipl, respectively]), lipid beta-oxidation (Ppara and Cpt1a) and secretion from liver (ApoB100 and Mtp [also known as Apob and Mttp, respectively]). However, our quantitative PCR analysis revealed no significant differences in genes involved in lipid metabolism (p > 0.05; Fig. 5a). To identify regulated genes in a genome-wide scale we performed a microarray analysis of liver tissue from Palatinose- vs sucrose-fed mice. We identified 608 differentially expressed probes (p < 0.02; ESM Table 2), from which 136 had fold changes >1.3. To assess the pathways affected by the altered expression pattern we performed enrichment analyses (ESM Table 3). Among the genes with a higher expression in sucrose- vs Palatinose-treated animals was the Socs2 gene (2.3-fold), which is known to be transcriptionally regulated by GH signalling [23]. Quantitative PCR analysis validated the microarray data showing augmented expression of the Socs2 gene (p < 0.05; Fig. 5b). In accordance with this finding we observed that plasma GH levels were 55% lower in Palatinose-fed compared with sucrose-fed animals (p = 0.07; Fig. 5c). FL is often associated with inflammation. However, we did not find significant regulation of inflammatory marker genes in either the global transcriptome analysis or in the quantitative PCR validation (ESM Table 3; ESM Fig. 3).
Palatinose intake enhanced fat uptake and oxidation in muscle
Palatinose intake significantly increased gene expressions of Cd36 (fatty acid transporter) and Ppara (beta-oxidation) in muscle (both p < 0.05), indicating improved fatty acid uptake and oxidation by muscle (Fig. 5d).
Discussion
The main finding of our study was that Palatinose feeding prevented the development of FL and improved glucose metabolism in the setting of a high fat diet (HFD), without differences in energy intake and BW between groups. The highly significant prevention of hepatic fat accumulation was mediated by reduced GIP response, avoiding postprandial hyperinsulinaemia. The results in Gipr −/− mice suggest that GIP may mediate the deleterious metabolic effects of sucrose induced IR and FL.
Palatinose, compared with sucrose, is slowly and completely absorbed in the more distal small intestine [24], which can differently influence GIP secretion. Although Palatinose is well-established as a low-GI sugar [25, 26], we provide evidence that its effect on intestinal hormone release is central to its insulin-sparing properties in mice.
We first investigated the acute response of Palatinose and sucrose in glycaemic homeostasis and in the release of intestinal incretins. Sucrose caused an expected rapid increase in glucose accompanied by GIP and insulin release, while GLP-1 levels did not differ between groups. By contrast, Palatinose caused a more delayed increase in glucose, which resulted in little GIP secretion and, accordingly, much lower insulin secretion.
Our next question was whether or not these differences might be maintained by diet. Indeed, the HFD containing sucrose induced a rapid and strong increase of GIP and insulin, whereas Palatinose was not associated with a major increase of GIP and, accordingly, resulted in a smaller increase of insulin. The differences observed in oral and meal tests on plasma glucose are well-known [27] and relate to the content of fat and protein in the whole diet, which slows gastric emptying and thereby delays glucose absorption in the small intestine. The most likely explanation for the differences in GIP release refers to the more distal absorption of Palatinose, which bypasses the proximally located GIP-producing K cells in the small intestine.
We further analysed the long-term metabolic response of sugars in a hypercaloric diet. The Palatinose-fed mice exhibited reduced hepatic TG and were protected from diet-induced impaired glucose tolerance. A study in rats has reported that Palatinose-fed animals had higher hepatic insulin sensitivity [25], reduced hepatic TG, and lower postprandial insulin and glucose levels, which is consistent with our results although that study was confounded by differences in BW. Indeed, liver fat is associated with and is likely to be a cause of hepatic IR [4]. The 2-fold increase in liver TG levels with increased levels of ALT, a key indicator of hepatotoxicity, supports the presence of liver damage in sucrose-fed mice. Palatinose intake resulted in a modest reduction in postprandial glucose levels and a 40% reduction in glucose-stimulated insulin response. The latter may also reduce cardiovascular disease risk [28] and the development of diabetes [29].
In our study, comparable BW and FI confirm similar energy intake and digestibility of diets; therefore, the observed metabolic and hormonal differences are related to absorption differences in the gut. In contrast to amylose containing diets, which are not fully digested in the small intestine and fermented in the colon or excreted in the faeces [30, 31], Palatinose and sucrose were fully hydrolysed and absorbed in the small intestine, confirming findings from previous studies [32].
The most compelling evidence in support of the contribution of GIP to FL was the in vivo experiment in Gipr −/− and WT mice. Gipr −/− mice behaved similarly to WT mice fed Palatinose, and were protected from FL. In addition, there was a tendency for protection from glucose intolerance and hyperinsulinaemia in Gipr −/− mice. It is reported that HFD in mice results both in hypersecretion of GIP and extreme visceral fat deposition, while inhibition of GIP signalling protects from obesity, IR and hepatic steatosis [33]. It has also been shown that GIP antagonism is able to reverse liver, muscle and adipose tissue TG infiltration caused by HFD [34].
The peripheral putative mechanism of reduced liver TG levels in Gipr −/− mice might be linked to adipokines. The Gipr −/− mice exhibited slightly increased adiponectin levels compared with WT mice, whereas the amount of circulating leptin was significantly reduced by 45% in Gipr −/− mice. Leptin is secreted by adipocytes in proportion to their size and number [35, 36], which might explain the reduced leptin levels in Gipr −/− mice. Although not significant, Palatinose feeding enhanced leptin release, suggesting higher energy expenditure [37]. Leptin exerts crucial metabolic effects by preventing TG storage in peripheral tissues [38]. High leptin levels in the Palatinose-fed mice did occur independently of changes in fat mass and FI most likely as a consequence of central regulation. Our data suggest that GIP may partially affect FL development by regulating adipokine secretion, which needs further attention.
Indeed, Palatinose improved glucose homeostasis and liver fat metabolism in the context of HFD in part by enhancing muscular fatty acid uptake and causing a shift towards fat oxidation instead of fat deposition in the liver, as suggested by increased expression of Cd36 and Ppara.
For additional mechanisms we performed microarray analyses in liver and observed that Palatinose reduced 2.3-fold the mRNA expression of Socs2, an inhibitor of cytokine signalling. Studies have revealed changes in SOCS2 mRNA levels in human steatotic livers [39, 40]. Recently, it was shown that Socs2 −/− mice are protected from HFD-induced hepatic steatosis [18]. Moreover, SOCS2 has been implicated in GH signal transduction [23] and the development of NAFL [41]. Because Socs2 expression was shown to be induced by GH in primary cultures of hepatocytes [23], the reduced expression of Socs2 mRNA observed in the Palatinose-fed mice could be due to reduced plasma GH levels. It is reported that the GIP receptor mediates an increase in GH after glucose challenge [42, 43]. This suggests that higher plasma GIP levels and GIP receptor expression could lead to GH synthesis and secretion. Our results suggest that Palatinose might indirectly contribute to GH modulation by inhibiting postprandial GIP release and Socs2 expression in the liver.
The expression of the key lipogenic transcription factors Srebp1c and Chrebp as well as factors involved in beta-oxidation (Ppara and Cpt1a) were not altered. This finding is in contrast to other reports in which increased Ppara expression and hepatic fat oxidation were observed for the reduced liver fat in Palatinose-fed animals. In these studies, BW differed between Palatinose- and sucrose-fed animals, which may explain the contrasting results [16]. However, pathway analysis of microarray data indicated upregulation of glycogen and NAD biosynthesis pathways (ESM Table 3). Liver NAD biosynthesis is controlled by a salvage pathway using nicotinamide as a precursor and a de novo pathway using tryptophan. Increased NAD in the liver may enhance gluconeogenesis [44]. Although not significant, glycogen content was decreased in fasting mice on Palatinose vs sucrose. Our data indicate higher metabolic flexibility in the Palatinose-fed compared with sucrose-fed mice.
A limitation of this study is the incomplete evaluation of functional and mechanistic behaviour/aspects of Gipr −/− mice on diets. Since metabolic responses to nutritional challenges might differ between humans and rodents, controlled interventions are needed in humans to investigate whether or not Palatinose vs sucrose intake can affect hepatic insulin clearance and glucose metabolism.
In conclusion, Palatinose as a food ingredient reduces postprandial GIP secretion by evading upper intestinal absorption. By this mechanism, Palatinose feeding results in reduced glucose and insulin levels. Therefore, nutritional strategies capable of reducing postprandial GIP release seem to represent a promising approach for the prevention and/or treatment of FL and IR in humans.
Abbreviations
- ALT:
-
Alanine aminotransferase
- BW:
-
Body weight
- FI:
-
Food intake
- FL:
-
Fatty liver
- GH:
-
Growth hormone
- GI:
-
Glycaemic index
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon like peptide 1
- GTT:
-
Glucose tolerance test
- HFD:
-
High fat diet
- IR:
-
Insulin resistance
- NAFL:
-
Non-alcoholic fatty liver
- SOCS2:
-
Suppressor of cytokine signalling 2
- TG:
-
Triacylglycerol
- TEE:
-
Total energy expenditure
References
Williams CD, Stengel J, Asike MI et al (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140:124–131
Krawczyk M, Bonfrate L, Portincasa P (2010) Nonalcoholic fatty liver disease. Best Pract Res Clin Gastroenterol 24:695–708
Marchesini G, Bugianesi E, Forlani G et al (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37:917–923
Birkenfeld AL, Shulman GI (2014) Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 59:713–723
Rudovich NN, Weickert MO, Machann J, Pfeiffer AF (2010) Combination of acarbose and ezetimibe prevents non-alcoholic fatty liver disease: a break of intestinal insulin resistance? J Hepatol 52:952–953
Sato K, Arai H, Mizuno A et al (2007) Dietary palatinose and oleic acid ameliorate disorders of glucose and lipid metabolism in Zucker fatty rats. J Nutr 137:1908–1915
Daly M (2003) Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr 78:865S–872S
Kawai K, Yoshikawa H, Murayama Y, Okuda Y, Yamashita K (1989) Usefulness of palatinose as a caloric sweetener for diabetic patients. Horm Metab Res 21:338–340
Isken F, Klaus S, Petzke KJ, Loddenkemper C, Pfeiffer AF, Weickert MO (2010) Impairment of fat oxidation under high- vs. low-glycemic index diet occurs before the development of an obese phenotype. Am J Physiol Endocrinol Metab 298:E287–E295
Mortensen K, Christensen LL, Holst JJ, Orskov C (2003) GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196
Yip RG, Wolfe MM (2000) GIP biology and fat metabolism. Life Sci 66:91–103
Shimotoyodome A, Suzuki J, Fukuoka D, Tokimitsu I, Hase T (2010) RS4-type resistant starch prevents high-fat diet-induced obesity via increased hepatic fatty acid oxidation and decreased postprandial GIP in C57BL/6J mice. Am J Physiol Endocrinol Metab 298:E652–E662
Musso G, Gambino R, Pacini G, De Michieli F, Cassader M (2009) Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr 89:558–567
Isken F, Weickert MO, Tschop MH et al (2009) Metabolic effects of diets differing in glycaemic index depend on age and endogenous glucose-dependent insulinotrophic polypeptide in mice. Diabetologia 52:2159–2168
Haberer D, Thibault L, Langhans W, Geary N (2009) Beneficial effects on glucose metabolism of chronic feeding of isomaltulose versus sucrose in rats. Ann Nutr Metab 54:75–82
Matsuo K, Arai H, Muto K et al (2007) The anti-obesity effect of the palatinose-based formula Inslow is likely due to an increase in the hepatic PPAR-alpha and adipocyte PPAR-gamma gene expressions. J Clin Biochem Nutr 40:234–241
Rico-Bautista E, Flores-Morales A, Fernandez-Perez L (2006) Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev 17:431–439
Zadjali F, Santana-Farre R, Vesterlund M et al (2012) SOCS2 deletion protects against hepatic steatosis but worsens insulin resistance in high-fat-diet-fed mice. FASEB J 26:3282–3291
Preitner F, Ibberson M, Franklin I et al (2004) Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 113:635–645
Isken F, Klaus S, Osterhoff M, Pfeiffer AF, Weickert MO (2010) Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J Nutr Biochem 21:278–284
Valentini L, Wirth EK, Schweizer U et al (2009) Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition 25:172–181
Rainer J, Sanchez-Cabo F, Stocker G, Sturn A, Trajanoski Z (2006) CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Res 34:W498–W503
Tollet-Egnell P, Flores-Morales A, Stavreus-Evers A, Sahlin L, Norstedt G (1999) Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology 140:3693–3704
Lina BA, Jonker D, Kozianowski G (2002) Isomaltulose (Palatinose): a review of biological and toxicological studies. Food Chem Toxicol 40:1375–1381
Arai H, Mizuno A, Matsuo K et al (2004) Effect of a novel palatinose-based liquid balanced formula (MHN-01) on glucose and lipid metabolism in male Sprague-Dawley rats after short- and long-term ingestion. Metabolism 53:977–983
Takeda E, Arai H, Yamamoto H, Okumura H, Taketani Y (2005) Control of oxidative stress and metabolic homeostasis by the suppression of postprandial hyperglycemia. J Med Invest 52(Suppl):259–265
Park JH, Kwon OD, Ahn SH, Lee S, Choi BK, Jung KY (2013) Fatty diets retarded the propulsive function of and attenuated motility in the gastrointestinal tract of rats. Nutr Res 33:228–234
Goff LM, Cowland DE, Hooper L, Frost GS (2013) Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials. Nutrition, metabolism, and cardiovascular diseases. Nutr Metab Cardiovasc Dis 23:1–10
Kendall DM, Sobel BE, Coulston AM et al (2003) The insulin resistance syndrome and coronary artery disease. Coron Artery Dis 14:335–348
Cummings JH, Englyst HN (1995) Gastrointestinal effects of food carbohydrate. Am J Clin Nutr 61:938S–945S
Chinda D, Nakaji S, Fukuda S et al (2004) The fermentation of different dietary fibers is associated with fecal clostridia levels in men. J Nutr 134:1881–1886
Tonouchi H, Yamaji T, Uchida M et al (2011) Studies on absorption and metabolism of palatinose (isomaltulose) in rats. Br J Nutr 105:10–14
Naitoh R, Miyawaki K, Harada N et al (2008) Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochem Biophys Res Commun 376:21–25
McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR (2007) GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 293:E1746–E1755
Knight ZA, Hannan KS, Greenberg ML, Friedman JM (2010) Hyperleptinemia is required for the development of leptin resistance. PLoS One 5:e11376
Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314
Kadowaki T, Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26:439–451
Shimabukuro M, Koyama K, Chen G et al (1997) Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A 94:4637–4641
Elam MB, Cowan GS Jr, Rooney RJ et al (2009) Hepatic gene expression in morbidly obese women: implications for disease susceptibility. Obesity (Silver Spring) 17:1563–1573
Tateno C, Kataoka M, Utoh R et al (2011) Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology 152:1479–1491
Fan Y, Menon RK, Cohen P et al (2009) Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 284:19937–19944
Umahara M, Okada S, Ohshima K, Mori M (2003) Glucose-dependent insulinotropic polypeptide induced growth hormone secretion in acromegaly. Endocr J 50:643–650
Occhi G, Losa M, Albiger N et al (2011) The glucose-dependent insulinotropic polypeptide receptor is overexpressed amongst GNAS1 mutation-negative somatotropinomas and drives growth hormone (GH)-promoter activity in GH3 cells. J Neuroendocrinol 23:641–649
Kraus D, Yang Q, Kong D et al (2014) Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508:258–262
Acknowledgements
The authors would like to acknowledge the excellent technical assistance of S. Richter and K. Sprengel (Department of Clinical Nutrition, German Institute of Human Nutrition, Nuthetal, Germany) for metabolite measurements, A. Bettenbrock (Institute of Experimental Genetics and German Mouse Clinic, Helmholtz Zentrum München GmbH, Neuherberg, Germany) for performing microarray experiments.
Funding
This study was partially supported by a grant from the German Research Foundation (PF164/14-2) to AFHP, by a grant from the German Research Foundation (BI1292/4-1) to ALB, and by a grant from the Helmholtz Alliance ICEMED to JB.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
FKN designed experiments, researched data, wrote and edited the manuscript. FI designed experiments and researched data. MI researched and analysed microarray data, wrote and edited the manuscript. EKW researched data, wrote and edited the manuscript. JB researched and analysed microarray data, wrote and edited the manuscript. ALB researched data, wrote and edited the manuscript. AFHP designed experiments, wrote and edited the manuscript and obtained funding supporting the research. AFHP has full access to all data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. All authors approved the final version of the manuscript.
FI passed away and was not involved in drafting the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr Frank Isken, who contributed to this research, passed away on 4 October 2010 prior to the publication of this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 6 kb)
ESM Table 1
(PDF 87 kb)
ESM Table 2
(XLS 158 kb)
ESM Table 3
(PDF 167 kb)
ESM Fig. 1
(PDF 17 kb)
ESM Fig. 2
(PDF 23 kb)
ESM Fig. 3
(PDF 10 kb)
Rights and permissions
About this article
Cite this article
Keyhani-Nejad, F., Irmler, M., Isken, F. et al. Nutritional strategy to prevent fatty liver and insulin resistance independent of obesity by reducing glucose-dependent insulinotropic polypeptide responses in mice. Diabetologia 58, 374–383 (2015). https://doi.org/10.1007/s00125-014-3423-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3423-5