Abstract
Key message
The Br locus confers bruchid resistance in mungbean; VrPGIP2 (encoding a polygalacturonase inhibitor) is a strong candidate gene for this resistance. The VrPGIP2 sequence differs between resistant and susceptible lines.
Abstract
Azuki bean weevil (Callosobruchus chinensis) and cowpea weevil (Callosobruchus maculatus) are serious insect pests of mungbean during storage. Bruchid resistance in mungbean is controlled by a single dominant locus, Br. Although the Br locus has been located on a genetic map, molecular basis and function of the gene remain unknown. In this study, high-resolution mapping using a BC11F2 population of 418 plants derived from a cross between ‘Kamphaeng Saen 1’ (KPS1; susceptible) and ‘V2802’ (resistant) using simple sequence repeat (SSR) markers delimited the Br locus to a genomic region of 38 Kb of chromosome 5 containing two annotated genes. EST-SSR marker DMB-SSR158 co-segregated perfectly with the Br locus. Bioinformatics analyses revealed that DMB-SSR158 corresponds to a gene encoding a polygalacturonase inhibitor (polygalacturonase-inhibiting protein PGIP) and was designated as VrPGIP2. Comparison of VrPGIP2 coding sequences between four bruchid-resistant (V2802, V1128, V2817 and TC1966) and four bruchid-susceptible (KPS1, Sulu-1, CM and an unknown accession) mungbean lines revealed six single nucleotide polymorphisms (SNPs) between the resistant and susceptible groups. Three of the six SNPs resulted in amino acid changes; namely, alanine (A) to serine (S) at position 320, leucine (L) to proline (P) at position 332, and threonine (T) to P at position 335 of the VrPGIP2 sequence in resistant lines, compared with that in susceptible lines. The A to S change at position 320 may affect the interaction between PGIP and polygalacuronase. These results indicate that VrPGIP2 is very likely the gene at the Br locus responsible for bruchid resistance in mungbean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mungbean (Vigna radiata (L.) Wilczek) is among the most important grain legumes in Asia with an annual planting area of about 6 million hectares (Nair et al. 2012). It is a popular crop because of its short life cycle, drought tolerance, and ability to fix nitrogen in association with soil bacteria. It is mainly cultivated in rotation after rice or wheat (Somta and Srinives 2007). Mungbean seeds are important sources of dietary proteins and carbohydrates. The seeds are consumed directly or used to produce sprouts, noodles and starches.
The most important insect pest of stored mungbean seeds is bruchids or seed weevils (Coleoptera: bruchidae). Bruchids damage seeds in the field and after harvest. In storage, they can cause a total loss of the seed lot within 3–4 months (Srinives et al. 2007). Azuki bean weevil (Callosobruchus chinensis L.) and cowpea weevil (Callosobruchus maculatus F.) are the main bruchid pests of mungbean. These bruchids also damage seeds of several other important Vigna crops such as cowpea (Vigna unguiculata L.), black gram (Vigna mungo (L.) Hepper), Bambara groundnut (Vigna subterranea (L.) Verdc.), and azuki bean (Vigna angularis (Willd.) Ohwi and Ohashi). The bruchid-damaged seeds are not suitable for consumption, or for agricultural or commercial uses. Therefore, breeding for resistance to bruchids is a major goal in mungbean breeding programs (Srinives et al. 2007).
Several sources of resistance to bruchids have been identified in mungbean. Fujii and Miyazaki (1987) showed that wild mungbean (V. radiata var. sublobata) accession TC1966 is completely resistant to C. chinensis and C. maculatus. Lambrides and Imrie (2000) found that wild mungbean accessions ACC23 and ACC41 are resistant to both bruchid species. Talekar and Lin (1992) reported that cultivated mungbean accessions V2709 and V2802 are highly resistant to C. chinensis. Previously, we found that V2709 and V2802 are also highly resistant to C. maculatus, while the cultivated accessions V1128 and V2817 are completely resistant to both bruchid species (Somta et al. 2008). The resistance in TC1966, V2709, V2802, V1128, and V2817 is due to biochemical compounds in the seeds (Talekar and Lin 1992; Somta et al. 2008).
Resistance to C. chinensis in the wild mungbean accession TC1966 is controlled by a single dominant gene locus, designated as Br, with modifying factor(s), and the degree of resistance depends on the seed genotype (Kitamura et al. 1988). Resistance to C. chinensis and C. maculatus in V2709 and V2802 is also controlled by a single dominant gene with modifiers (Somta et al. 2007). Since V. radiata var. sublobata is proposed to be the ancestor of cultivated mungbean (Maréchal et al. 1978), the bruchid resistance gene in V2709 and V2802 may have been inherited from the V. radiata var. sublobata progenitor. Gene mapping for C. chinensis resistance in TC1966 using restriction fragment length polymorphism (RFLP) markers revealed that the Br locus is located on linkage group (LG) VIII between the markers (probes) pA882 and pM151a (Young et al. 1992). Later, additional RFLP markers were added to the mungbean genetic map and LG VIII containing the Br gene was re-named LG9. The Br locus was labeled as the Bruc locus on this map (Menancio-Hautea et al. 1993). Kaga and Ishimoto (1998) narrowed down the Br locus to a region of 0.7 cM between the RFLP probes Bng110 and Bng143. Bng143 is tightly linked to Br, being 0.2 cM away from this locus. Using ACC41 as the resistance source, the RFLP marker mgM213 mapped onto LG9 was identified as being closely associated with Br (1.3 cM apart Miyagi et al. 2004; Mei et al. 2009). A few simple sequence repeat (SSR) and sequence-tagged site (STS) markers for the Br locus in ACC41 were developed from a bacterial artificial chromosome (BAC) clone containing marker mgM213 (Miyagi et al. 2004). One of those markers, STSbr2, was shown to be associated with Br in V2709 (Sun et al. 2008); Chen et al. (2007) developed seven cleaved amplified polymorphism (CAP) markers closely linked with Br in TC1966. Chotechung et al. (2011) reported that the mungbean SSR marker DMB-SSR158 (DMB158 in the original paper) perfectly associated with Br in V2802. Based on QTL mapping, Chen et al. (2013) showed that DMB-SSR158 is tightly linked with the major QTL for bruchid resistance in TC1966. The distance between DMB-SSR158 and this major QTL was <0.1 cM. These reports suggested that the same Br locus controls bruchid resistance in TC1966, ACC41, V2709 and V2802.
Although DNA markers closely linked to the Br locus have been reported, most of them are RFLP markers which are not suitable for marker-assisted selection (MAS) in a large population. In addition, the molecular basis of bruchid resistance is still unknown. Recently, a draft genome sequence of mungbean was reported (Kang et al. 2014). This sequence is useful for gene identification and development of DNA markers for trait(s) of interest in mungbean breeding. In this paper, we report high-resolution mapping of the Br locus in the cultivated mungbean accession V2802. The objectives of this study were to narrow down the genomic region of the Br locus and to identify candidate gene(s) for resistance.
Materials and methods
Plant materials and DNA extraction
Four bruchid-resistant mungbean accessions (V1128, V2802, V2817, and TC1966) and one bruchid-susceptible mungbean cultivar ‘Kamphaeng Saen 1’ (KPS1) were used in this study. A BC11F2 population developed from crossing KPS1 as the recipient parent and V2802 as the donor parent was used as the mapping population. Population development procedures are shown in Fig. 1. The population comprised 418 individuals from five BC11F1 plants. The BC11F2 plants were grown together with the parents in an experimental field of Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom, Thailand, from February to April 2013. Seeds produced from each of the BC11F2 and parental plants were individually harvested and used to evaluate bruchid resistance.
Genomic DNA was extracted from young leaf tissue of all mungbean plants using a modified CTAB method (Lodhi et al. 1994).
Evaluation of bruchid resistance
Mature seeds were evaluated by exposure to C. chinensis and C. maculatus reared on seeds of KPS1. The resistance evaluation was conducted as described previously (Somta et al. 2007) with modifications. Briefly, 30–40 seeds from each BC11F2 plant were placed in a plastic box. Then, 40 adult bruchids (20 pairs of 1–3 day old males and females) were added to the box and kept there for 7 days to lay eggs and then removed. At least three eggs were laid on each seed. Parental seeds were tested and replicated five times. The infested seeds were maintained at 28 °C and 70 % RH. The number of damaged seeds was counted at 60 days after introducing the insects and then used to calculate damage percentages.
Segregation analysis
To confirm the monogenic inheritance of bruchid resistance in V2802 (Somta et al. 2007; Chotechung et al. 2011), BC11F2 plants showing 0–80 % damaged seeds were characterized as resistant. These included the homozygous resistance genotype (highly resistant with 0–20 % damaged seeds) and the heterozygous resistance genotype (moderately resistant with 21–80 % damaged seeds), while BC11F2 plants showing 81–100 % damaged seeds were considered to be homozygous for the susceptible genotype (Somta et al. 2007). A Chi-squared (χ 2) test was used to determine the goodness of fit to a 3 (resistant):1 (susceptible) ratio. The test was conducted using software R-program 2.0.10 (R Development Core Team 2010).
The correlation between percentages of seeds damaged by C. chinensis and C. maculatus was calculated using R-program 2.0.10.
Bioinformatics analyses of Br locus location on mungbean genome
In a previous study, the Br locus was found to be located between the RFLP markers Bng110 and Bng143 (Kaga and Ishimoto 1998). Therefore, we used mungbean cDNA sequences of the probes Bng110 (GenBank accession AB020611) and Bng143 (GenBank accession AB020610) to conduct BLASTN searches against the mungbean reference sequence (Kang et al. 2014; http://plantgenomics.snu.ac.kr/sequenceserver). Once identified, the genome sequence between the probes Bng110 and Bng143 was downloaded and searched for SSRs using SSRIT software (Temnykh et al. 2001). The primers were designed using Primer3 software (Untergasser et al. 2012).
We also conducted a BLASTN search for the location of the genic SSR marker DMB-SSR158 on the reference genome, because this marker was reported to completely co-segregate with the Br locus in V2802 (Chotechung et al. 2011). The cDNA sequence of this marker was used in the BLASTN search (GenBank accession AM696512; Somta et al. 2009).
DNA marker analysis
The SSR markers used to screen for polymorphisms between KPS1 and V2802 were those located on the same linkage group as the RFLP markers Bng110 and Bng143 [LG9 of mungbean reported by Kaga and Ishimoto (1998), Zhao et al. (2010) and LG2 of mungbean, azuki bean and cowpea reported by Isemura et al. (2012), Han et al. (2005) and Kongjaimun et al. (2012), respectively] together with the new SSR markers developed in this study (Supplementary Table S1). We also included one EST marker, RP, which was shown to be closely linked to Br in mungbean accession V2709 (Hong et al. 2015). The PCR amplifications, gel electrophoresis, and DNA band visualization were carried out as described previously (Somta et al. 2009). Polymorphic markers were used to analyze the DNA of the BC11F2 plants.
QTL mapping for bruchid resistance
A genetic linkage map was constructed using QTL IciMapping 4.0 software (Meng et al. 2015). Markers were grouped with log of odds (LOD) value of 3.0 and anchored based on their positions on the reference sequence. Distances between markers were calculated using Kosambi’s mapping function. A QTL controlling bruchid resistance was located onto the linkage map by the inclusive composite interval mapping method (Li et al. 2007) using QTL IciMapping 4.0. The LOD threshold for declaring the QTL was determined by a 5000 permutation test (at P = 0.001).
Sequencing of candidate genes for the Br locus
Based on the results of QTL mapping Vradi05g03940 and Vradi05g03950 were considered as candidate genes for Br (see “Results”). Primer pairs were designed (Supplementary Table S2) to amplify all exonic regions of the genes in all mungbean accessions/cultivars used in this study. The PCR mixture (10 µl) contained 10 ng genomic DNA, 5 pmol each forward and reverse primers, 1 U Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA), 1× Taq Buffer, 2 mM dNTPs, and 0.8 mM MgCl2. The thermal cycling program (using a GeneAmp 9700 system) was as follows: 94 °C for 2 min, 40 cycles of 94 °C for 15 s, 57.5 °C for 30 s, 72 °C for 90 s, and 72 °C for 2 min. The amplified products were electrophoresed on a 1.0 % agarose gel, and then DNA bands with expected size were cut and purified with E.Z.N.A.® Gel Extraction Kit (Omega, Doraville, GA, USA) following the manufacturer’s instructions. The purified DNA was sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit on an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) by Macrogen Inc. (Seoul, Korea).
Re-annotation of Vradi05g03950
The cDNA sequence of the marker DMB-SSR158 (GenBank accession AM696512) was used in BLASTN searches against the transcriptome shortgun assembly (TSA) of mungbean in GenBank and our in-house mungbean transcriptome sequence data generated from the susceptible mungbean accessions ‘Sulu_1’ and ‘CM’. A transcript sequence from GenBank (GenBank accession GBXO01011683) and two transcript sequences from our laboratory were aligned with the Vradi05g03950 sequence from the reference sequence (VC1973A), KPS1, V2802, V1128 and TC1966 using Clustal 2.1 to obtain a new annotated CDS and identify mutations among the mungbean accessions. The predicted protein sequences of Vradi05g03950 from all these mungbean lines were also aligned.
Results
Bruchid resistance in the BC11F2 population
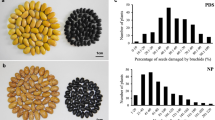
Seeds of V2802 were completely resistant to C. chinensis and C. maculatus, while all seeds of KPS1 were damaged by both bruchid species. In the BC11F2 population, the frequency distribution of the percentage of damaged seeds was non-continuous and bimodal, and skewed toward KPS1 (Fig. 2). The proportions of damaged seeds caused by C. chinensis and C. maculatus were similar, ranging from 0 to 100 % with a mean of 48.65 % for C. chinensis and 48.58 % for C. maculatus, with a correlation coefficient of 0.97 (df = 402, P < 0.0001). The high correlation coefficient confirmed the results reported by Somta et al. (2007) and Chotechung et al. (2011) and suggested that resistance to C. chinensis and to C. maculatus in V2802 is controlled by the same locus.
We classified resistant and susceptible plants in the BC11F2 population using 80 % damaged seeds as the cutoff level. This analysis revealed that resistance to C. chinensis and to C. maculatus did not fit a 3 (resistant):1 (susceptible) ratio (χ 2 = 25.27, P < 0.0001 and χ 2 = 27.57, P < 0.0001, respectively). These findings contrasted with those reported by Somta et al. (2007) and Chotechung et al. (2011) that a single dominant gene with modifiers controls bruchid resistance in V2802.
Location of Br locus on mungbean reference genome
The mungbean chromosome region containing Br was identified by BLAST searches using the sequences of RFLP markers Bng143 and Bng110. The results showed that these markers corresponded to two annotated genes, Vradi05g03740 (Vr05:5,049,905..5,054,628) and Vradi05g04410 (Vr05:6,775,804..6,780,603), on chromosome 5 of mungbean, respectively (Fig. 3a). The markers Bng143 and Bng110 were found to be 1.72 Mbp apart on the mungbean reference genome. The search also revealed that the RFLP marker mgM213 and SSR marker DMB-SSR158 corresponded to Vradi05g06080 (Vr05:12,103,656..12,111,809) and Vradi05g03950 (Vr05:5,590,573..5,598,731), respectively, on chromosome 5 of mungbean (Fig. 3). There were 76 annotated genes in the 1.72 Mb region.
Location of Br locus in wild mungbean accession TC1966 (as reported by Kaga and Ishimoto (1998)) on chromosome 5 of mungbean reference genome based on BLAST search of RFLP markers, Bng110 and Bng143 (a). Current map of the Br locus redrawn based on the map reported by the same authors. Location of the Br locus from cultivated mungbean accession V2802 detected in the BC11F2 population in this study, and its position and corresponding genes on chromosome 5 of the mungbean reference genome (b). Numbers in parentheses indicate the position of marker on the linkage map in centiMorgan units
We developed 40 SSR markers located between Vradi05g03740 and Vradi05g04410 (Supplementary Table S1). These new SSR markers, seven previously developed SSR markers, and one STS marker previously mapped to the same linkage group with the Br locus (48 markers in total) were used to detect polymorphisms between V2802 and KPS1. Of the 48 markers, 45 were able to amplify mungbean DNA and 10 were polymorphic between the two mungbean cultivars (Supplementary Fig. S1). Marker DMB-SSR158 detected two loci: one monomorphic and the other polymorphic. The polymorphic locus was inherited as a dominant marker (DNA band was present only in V2802).
High-resolution mapping of the Br locus
A genetic map for mungbean chromosome 5 was constructed using polymorphic DNA markers. The linkage group comprised ten markers and spanned a length of 60.9 cM. A QTL analysis identified one major QTL for resistance to each bruchid species (Table 1; Supplementary Fig. S2). These QTLs were located at the same position between markers VrBr-SSR013 and DMB-SSR158 and thus were named qBr. The qBr was only 0.1 cM away from DMB-SSR158 (Supplementary Fig. S2), and explained 93.34–93.84 % of the total variation in the proportion of damaged seeds caused by C. chinensis and C. maculatus, respectively.
When the genotypes were classified according to bruchid damage as resistant (Br/Br and Br/br) and susceptible (br/br), we found that Br co-segregated perfectly with the marker DMB-SSR158 (Vradi05g03950) (Supplementary Table S3). This result together with the QTL mapping result suggested that Br and Vradi05g03950 are the same locus. Figure 3 shows the location of Br and the markers associated with it on chromosome 5. A BLASTP search against the NCBI database revealed that Vradi05g03950 showed the strongest similarity to a polygalacturonase-inhibiting protein (PGIP) in Medicago (Medicago truncatula Gaertn.) (accession XP_003625218.1; e-value = 1e−118, score = 993, identity = 68.0 %).
Sequencing and re-annotation of candidate gene(s)
The coding DNA sequences (CDSs) of Vradi05g03940 and Vradi05g03950 in V2802 and KPS1 were determined. Vradi05g03940 was sequenced because it is located next to Vradi05g03950 and encodes a PGIP. In the mungbean reference sequence, the full CDS of these two genes consisted of 1302 and 1431 nucleotides, respectively.
A comparison of the CDSs showed that Vradi05g03940 from VC1973A and KPS1 (both susceptible to bruchids) were the same, whereas the sequences of V2802, V1128, V2817, and TC1966 (all resistant to bruchids) were identical but different from that in susceptible mungbean. There were 12 single nucleotide polymorphisms (SNPs) between the two mungbean groups (Supplementary Fig. S3). The SNPs at positions 854 and 913 caused amino acid changes in the bruchid-resistant mungbean accessions, while the SNP at position 1087 resulted in a premature stop codon in these accessions. The predicted PGIP encoded by Vradi05g03940 in the susceptible mungbeans consisted of 433 amino acids, while that in the resistant mungbeans consisted of only 362 amino acids (Supplementary Fig. S4). There were two changes in amino acids at positions 285 [asparagine (N) to serine (S)] and 305 [glutamic acid (E) to lysine (K)]. Vradi05g03940 showed the strongest similarity to PGIP from M. truncatula (XP_003625218.1; identities = 69 %, E-value = 3 × 10−150); therefore, we named it VrPGIP1.
We re-annotated the CDS of Vradi05g03950 because a BLAST search for the position of DMB-SSR158 showed that the transcript sequence (GenBank accession AM696152) used to develop this marker aligned to both exonic and intronic regions of this gene. We conducted a BLAST search against mungbean TSA database together with our in-house transcriptome data and found that AM696512 was almost identical to transcripts of GBXO01011683, Sulu_1 and CM. We aligned these three transcript sequences and AM696152 to the reference sequence of VC1973A and obtained a new annotation for Vradi05g03950 (Supplementary Fig. S5). The new annotated gene was found to be intronless with 1011 nucleotides encoding a protein of 336 amino acids. A blast search of this protein against the GenBank database demonstrated that, similar to VrPGIP1, it showed the highest similarity to PGIP from M. truncatula (XP_003625218.1; identities = 69 %, E-value = 3 × 10−150). Thus, we named this gene VrPGIP2.
We compared the nucleotide sequences of VrPGIP2 from GBXO01011683, Sulu-1, CM, KPS1, V2802, V1128, V2817, and TC1966, and found that it was of the same length in all of the accessions. The VrPGIP2 sequences in GBXO01011683, Sulu_1, CM, and KPS1 (all susceptible to bruchids) were identical. The VrPGIP2 sequences in V2802, V1128, V2817, and TC1966 (all resistant to bruchids) were also identical. However, the VrPGIP2 sequences differed between the resistant and the susceptible groups of cultivars (Supplementary Fig. S6). There were six SNPs (at nucleotide positions 958, 969, 972, 995, 1003, and 1008) between the two mungbean groups (Supplementary Fig. S6). The SNPs at positions 958, 995, and 1003 caused amino acid changes in VrPGIP2 at positions 320 [from alanine (A) to S], 332 [from leucine (L) to proline (P)], and 335 [from threonine (T) to P] in the resistant mungbean lines, compared with the sequence in the susceptible lines (Fig. 4).
Alignment of deduced amino acid sequences encoded by VrPGIP2 in Sulu_1, CM, an unknown accession (GBXO01011683), KPS1, V2802, V2817, V1128, and TC1966. Sulu_1, CM, GBXO01011683 and KPS1 are susceptible to bruchids, while V2802, V2817, V1128, and TC19966 are resistant to bruchids. Polymorphic sites are shown in bold
Discussion
Legume breeders and scientists have long been interested in identifying the bruchid resistance gene in mungbean. The Br locus confers resistance against five bruchid species including C. chinensis, C. maculatus, C. phaseoli (Gyllenhal), Zabrotes subfasciatus (Boheman), and Acanthoscelides obtectus (Say) (Fujii and Miyazaki 1987; Fujii et al. 1989; Lambrides and Imrie 2000), which are major storage pests of several legume crops around the world. Despite fine mapping of the Br locus 18 years ago (Kaga and Ishimoto 1998), identification of the gene(s) or candidate gene(s) responsible for resistance has been impossible due to the lack of genomic resources for mungbean. Recently, the whole-genome sequence of mungbean has been reported (Kang et al. 2014). Using this sequence together with QTL mapping and bioinformatics analyses, we were able to identify a candidate gene for Br in mungbean accession V2802.
Previous studies have shown that resistance to C. chinensis and C. maculatus in V2802 is controlled by a single dominant gene with some modifiers (Somta et al. 2007; Chotechung et al. 2011). In this study, the segregation of resistance to both insect species in BC11F2 was not consistent with monogenic inheritance, although the BC11F2 population used in this study was derived from the same BC4F2 population as that used in the study by Chotechung et al. (2011) (Fig. 1). These contrasting results may be because the BC11F2 population was a bulked population derived from five BC11F1 families, each consisting of 45–121 BC11F2 plants. The small number of plants in some families may have resulted in a deviation from the expected monogenic segregation.
Although segregation distortion of Br was evident in the BC11F2 population, QTL mapping detected a major QTL for resistance to both bruchids in V2802 in the genomic region previously identified to contain Br, as reported by Kaga and Ishimoto (1998) (Fig. 3). The QTLs for both bruchid species were localized to the same position and explained approximately 90 % of the variation in the proportion of damaged seeds. These results agreed with the high correlation between the two bruchids in terms of the proportion of damaged seeds found in this study and previously (Somta et al. 2007). The source of resistance used by Kaga and Ishimoto (1998) was the accession TC1966. Thus, the resistance gene in V2802 and that in TC1966 were predicted to be the same. The physical genome distance between markers VrBr-SSR13 and DMB-SSR158, which flank the bruchid-resistance QTL on the mungbean reference sequence, is only 38 kb, and contains two annotated genes, Vradi05g03940 and Vradi05g03950, considered as candidates for Br. Vradi05g03950 corresponding to DMB-SSR158 which co-segregated perfectly with bruchid resistance (Supplementary Table S3) and confirmed our previous result (Chotechung et al. 2011). Recently, Hong et al. (2015) reported that C. chinensis resistance in a mungbean cultivar that derived its resistance from V2709 is controlled by two tightly linked major QTLs, although our research group concluded that the resistance in this accession is controlled by a single gene (Somta et al. 2007; Chotechung et al. 2011). Nonetheless, based on blast searches of the primer sequences of the markers GBssr-MB87 and RP that were linked to each QTL as reported by Hong et al. (2015), we found that the position of GBssr-MB87 on chromosome 5 (5,602,082; identities = 94 %; E-value = 4.1) is very close to Vradi05g03950, while RP on chromosome 5 has multiple positions corresponding to the region around 5,210,000 to 5,520,000 (data not shown). Further research is required to explore the reasons for these contrasting results.
Both Vradi05g03940 and Vradi05g03950 encode a PGIP. Such enzyme inhibitor(s) play important roles in plant defense against phytophagous insects (Lawrence and Koundal 2002). For example, an α-amylase inhibitor in common bean seeds negatively affects the growth and development of C. chinensis and C. maculatus (Ishimoto and Kitamura 1989; Shade et al. 1994; Ishimoto et al. 2006). Polygalacturonase (PG) is a pectin-degrading enzyme that hydrolyzes α-1,4-glycosidic bonds between galacturonic acid units and acts preferentially on pectic acid. These proteins belong to the leucine-rich-repeat protein family. In plants, PGIPs are known to play roles in defense against disease and insects. Pectinesterases and PGs degrade pectin into oligosaccharides that can be absorbed in the insect gut. The main causes of plant damage by phytophagous insects are PGs from salivary glands (Girard and Jouanin 1999; Boyd et al. 2002). In C. maculatus, PGs are involved in digestion (Pedra et al. 2003; Pauchet et al. 2010; Nogueira et al. 2012; Santana 2013). Nogueira et al. (2012) noted that a high number of PG isoforms in larval C. maculatus found in their study and that of Pauchet et al. (2010) could be explained by the presence of PGIPs in cowpea seeds fed to the insects. They also noted that plants accumulate or induce enzyme inhibitors involved in polymer digestion, such as α-amylase inhibitors for starch and PGIPs for hemicellulose or pectin. Thus, PGs appear to play an important role in digestion in C. maculatus. In rice weevil [Sitophilus oryzae (Coleoptera: curculionidae)] feeding on cereal seeds, especially rice and wheat, pectinases including PG function as digestive enzymes to degrade pectin in the seeds as an energy source (Shen et al. 1996). In addition, degrading pectin helps other digestive enzymes to gain access to their substrates in insect food (Shen et al. 1996). The same is likely to be true for PGs in Callosobruchus weevils.
Although the VrPGIP1 protein encoded by Vradi05g03940 (VrPGIP1) differs between KPS1 and V2802, VrPGIP1 is not likely to be the gene responsible for bruchid resistance in V2802 because the SSR marker VrBr-SSR13 within this gene did not completely co-segregate with resistance (Supplementary Table S3). Also, Vradi05g03940 encodes an incomplete PGIP in V2802 and all other bruchid-resistant accessions (Supplementary Fig. S3). However, further research is necessary to determine whether the incomplete VrPGIP1 plays any role in bruchid resistance. In this study, Vradi05g03950 (VrPGIP2) was re-annotated and was found to encode a polymorphic VrPGIP2 between KPS1 and V2802. The marker DMB-SSR158, which was developed from this gene’s sequence, co-segregated perfectly with resistance, suggesting that VrPGIP2 is the gene responsible for bruchid resistance at the Br locus. However, Liu et al. (2016) recently showed that DMB-SSR158 was not completely associated with Br in recombinant inbred lines of NM92 × TC1966. The contrasting results between our study and that of Liu et al. (2016) are worthy of further investigation. Among the three polymorphic amino acids in VrPGIP2 between V2802 and KPS1 (between resistant and susceptible mungbeans) encoded by VrPGIP2, the change from S to A at position 320 is likely to play an important role in bruchid resistance. Leckie et al. (1999) showed that an S to A mutation and vice versa at this position (equivalent to position 326 of the common bean PGIP1 and PGIP2) affects the PGIP–PG interaction. Nonetheless, the perfect co-segregation between DMB-SSR158 (VrPGIP2) and Br and the VrPGIP2 polymorphisms between KPS1 and V2802 found in this study indicate that VrPGIP2 is a very strong candidate as the gene responsible for resistance at the Br locus. Gene cloning and complementation analyses should be conducted to confirm the role of VrPGIP2 in bruchid resistance.
Previous studies have shown that PGIPs play roles in the feeding behavior of Heteropteran insects. For example, PGIP3 and PGIP4 from common bean inhibit PGs of mirid bugs (Frati et al. 2006). VrPGIP2 may also confer resistance to other piercing–sucking insects by preventing the digestion of plant tissue by insect PGs. This could explain why Br confers resistance to not only bruchids, but also to pod-sucking bug [Riptortus clavatus (Thunb.)] (Heteroptera: alydidae) (Kaga and Ishimoto 1998).
The SSR marker system has become standard for MAS using standard laboratory facilities because SSRs are easy to detect (PCR-based), multi-allelic, highly polymorphic, and highly reproducible. Previously, only a few SSR markers linked to bruchid resistance have been reported for mungbean, including DMB-SSR158 (Chotechung et al. 2011; Chen et al. 2013), GBssr-MB87 (Chotechung et al. 2011; Hong et al. 2015), and SSRbr1 (Miyagi et al. 2004). In this study, we developed several SSR markers located physically very close to the Br locus, and the marker DMB-SSR158 located within the candidate gene sequence (Supplementary Table S1and Fig. 3). These SSR markers will be very useful for MAS for bruchid resistance and will save 3–4 months in each selection cycle as compared to phenotypic selection.
Author contribution statement
S.C. carried out major parts of the experiments and analyzed data. S.C., P. Somta, T.Y., and P. Srinives prepared plant materials. S.C and T.Y. evaluated bruchid resistance, J.C. and X.C. developed markers and sequenced DNA. P. Somta initiated and coordinated the study, and analyzed the results. P. Somta and P. Srinives wrote the manuscript. All authors approved the manuscript.
References
Benchimol LL, de Campos T, Carbonell SAM, Colombo CA, Chioratto AF, Formighieri EF, Gouvêa LRL, de Souza AP (2007) Structure of genetic diversity among common bean (Phaseolus vulgaris L.) varieties of Mesoamerican and Andean origins using new developed microsatellite markers. Genet Resour Crop Evol 54:1747–1762
Boyd DW Jr, Cohen AC, Alverson DR (2002) Digestive enzymes and stylet morphology of Deraeocoris nebulosus (Hemiptera: miridae), a predacious plant bug. Ann Entomol Soc Am 95:395–401
Chen HM, Liu CA, Kuo GC, Chien CM, Sun HC, Huang CC, Lin YC, Ku HM (2007) Development of a molecular marker for a bruchid (Callosobruchus chinensis L.) resistance gene in mungbean. Euphytica 157:113–122
Chen HM, Ku HS, Schafleitner R, Bains TS, Kuo GC, Liu CA, Nair RM (2013) The major quantitative trait locus for mungbean yellow mosaic Indian virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata. Euphytica 192:205–216
Chotechung S, Somta P, Chankaew S, Srinives P, Somta P (2011) Identification of DNA markers associated with bruchid resistance in mungbean. Khon Khan Agri J 39(S3):221–226 (in Thai with English abstract)
Development Core Team R (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Frati F, Galletti R, De Lorenzo G, Salerno G, Conti E (2006) Activity of endo- polygalacturonases in mirid bugs (Heteroptera: miridae) and their inhibition by plant cell wall proteins (PGIPs). Eur J Entomol 103:515–522
Fujii K, Miyazaki S (1987) Infestation resistance of wild legumes (Vigna sublobata) to azuki bean weevil, Callosobruchus chinensis (L.) (Coleoptera: bruchidae) and its relationship with cytogenetic classification. Appl Ent Zool 22:229–230
Fujii K, Ishimoto M, Kitamura K (1989) Patterns of resistance to bean weevils (bruchidae) in Vigna radiata-sublobata complex inform the breeding of new resistant varieties. Appl Entomol Zool 24:126–132
Gaitán-Solís E, Duque MC, Edwards KJ, Tohme J (2002) Microsatellite repeats in common bean (Phaseolus vulgaris): isolation, characterization and cross-species amplification in Phaseolus ssp. Crop Sci 42:2128–2136
Girard C, Jouanin L (1999) Molecular cloning of cDNAs encoding a range of digestive enzymes from a phytophagous beetle, Phaedon cochleariae. Insect Biochem Mol Biol 29:1129–1142
Grisi MCM, Blair MW, Gepts P, Brondani C, Pereira PAA, Brondani RPV (2007) Genetic mapping of a new set of microsatellite markers in a reference common bean (Phaseolus vulgaris) population BAT93 × Jalo EEP558. Genet Mol Res 6:691–706
Han OK, Kaga A, Isemura T, Wang XW, Tomooka N, Vaughan DA (2005) A genetic linkage map for azuki bean [Vigna angularis (Willd.) Ohwi & Ohashi]. Theor Appl Genet 111:1278–1287
Hong MG, Kim KH, Ku JH, Jeong JK, Seo MJ, Park CH et al (2015) Inheritance and quantitative trait loci analysis of resistance genes to bruchid and bean bug in mungbean (Vigna radiata L. Wilczek). Plant Breed Biotechnol 3:39–46
Isemura T, Kaga A, Tabata S, Somta P, Srinives P, Shimizu T et al (2012) Construction of a genetic linkage map and genetic analysis of domestication related traits in mungbean (Vigna radiata). PLoS ONE 7:e41304
Ishimoto M, Kitamura K (1989) Growth inhibitory effects of an α-amylase inhibitor from kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: bruchidae). Appl Ent Zool 24:281–286
Ishimoto M, Sato T, Chrispeels MJ, Kitamura K (2006) Bruchid resistance of transgenic azuki bean expressing seed α-amylase inhibitor of common bean. Entomol Exp Appl 79:309–315
Kaga A, Ishimoto M (1998) Genetic localization of a bruchid resistance gene and its relationship to insecticidal cyclopeptide alkaloids, the vignatic acids in mungbean (V. radiata L.Wilczek). Mol Gen Genet 258:378–384
Kang YJ, Kim S, Kim MY, Lestari P, Kim KH, Ha BK et al (2014) Genome sequence of mungbean and insights into evolution within Vigna species. Nat Commun 5:5443. doi:10.1038/ncomms6443
Kitamura K, Ishimoto M, Sawa M (1988) Inheritance of resistance to infestation with azuki bean weevil in Vigna sublobata and successful incorporation to V. radiata. Jpn J Breed 38:459–464
Kongjaimun A, Kaga A, Tomooka N, Somta P, Shimizu T, Shu Y et al (2012) An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis group) and QTL analysis of pod length. Genome 55:81–92
Lambrides CJ, Imrie BC (2000) Susceptibility of mungbean varieties to the bruchid species Callosobruchus maculatus (F.), C phaseoli (Gyll.), C chinensis (L.), and Acanthoscelides obtectus (Say) (Coleoptera: chrysomelidae). Aust J Agric Res 51:85–89
Lawrence PK, Koundal KR (2002) Plant protease inhibitors in control of phytophagous insects. Electron J Biotechnol 5(1):3. doi:10.2225/vol5-issue1-fulltext-3Electronic
Leckie F, Mattei B, Capodicasa C, Hemmings A, Nuss L, Aracri B et al (1999) The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed beta-strand/beta-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J 418:2352–2363
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374
Liu MS, Kuo TCY, Ko CY, Wu DC, Li KY, Lin WJ et al (2016) Genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol 16:46
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13
Maréchal R, Mascherpa JM, Stainier F (1978) Etude taxonomiqe d’un group d’espécies des geners Phaseolus et Vigna (Papilionaceae) sur la basedenees morphologiques, traitees, pour 1’ analyse informatique. Boisseira 28:1–278
Mei L, Cheng XZ, Wang SH, Wang LX, Liu CY, Sun L et al (2009) Relationship between bruchid resistance and seed mass in mungbean based on QTL analysis. Genome 52:589–596
Menancio-Hautea D, Kumar L, Danesh D, Young ND (1993) A genome map for mungbean (Vigna radiata L. Wilczek) based on DNA genetic markers (2 N = 2X = 22). In: O’Brien SJ (ed) Genetic maps: locus maps of complex genomes, Cold Spring Harbor Laboratory Press, New York, pp 6.259–6.261
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Miyagi M, Humphry M, Ma ZY, Lambrides CJ, Bateson M, Liu CJ (2004) Construction of bacterial artificial chromosome libraries and their application in developing PCR-based markers closely linked to a major locus conditioning bruchid resistance in mungbean (Vigna radiata L. Wilczek). Theor Appl Genet 110:151–156
Nair RM, Schafleiner R, Kenyon L, Srinivasan R, Easdown W, Ebert AW, Hanson P (2012) Genetic improvement of mungbean. SABRAO J Breed Genet 45:177–190
Nogueira FCS, Silva CP, Alexandre D, Samuels RI, Soares EL, Aragão FJL et al (2012) Global proteome changes in larvae of Callosobruchus maculatus (Coleoptera: chrysomelidae: bruchinae) following ingestion of a cysteine proteinase inhibitor. Proteomics 12:2704–2715
Pauchet Y, Wilkinson P, Chauhan R, Ffrench-Constant RH (2010) Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One 5:e15635
Pedra JHF, Brandt A, Westerman R, Lobo N, Li HM, Romero-Severson J, Murdock LL, Pittendrigh BR (2003) Transcriptome analysis of the cowpea weevil bruchid: identification of putative proteinases and alpha-amylases associated with food breakdown. Insect Mol Biol 12:405–412
Santana MC (2013) Identificação de poligalacturonases expressas no sistema digestório de Callosobruchus maculatus (Coleoptera: chrysomelidae: bruchinae). Ph.D. Thesis, Universidade Federal de Santa Catarina, Brazil
Shade RE, Schroeder HE, Pueyo JJ, Tabe LL, Murdock TJV, Higgins MJ, Chrispeels MJ (1994) Transgenic pea seeds expressing the α-amylase inhibitor of the common bean are resistant to bruchid beetles. Bio/Technol 12:793–796
Shen Z, Reese JC, Reeck GR (1996) Purification and characterization of polygalacturonase from the rice weevil, Sitophilus oryzae (Colecoptera: curculionidae). Insect Biochem Mol Biol 26:427–433
Somta P, Srinives P (2007) Genome research in mungbean [Vigna radiata (L.) Wilczek] and blackgram [V. mungo (L.) Hepper]. Sci Asia 33(S1):69–74
Somta P, Ammaranan C, Ooi PAC, Srinives P (2007) Inheritance of seed resistance to bruchids in cultivated mungbean (Vigna radiata (L) Wilzcek). Euphytica 155:49–55
Somta C, Somta P, Tomooka N, Ooi PAC, Vaughan DA, Srinives P (2008) Characterization of new sources of mungbean (Vigna radiata (L) Wilczek) resistance to bruchids, Callosobruchus spp (Coleoptera: bruchidae). J Stored Prod Res 44:316–321
Somta P, Seehalak W, Srinives P (2009) Development, characterization and cross-species amplifcation of mungbean (Vigna radiata) genic microsatellite markers. Conserv Genet 10:1939–1943
Srinives P, Somta P, Somta C (2007) Genetics and breeding of resistance to bruchids (Callosobruchus spp) in Vigna crops: a review. NU Int J Sci 4:1–17
Sun L, Cheng XZ, Wang SH, Wang LX, Liu CY, Mei L, Xu N (2008) Heredity analysis and gene mapping of bruchid resistance of a mungbean cultivar V2709. Agric Sci China 7:672–677
Talekar NS, Lin CL (1992) Characterization of Callosobruchus chinensis (Coleoptera: bruchidae) resistance in mungbean. J Econ Entomol 85:1150–1153
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40(15):e115
Young N, Kumar L, Menancio-Hautea D, Danesh D, Talekar NS, Shanmugasundaram S, Kim DH (1992) RFLP mapping of a major bruchid resistance gene in mungbean (Vigna radiata, L Wilczek). Theor Appl Genet 84:839–844
Yu K, Park SJ, Poysa V, Gepts P (2000) Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J Hered 91:429–434
Zhao D, Cheng XZ, Wang LX, Wang SH, Ma YL (2010) Integration of mungbean (Vigna radiata) genetic linkage map. Acta Agron Sin 36:932–939
Acknowledgments
This research was funded by the Center for Advanced Studies for Agriculture and Food (CASAF), Kasetsart University under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand to P. Somta. We also acknowledge initial support from Thailand’s National Science and Technology Development Agency to P. Srinives.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The experiments carried out in this study comply with the current laws of Thailand.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Matthew N Nelson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chotechung, S., Somta, P., Chen, J. et al. A gene encoding a polygalacturonase-inhibiting protein (PGIP) is a candidate gene for bruchid (Coleoptera: bruchidae) resistance in mungbean (Vigna radiata). Theor Appl Genet 129, 1673–1683 (2016). https://doi.org/10.1007/s00122-016-2731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2731-1