Abstract
Key message
Three novel QTL for peroxidase activity were mapped, and gene-specific markers for TaPod-A1 were developed and validated using RILs derived from the Doumai/Shi 4185 cross and 281 wheat cultivars. TaPod-A1 is within one of the three QTL.
Abstract
Peroxidase (POD) activity in grain is an important factor determining the color of flour and end-use products of wheat, such as noodles and steamed bread. Mapping QTL for POD activity, characterization of POD genes and development of gene-specific markers are important for molecular marker-assisted selection in wheat breeding. Quantitative trait loci (QTL) for POD activity in common wheat were mapped using a recombinant inbred line (RIL) population derived from a Doumai/Shi 4185 cross grown in four environments and genotyped using the wheat 90 K iSelect assay. Three novel QTL for POD activity, QPod.caas-3AL, QPod.caas-4BS and QPod.caas-5AS, were identified on chromosomes 3AL, 4BS and 5AS, explaining 5.3–21.2 % of phenotypic variance across environments. The full-length genomic DNA (gDNA) sequence of a POD gene, designated TaPod-A1, on chromosome 3A was characterized by homolog cloning and PCR verification. Two complementary dominant sequence-tagged site (STS) markers, POD-3A1 and POD-3A2, were developed based on single nucleotide polymorphisms (SNPs) between two alleles at the TaPod-A1 locus, amplifying 291- and 766-bp fragments in cultivars with lower and higher POD activities, respectively. The two gene-specific markers were mapped on chromosome 3AL using a set of Chinese Spring (CS) nulli-tetrasomic lines, and ditelosomic lines 3AL and 3AS. QTL analysis indicated that QPod.caas-3AL co-segregated with the gene-specific markers POD-3A1 and POD-3A2. POD-3A1 and POD-3A2 were verified on 281 wheat cultivars and advanced lines, and showed significant (P < 0.05) associations with POD activities. POD-3A1 and POD-3A2 may be useful as markers for improving color attributes in wheat breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Class III plant POD is a plant-specific oxidoreductase (Hiraga et al. 2001). The plant POD superfamily is a ubiquitous group of heme-containing cytochrome b proteins, classified on the basis of conserved domains as class III secretory peroxidases to distinguish them from class I plant ascorbate peroxidases, and class II fungal peroxidases (Blee et al. 2001). It is particularly abundant in three major kernel fractions of durum wheat, viz. semolina, bran and embryo (Fraignier et al. 2000). POD contains an active site cysteine that is sensitive to oxidation by H2O2 (Rhee et al. 2012). Class III POD in grain is involved in seed development, and is associated with flour quality (Borrelli et al. 2003; Takasaki et al. 2005).
Ferulic acid is a substrate for POD. In fact, POD catalyzes the formation of diferulic acid to produce dark-colored products, which are undesirable for most classes of noodles and steam breads (Fry 1986; Taha and Sagi 1987; Fraignier et al. 2000). POD activity is highly and positively correlated with the brown index of pasta products (r = 0.84–0.97) (Kobrehel et al. 1974; Hemalatha et al. 2007). POD can lead to an increased brown hue, which reduces semolina and pasta yellowness (Kobrehel et al. 1974; Matsuo and Dexter 1980; Taha and Sagi 1987; Borrelli et al. 1999). Fortunately, POD shows no activity during pasta processing, probably due to the lack of availability of hydrogen peroxide (Icard-Vernière and Feillet 1999; Feillet et al. 2000). Carotenoid pigment degradation is also influenced by POD, which can oxidize a large number of compounds at the expense of hydrogen peroxide (Fraignier et al. 2000). Therefore, POD can influence the degree of yellowness in flour end-products (Borrelli et al. 1999; Trono et al. 1999; Feillet et al. 2000; Hessler et al. 2002). It can replace benzoyl peroxide as a bread dough-bleaching agent, but pure and commercial POD is screened based on degradation of β-carotin. POD has the best bleaching activity in bread dough. For 100 g of flour, combinations of peroxidase (3000 U), lipase (815–1630 U), and linoleic acid (0–300 mg) can completely bleach bread dough (Gélinas et al. 1998). POD activity is higher in bran than that in wheat debranned flour, and POD can significantly increase dough hardness and decrease adhesiveness (Žilić et al. 2012; Revanappa et al. 2014). Thus, the bran fraction of wheat potentially provides naturally occurring antioxidants. For health benefits, a small amount of bran incorporated in bread can also increase dietary fiber and phytonutrients in the diet (Žilić et al. 2012). Therefore, POD can be a more important trait for wheat products made from whole meal flours.
POD activity in wheat depends on genotype and is slightly modified by growing conditions (Feillet et al. 2000). Among gramineous crops, POD activity is the highest in common wheat grain, threefold that of oat (Avena sativa L.), sixfold that of rice (Oryza sativa L.) and sevenfold that of corn (Zea mays L.) (Maksimov et al. 2010). POD activity in common wheat is higher than durum and there are large differences between common wheat cultivars (Borrelli et al. 2003; Hemalatha et al. 2007; Žilić et al. 2012).
Class III POD genes have been cloned and sequenced in rice (O. sativa L.) (Passardi et al. 2004), barley (Hordeum vulgare L.) (Johansson et al. 1992; Theilade and Rasmussen 1992) and common wheat (http://www.ncbi.nlm.nih.gov/nuccore/AF525425). The POD gene prx23 (GenBank accession BN000552) located on chromosome 1 was isolated from rice (Passardi et al. 2004). Johansson et al. (1992) cloned POD gene Prx5 (M73234) from barley, and mapped it on chromosome 3H. Theilade and Rasmussen (1992) cloned another POD gene, Prx6 (M83671), also on chromosome 3H. A complementary DNA (cDNA) sequence, WSP1 (AF525425), was cloned from common wheat. POD genes were also reported on chromosomes 2A, 2B, 2D, 4B, 7A and 7D using CS nulli-tetrasomic lines (Kobrehel and Feillet 1975; Li et al. 1999). Endosperm peroxidases were located on chromosomes 7DS, 4AL and 7AS, whereas embryo plus scutellum isozymes were on chromosomes 3AL, 3BL and 3DS (Bosch et al. 1987). Recently, the bread wheat A-genome progenitor Triticum urartu and D-genome progenitor Aegilops tauschii draft genome sequences were reported (Brenchley et al. 2012; Jia et al. 2013; Ling et al. 2013). These can greatly aid gene cloning in the large and complicated genomes of various wheat species.
Gene-specific markers can be used for accurate discrimination of contrasting alleles and are widely used in marker-assisted selection for quality improvement in wheat (Liu et al. 2012). However, up to now, there is no information available on QTL analyses of POD activity, gDNA sequences of POD genes, and development of gene-specific markers in common wheat and durum. Thus, the objectives of this study were to map QTL for POD activity in common wheat, clone the gDNA of a POD gene, identify allelic variants, and develop gene-specific markers based on reported wheat genomics sequencing.
Materials and methods
Plant materials
Two hundred and fourteen recombinant inbred lines (RILs) derived from the cross Doumai/Shi 4185 were used for QTL mapping of POD activity. Doumai is a common wheat line with relatively high POD activity (506.5 Ug−1 min−1), whereas Shi 4185 has lower POD activity (421.5 Ug−1 min−1). A set of CS nulli-tetrasomic and ditelosomic 3AL and 3AS lines was used to verify the chromosomal location of STS markers. Two hundred and eighty-one leading cultivars and advanced lines from China and other countries (42 foreign winter wheat cultivars, 40 Chinese cultivars from the Northern China Plain Winter Wheat Region (Region I, Table S2), 19 foreign facultative cultivars, 132 Chinese cultivars from the Yellow and Huai River Valley Facultative Wheat Region (Region II, Table S2), and 48 Chinese cultivars from the Yangtze River Valley and Southwestern China Autumn-sown Spring Wheat Region (Region III, Table S2) were used to determine the association between the allelic variation and POD activity. The three regions mentioned produce around 95 % of Chinese wheat production.
Field trials
During the 2012–2013 and 2013–2014 cropping seasons, 214 RILs from Doumai/Shi 4185 were sown at Beijing and Shijiazhuang in Hebei province, providing data for four environments. During the two seasons, 82 cultivars and advanced lines from Region I were sown at Beijing and Shijiazhuang, 151 cultivars and advanced lines from Region II were sown at Anyang in Henan province and Suixi in Anhui province, and 48 cultivars and advanced lines from Region III were sown at Chengdu in Sichuan province and Suixi. All field trials were managed in randomized complete blocks with three replicates, and grains from two replicates were used for POD activity assay. Each plot contained three 2-m-length rows spaced 20 cm apart, with 50 plants in each row. All tested samples were sound and free of sprouting damage.
POD activity assay
POD activity in wheat grains was assayed following Fraignier et al. (2000), Borrelli et al. (2003) and Hemalatha et al. (2007) with some modifications. Wheat grains (about 1.2 g) were milled to whole meal with a high-throughput tissue grinder (Spex 2010 Geno/Grinder, USA; http://www.spexsampleprep.com) and used for estimating POD activity within 1 day. Enzyme was extracted by mixing 0.5 g meal with 5 mL of 0.1 M sodium phosphate buffer (pH 7.5), stirring for 2 h in an ice-water bath and centrifuging at 10,000 rpm at 4 °C for 15 min. The supernatant containing POD was used for assaying POD activity with 25 μL of 2 % hydrogen peroxide, 5 μL of 2 % guaiacol in 175 μL of reaction mixture at its optimum pH of 5.0 in 50 mM citrate phosphate buffer. After blending, 5 μL of POD extract was added. Each sample was assayed in duplicate. If the difference between two repeats was more than 10 % for the spectrophotometric assay of POD activity, a third test was conducted (Geng et al. 2012).
POD activity was determined by measuring increased absorbance at 470 nm with an Absorbance Microplate Reader (SpectraMax Plus 384, Molecular Devices, LLC, USA; http://www.moleculardevices.com ). The total time of test was 150 s and the interval reading time was 10 s. An enzyme linked immunosorbent assay (ELISA) plate (96 Well EIA/RIA Plate, Costar 3590, USA; http://www.corning.com/lifesciences) was used. One unit of POD activity was defined as a one-hundredth increase in absorbance at 470 nm min−1 g−1 of whole wheat meal between 50 and 150 s under assay conditions. For each score, POD activity was determined in duplicate extracts of whole wheat meal with parallel spectrophotometric measurements, and mean values are reported.
SNP genotyping and QTL detection
The 214 RILs and their parents were genotyped by the 90 K iSelect SNP assay at CapitalBio Corporation (Beijing, China; http://www.capitalbio.com). Genotypic clusters for each SNP were determined using the manual option of Genome Studio version 1.9.4 with the polyploid clustering version 1.0.0 (Illumina) (http://www.illumina.com), based on data from all genotypes. Markers not polymorphic between Doumai and Shi 4185 and those with high percentages of heterozygous genotypes were discarded. Markers with over 20 % of missing data were also removed. Linkage groups were established with the software JoinMap 4.0 (Stam 1993; http://www.kyazma.nl) and maps were made by MapChart 2.2 (Voorrips 2002; http://www.earthatlas.mapchart.com). Map distances (in centiMorgans, cM) between markers were calculated by the Kosambi (Kosambi 1944) mapping function. POD activities of the Doumai/Shi 4185 RIL population were measured in four environments during two cropping seasons for QTL analysis, which was conducted using the software IciMapping 4.0 (Li et al. 2007; http://www.isbreeding.net), and a logarithm of odds (LOD) value of 2.5 was used for declaring definitive QTL. Individual environment QTL overlapping within a 20-cM interval were considered common.
Cloning and identifying allelic variants of a POD gene on chromosome 3AL
The corresponding cDNA sequences of common wheat POD gene wsp1 (GenBank accession AF525425), rice POD gene prx23 (BN000552) and barley POD gene Prx6 (M83671) were used for BLAST searching against the wheat genome sequences database in the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena). All wheat genome scaffold sequences sharing high similarity with the reference gene were subjected to overlapping sequence assembly (E value <10−50, score >400 bp, and identify >70 %, ENA, 2013). Based on sequence alignment, two primer sets, POD1 and POD2 (Table S1), were designed with the expectation that amplified fragments from gDNA could cover the whole coding sequence (CDS) using the two primer sets. Primers were designed using Primer Premier version 5.0 (Premier Biosoft, 2013). All primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China; http://www.sangon.com).
Genomic DNA was extracted from seeds using a method modified from Lagudah et al. (1991). PCR were performed in an MJ Research PTC-200 thermal cycler in volumes of 20 μL including 10 μL 2 × GC buffer I, 100 μM of each dNTP, 2 pmol of each primer, 1 U of ExTaq, and 80 ng of template DNA. All reagents were obtained from Takara Biotechnology (Dalian) Co., Ltd. (Dalian, China; http://www.takara.com.cn). PCR conditions were 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 64 °C for 30 s, and 72 °C for 1 min, with a final extension of 72 °C for 8 min. The PCR products were separated by electrophoresis in a 1.5 % agarose gel, stained with ethidium bromide and visualized using ultraviolet (UV) light. Fragments with the expected sizes were recovered and cloned into the pEASY-T1 Simple Cloning Vector (Beijing TransGen Biotech Co., Ltd., Beijing, China; http://www.transgen.com.cn) and sequenced by Beijing Genomics Technology Co., Ltd. (Beijing, China; http://www.genomics.cn) with at least three repeats for each clone.
Cultivars Linmai 2, Zhongyou 9507, Aikang 58 and Xiaoyan 22 with a diverse range of POD activities were used to clone the full-length sequence of the TaPod-A1 gene on chromosome 3A, and to identify allelic variations at the locus. Each primer combination was confirmed to be chromosome-specific using CS nulli-tetrasomic lines. The PCR and DNA sequencing were repeated three to four times for each primer set to ensure the accuracy of nucleotide sequences. The genomic DNA sequences and deduced amino acids for different allelic variants of the TaPod-A1 gene were aligned using the software Geneious (Biomatters Ltd., New Zealand; http://www.geneious.com). Intron positions were also determined by alignment of the amplified genomic DNA sequence and cDNA sequence using the software Geneious (Biomatters Ltd., New Zealand; http://www.geneious.com ).
Sequence-tagged site marker analysis
Five cultivars with higher POD activities and five cultivars with lower POD activity were used for developing STS markers. Based on the sequence polymorphisms between the two alleles at the TaPod-A1 locus, two primer sets were designed to identify variants among these cultivars. The divergence of phenotypic values was assumed to be associated with TaPod-A1, which was then validated using 281 cultivars and advanced lines and also confirmed with 214 RILs. The PCR for one primer set was 94 °C for 5 min followed by 35 cycles of touchdown PCR at 94 °C for 30 s, 68–57.5 °C for 30 s with 0.3 °C decreases per cycle, and 72 °C for 1 min, with a final extension of 72 °C for 8 min, and the other was 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 1 min, with a final extension of 72 °C for 8 min.
Statistical analyses
For the 214 RILs derived from Doumai/Shi 4185 and 281 cultivars and advanced lines, the POD activity of each line or cultivar was measured in four environments, and the arithmetic means of each line were used for subsequent QTL mapping or analysis of variance (ANOVA). ANOVA and computation of correlation coefficients were conducted by the SAS System for Windows version 9.0 (SAS Institute, http://www.sas.com). The contributions of lines (RILs) and environments were evaluated by PROC MIXED, where environments were treated as fixed effects, and line, line × environment interaction and replicate nested in environments were all treated as random. Broad-sense heritability (h 2) for POD activity was calculated using the formula; h 2 = σ 2g /(σ 2g + σ 2ge /r +σ 2ε /re), where σ 2g , σ 2ge and σ 2ε were estimates of line, line × environment interaction and residual error variances, respectively, and e and r were the numbers of environments and replicates per environment, respectively. A total of 281 cultivars and advanced lines were used to verify the association between POD activity and allelic variation. Differences in POD activity among genotypes (TaPod-A1 alleles) with different PCR band profiles were also conducted by PROC MIXED, with genotypes as a categorical variable to derive the mean POD activity for each class and to test the differences for the two alleles. The genotypes indicated by two TaPod-A1 alleles, environments, and their interaction were treated as fixed effects, while cultivars nested in TaPod-A1 alleles, cultivar (nested in TaPod-A1 alleles) × environment interaction and replicates nested in environments were random. The random effect was analyzed with a Z test in the MIXED model.
Results
Phenotypic evaluation of the Doumai/Shi 4185 RIL population
POD activities were significantly (P < 0.0001) affected by line, environment and their interaction (Table 1). They were significantly correlated (P < 0.0001) across four environments, with correlation coefficients ranging from 0.41 to 0.64. Heritability of POD activity was 0.77, indicating that POD activity is mainly affected by genetic factors and that selection for POD activity can be effectively applied at the early generation stage of a wheat breeding program. The frequency distribution of POD activity in the RIL population in different environments showed continuous variation (Fig. S1).
QTL for POD activity
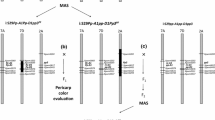
Of 81,587 wheat SNP markers in the 90 K chip, 74,196 were either non-polymorphic between two parents Doumai and Shi 4185 or had a high percentage of heterozygous genotypes or missing data. The RIL population was finally genotyped with 7391 SNP markers and a new STS marker developed in this study. These were assigned into 21 linkage groups. Three QTL for POD activity detected in the RIL population across the four environments and the averaged data, were designated QPod.caas-3AL, QPod.caas-4BS and QPod.caas-5AS, on chromosomes 3AL, 4BS and 5AS, respectively (Table 2; Fig. 1; Fig. S2). The alleles for increasing POD activities at the QPod.caas-3AL and QPod.caas-4BS loci came from Doumai, and that at the QPod.caas-5AS locus came from Shi 4185. These QTL accounted for 7.3–31.4 % of the total phenotypic variance in a simultaneous fit across the four environments and averaged data from all environments (Table 2). QPod.caas-3AL was flanked by Excalibur_c6906_1167 and POD-3A1/POD-3A2 with a genetic distance of 2.3 cM, and explained 6.0, 7.3, 7.4, 5.3 and 9.2 % of the phenotypic variance for POD activity in Beijing 2013, Shijiazhuang 2013, Beijing 2014, Shijiazhuang 2014 and the averaged data, respectively (Fig. 1; Table 2). QPod.caas-4BS was flanked by BS00026143_51 and Excalibur_c17607_542 with a genetic distance of 9.8 cM, and explained 9.3, 13.3, 21.2 and 16.0 % of the phenotypic variance for POD activity in Beijing 2013, Beijing 2014, Shijiazhuang 2014 and the averaged data, respectively (Fig. 1; Table 2). QPod.caas-5AS was flanked by wsnp_Ex_c16551_25061517 and CAP8_s9855_165 with a genetic distance of 10.8 cM, and explained 11.7, 5.8 and 6.2 % of the phenotypic variance for POD activity in Beijing 2013, Beijing 2014 and the averaged data, respectively (Fig. 1; Table 2).
Chromosomes 3A, 4B and 5A linkage maps for the Doumai/Shi 4185 RIL population with QTL for POD activity indicated by green bars. LOD thresholds for declaring significant QTL were 2.5. The short arms of chromosomes were toward the top. The STS markers POD-3A1/POD-3A2 on chromosome 3AL developed in this study were linked to Excalibur_c6906_1167 with a genetic distance of 2.3 cM
Allelic variants at TaPod-A1 locus
One genomic scaffold, Triticum_urartu scaffold 33924, was detected through a BLAST search using the common wheat wsp1 (AF525425), rice prx23 (BN000552) and barley Prx6 (M83671) sequences as probes. Based on the sequence, two chromosome-specific primer sets were developed to clone the full-length gDNA sequence of a POD gene. Each primer set was tested with a set of CS nulli-tetrasomic lines. Primer sets POD1 and POD2 amplified PCR fragments of 743 and 657 bp, respectively, in CS nulli-tetrasomic lines except N3A-T3B and ditelosomic line 3AL; no PCR products were obtained in N3A-T3B and DT3AS (Fig. S3; Figs. S4a, b), indicating that the TaPod-A1 gene was located on chromosome 3AL. The PCR fragment amplified by POD1 had 150 bp overlap with that amplified by POD2.
The complete gDNA sequence of TaPod-A1 comprised 1250 bp, with two exons and one intron as well as the 5′ and 3′ flanking sequences. A comparison of the gDNA and cDNA (ENA accession GAKM01059098) sequences indicated two exons of TaPod-A1 of 270 and 834 bp, respectively, whereas the intron size was 76 bp. The intron sequences bordering the exon–intron junctions conformed to the GT–AG rule. The exon–intron structure of TaPod-A1 was very similar to those of rice prx23 (AF525425) and barley Prx6 (M83671) (Fig. S5), indicating a close phylogenetic relationship. The cDNA sequence of the cloned TaPod-A1 was 1,174 bp, possessing an intact open reading frame (ORF) of 1104 bp, a 34-bp 5′ untranslated region (UTR) and a 36-bp 3′ UTR. Two alleles, designated TaPod-A1a and TaPod-A1b (Fig. S6), were amplified from cultivars with lower and higher POD activities, respectively. Five single nucleotide polymorphisms were found between two alleles in the exons of TaPod-A1, with the first mutation from A to G at the 138th base, the second from G to T at the 237th, the third from A to G at the 462th, the fourth from A to G at the 769th and the fifth from T to A at the 866th base. The deduced peptide sequences of TaPod-A1a and TaPod-A1b comprised 367 amino acid residues with predicted molecular weights of 39.2 and 39.3 kDa, respectively. They shared 99.5 % sequence identity, with two different amino acid residues at positions 129 and 264.
Development and validation of gene-specific markers for TaPod-A1
Based on the first, second and fifth SNP in TaPod-A1a and TaPod-A1b (Fig. S6), two complementary dominant markers, designated POD-3A1 and POD-3A2 (Table S1), were developed. POD-3A1 designed from the TaPod-A1a sequence, amplified a 291-bp PCR fragment in cultivars with lower POD activities, but produced no PCR products in those with higher POD activities (Fig. S7). In contrast, POD-3A2, developed from TaPod-A1b, yielded a 766-bp fragment in cultivars with higher POD activities, but no PCR products in those with lower POD activities (Fig. S7). Thus, POD-3A1 and POD-3A2 were complementary markers. The locations of gene-specific markers POD-3A1 and POD-3A2 were verified with the CS nulli-tetrasomic and ditelosomic 3AL and 3AS lines. The results confirmed that POD-3A1, POD-3A2 and the corresponding TaPod-A1 were located on chromosome 3AL (Fig. S4c; Fig. S8), in agreement with the linkage analysis based on SNP markers (Fig. 1).
Among the 281 cultivars and lines screened by POD-3A1 and POD-3A2, TaPod-A1a and TaPod-A1b were observed in 156 and 125 cultivars, respectively (Table S2). The analysis of variance (ANOVA) indicated a significant influence of genotypes (TaPod-A1a and TaPod-A1b) on POD activities. The mean POD activities of cultivars with TaPod-A1a (707.7, 633.6, 636.1 U min−1 g−1 for Region I, II and III, respectively) were significantly lower than those with TaPod-A1b (763.4, 711.0, 719.0 U min−1 g−1 for Region I, II and III, respectively) (P < 0.0001 for Region II; P < 0.05 for Regions I and III) during two cropping seasons (Table 3).
Discussion
QTL analysis based on 90 K iSelect SNP assay
SNPs are the most abundant form of DNA sequence variation. Once SNPs are discovered, it is possible to genotype them using high-throughput automated methods (Ravel et al. 2006). A multiplex genotyping method could be used in polyploid wheat species. One way is to use existing high-throughput multiplex technologies, such as SNPlex™, for the analysis of polyploid genomes, such as wheat (Bérard et al. 2009). In common wheat, SNP studies have been limited to single gene or DNA fragments allowing association studies or genetic mapping (Giroux and Morris 1997; Peng et al. 1999; Morris 2002; Yanagisawa et al. 2003; Guillaumie et al. 2004; Boisson et al. 2005). The size of the common wheat genome and its allohexaploidy make verification of SNPs difficult, because variations in allelic sequences may be confounded by homoeologous (difference between the copies of the A, B and D genomes) and paralogous (difference in duplicate copies that may exist within a given genome) variation (Ravel et al. 2006). The 90 K iSelect SNP genotyping assay resulted in a dramatic improvement in genotyping populations and generation of genetic maps. In this study, we were able to map 7393 polymorphic and homozygous SNPs between the parent cultivars Doumai and Shi 4185 out of the total 81,587 markers in our mapping population. Unfortunately, this led to some gaps in the linkage map, resulting in difficulties for the linkage and QTL analysis.
QTL for wheat grain POD activity have not been reported previously, so the three QTL QPod.caas-3AL, QPod.caas-4BS and QPod.caas-5AS are new. Veldboom and Lee (1996) indicated that QTL identified across more than one environment or those identified using data pooled over environments are useful for marker-assisted selection. QTL effects across environments were influenced by the environment, leading to genotype by environment interaction (Malosetti et al. 2008). In the present study, all three QTL for POD activity, QPod.caas-3AL, QPod.caas-4BS and QPod.caas-5AS, were consistently detected across two or more environments, indicating that they were stable across environments and had a significant effect on POD activity. The QTL QPod.caas-3AL co-segregated with POD-3A1/POD-3A2, and the peak of LOD contours for the maximum LOD value corresponded with the gene-specific markers (Fig. S2). This indicated that TaPod-A1 may be the causal gene corresponding to QPod.caas-3AL for the POD activity. The association analysis also exhibited that cultivars with the allele TaPod-A1b had significantly higher (P < 0.05) POD activity than those with TaPod-A1a, validating the association of TaPod-A1 with POD activity (Table 3). TaPod-A1 was at the distal end of the linkage map (Fig. S2), but it was not in the telomere region on chromosome 3A according to the wheat consensus SNP map (Wang et al. 2014). On the consensus map, over ten SNP markers were present on the distal side of TaPod-A1; unfortunately, these were not polymorphic between the two parents of the Doumai/Shi 4185 population.
Sequence divergence of allelic variants with POD activity at the TaPod-A1 locus
Compared with the allele TaPod-A1a associated with lower POD activity, TaPod-A1b associated with higher POD activity has five point mutations, indicating that the POD gene on chromosome 3A is highly conserved in DNA sequence. The first, second and fourth SNPs are same-sense mutations, whereas the third and fifth SNPs are missense mutations, resulting in changes from asparagine to glycine at the 129th amino acid and from serine to threonine at the 264th amino acid in the second exon. The reduction potential of peroxidase molecules is regulated by the ligand state of axial ligands and heme (Goodin and McRee 1993; Poulos et al. 1995). A strong hydrogen bonding force between Ser and Asp makes the histidine residue deviate from the hemoglobin plane and greatly weakens the binding force with Fe (Piontek et al. 1993). The increased reduction potential of peroxidase molecules made oxidation by hydrogen peroxide difficult and this may result in a lower peroxidase activity. These putative shifts in function are consistent with an association to our POD QTL.
Based on the first, second and fifth SNPs between two allelic variants at TaPod-A1 locus, we designed the gene-specific markers POD-3A1/POD-3A2 and tested 281 cultivars from three wheat regions, and the results indicated that the cultivars with TaPod-A1b had significantly higher POD activity (P < 0.05) than those with TaPod-A1a allele during two seasons (Table 3). These results indicated that the gene-specific markers were able to differentiate two QTL haplotypes-associated low and high enzyme activity for wheat cultivars. Further investigation, such as gene complementation or in vitro assays of purified enzymes, is required to definitively determine the role of TaPod-A1 in the 3AL QTL. Because several genes are responsible for POD activities in common wheat (Kobrehel and Feillet 1975; Bosch et al. 1987; Li et al. 1999), TaPod-A1 is only one of them, and it can simply explain part of the phenotypic variances, thus the two alleles TaPod-A1a and TaPod-A1b do not precisely correspond to low and high enzyme activity in the cultivars. Sequence polymorphisms between two alleles may affect mRNA expression, which will be studied in the future. Further investigation is required to discover and validate additional POD modifying genes. In the meantime, POD-3A1 and POD-3A2 exhibited high associations with lower and higher POD activities, respectively, and can be used in wheat breeding programs aimed at improvement of flour color for various wheat-based products.
Author contribution statement
J. X. Wei performed the experiment and wrote the paper. J. D. Liu and W. E. Wen did field experiment. H. W. Geng, Y. Zhang, Y. Zhang, X. C. Xia, X. M. Chen and Z. H. He designed the experiment and wrote the paper.
Abbreviations
- ANOVA:
-
Analysis of variance
- cDNA:
-
Complementary DNA
- CDS:
-
Coding sequence
- CS:
-
Chinese Spring
- gDNA:
-
Genomic DNA
- LOD:
-
Logarithm of odds
- mRNA:
-
Message RNA
- ORF:
-
Open reading frame
- PCR:
-
Polymerase chain reaction
- POD:
-
Peroxidase
- QTL:
-
Quantitative trait loci/locus
- RIL:
-
Recombinant inbred line
- SNP:
-
Single nucleotide polymorphism
- STS:
-
Sequence-tagged site
- UTR:
-
Untranslated region
- UV:
-
Ultraviolet
References
Bérard A, Le Paslier MC, Dardevet M, Exbrayat-Vinson F, Bonnin I, Cenci A, Haudry A, Brunel D, Ravel C (2009) High-throughput single nucleotide polymorphism genotyping in wheat (Triticum spp.). Plant Biotechnol J 7:364–374
Blee KA, Jupe SC, Richard G, Zimmerlin A, Davies DR, Paul Bolwell G (2001) Molecular identification and expression of the peroxidase responsible for the oxidative burst in French bean (Phaseolus vulgaris L.) and related members of the gene family. Plant Mol Biol 47:607–620
Boisson M, Mondon K, Torney V, Nicot N, Laine AL, Bahrman N, Gouy A, Daniel-Vedele F, Hirel B, Sourdille P, Dardevet M, Ravel C, Gouis L (2005) Partial sequences of nitrogen metabolism genes in hexaploid wheat. Theor Appl Genet 110:932–940
Borrelli GM, Troccoli A, Di Fonzo N, Fares C (1999) Durum wheat lipoxygenase activity and other quality parameters that affect pasta colour. Cereal Chem 76:335–340
Borrelli GM, De Leonardis AM, Fares C, Platani C, Di Fonzo N (2003) Effect of modified processing conditions on oxidative properties of semolina dough and pasta. Cereal Chem 80:225–231
Bosch A, Vega C, Benito C (1987) The peroxidase isozymes of the wheat kernel: tissue and substrate specificity and their chromosomal location. Theor Appl Genet 73:701–706
Brenchley R, Spannagl M, Pfeifer M, Barker GLA, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Huo N, Luo MC, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dvorak J, McCombie WR, Hall A, Mayer KFX, Edwards KJ, Bevan MW, Hall N (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491:705–710
Feillet P, Autran JC, Icard-Vernière C (2000) Mini review pasta brownness: an assessment. J Cereal Sci 32:215–233
Fraignier MP, Michaux-Ferrière N, Kobrehel K (2000) Distribution of peroxidase in durum wheat (Triticum durum). Cereal Chem 77:11–17
Fry SC (1986) Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol 37:165–186
Gélinas P, Poitras E, McKinnon CM, Morin A (1998) Oxido-reductases and lipases as dough-bleaching agents. Cereal Chem 75:810–814
Geng HW, Xia XC, Zhang LP, Qu YY, He ZH (2012) Development of functional markers for a lipoxygenase gene TaLox-B1 on chromosome 4BS in common wheat. Crop Sci 52:568–576
Giroux MJ, Morris CF (1997) A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor Appl Genet 95:857–864
Goodin DB, McRee DE (1993) The Asp–His–iron triad of cytochrome c peroxidase controls the reduction potential electronic structure, and coupling of the tryptophan free radical to the heme. Biochemistry 32:3313–3324
Guillaumie S, Charmet G, Linossier L, Torney V, Robert N, Ravel C (2004) Colocation between a gene coding for the bZip factor SPA and an eQTL for a high-molecular-weight glutenin subunit in wheat (Triticum aestivum). Genome 47:705–713
Hemalatha MS, Manu BT, Bhagwat SG, Leelavathi K, Prasada Rao UJS (2007) Protein characteristics and peroxidase activities of different Indian wheat varieties and their relationship to chapatti-making quality. Eur Food Res Technol 225:463–471
Hessler TG, Thomson MJ, Benscher D, Nachit MM, Sorrells ME (2002) Association of a lipoxygenase locus, Lpx-B1, with variation in lipoxygenase activity in durum wheat seeds. Crop Sci 42:1695–1700
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Icard-Vernière C, Feillet P (1999) Effects of mixing conditions on pasta dough development and biochemical changes. Cereal Chem 76:558–565
Jia JZ, Zhao SC, Kong XY, Li YR, Zhao GY et al (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Johansson A, Rasmussen SK, Harthill JE, Welinder KG (1992) cDNA, amino acid and carbohydrate sequence of barley seed-specific peroxidase BP 1. Plant Mol Biol 18:1151–1161
Kobrehel K, Feillet P (1975) Identification of genomes and chromosomes involved in peroxidase synthesis of wheat seeds. Can J Bot 53:2336–2344
Kobrehel K, Laignelet B, Feillet P (1974) Study of some factors of macaroni brownness. Cereal Chem 51:675–684
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Lagudah ES, Appels R, McNeil D (1991) The Nor-D3 locus of Triticum tauschii: natural variation and genetic linkage to markers in chromosome 5. Genome 34:387–395
Li WL, Faris JD, Chittoor JM, Leach JE, Hulbert SH, Liu DJ, Chen PD, Gill BS (1999) Genomic mapping of defense response genes in wheat. Theor Appl Genet 98:226–233
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374
Ling HQ, Zhao SC, Liu DC, Wang JY, Sun H, Zhang C, Fan HJ, Li D, Dong LL, Tao Y, Gao C, Wu HL, Li YW, Cui Y, Guo XS, Zheng SS, Wang B, Yu K, Liang QS, Yang WL, Lou XY, Chen J, Feng MJ, Jian JB, Zhang XF, Luo GB, Jiang Y, Liu JJ, Wang ZB, Sha YH, Zhang BR, Wu HJ, Tang DZ, Shen QH, Xue PY, Zou SH, Wang XJ, Liu X, Wang FM, Yang YP, An XL, Dong ZY, Zhang KP, Zhang XQ, Luo MC, Dvorak J, Tong YP, Wang J, Yang HM, Li ZS, Wang DW, Zhang AM, Wang J (2013) Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 496:87–90
Liu YN, He ZH, Appels R, Xia XC (2012) Functional markers in wheat: current status and future prospects. Theor Appl Genet 125:1–10
Maksimov IV, Cherepanova EA, Kuzmina OI, Yarullina LG, Akhunov AA (2010) Molecular peculiarities of the chitin-binding peroxidases of plants. Russ J Bioorganic Chem 36:293–330
Malosetti M, Ribaut JM, Vargas M, Crossa J, Eeuwijk FA (2008) A multi-trait multi-environment QTL mixed model with an application to drought and nitrogen stress trials in maize (Zea mays L.). Euphytica 161:241–257
Matsuo RR, Dexter J (1980) Relationship between some durum wheat physical characteristics and semolina milling properties. Can J Plant Sci 60:49–53
Morris CF (2002) Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol Biol 48:633–647
Passardi F, Longet D, Penel C, Dunand C (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65:1879–1893
Peng JR, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Piontek K, Glumoff T, Winterhalter K (1993) Low pH crystal structure of glycosylated lignin peroxidase from Phanerochate chrysosporium at 2.5 Å resolution. FEBS Lett 315:119–124
Poulos TL, Patterson WR, Sundaramoorthy M (1995) The crystal structure of ascorbate and manganese peroxidase: the role of non-haem metal in the catalytic mechanism. Biochem Soc Trans 23:228–232
Ravel C, Praud S, Murigneux A, Canaguier A, Sapet F, Samson D, Balfourier F, Dufour P, Chalhoub B, Brunel D, Beckert M, Charmet G (2006) Single-nucleotide polymorphism frequency in a set of selected lines of bread wheat (Triticum aestivum L.). Genome 49:1131–1139
Revanappa SB, Salimath PV, Prasada Rao UJS (2014) Effect of peroxidase on textural quality dough and arabinoxylan characteristic isolated from whole wheat flour dough. Int J Food Microbiol 17:2131–2141
Rhee SG, Woo HA, Kil IS, Bae SH (2012) Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 287:4403–4410
Stam P (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J 3:739–744
Taha SA, Sagi F (1987) Relationships between chemical composition of durum wheat semolina and macaroni quality. II. Ash, carotenoid pigments and oxidative enzymes. Cereal Res Commun 15:123–129
Takasaki S, Kato Y, Murata M, Homma S, Kawakishi S (2005) Effects of peroxidase and hydrogen peroxide on the dityrosine formation and the mixing characteristics of wheat-flour dough. Biosci Biotechnol Biochem 69:1686–1692
Theilade B, Rasmussen SK (1992) Structure and chromosomal localization of the gene encoding barley seed peroxidase BP 2A. Gene 118:261–266
Trono D, Pastore D, Di Fonzo N (1999) Carotenoid dependent inhibition of durum wheat lipoxygenase. J Cereal Sci 29:99–102
Veldboom LR, Lee M (1996) Genetic mapping of quantitative trait loci in maize in stress and non-stress environments: I. Grain yield and yield components. Crop Sci 36:1310–1319
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang SC, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, IWGSC, Lillemo M, Mather D, Appels R, Dolferus R, Guedira GB, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo MC, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E (2014) Characterization of polyploidy wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol J 12:787–796
Yanagisawa T, Kiribuchi-Otobe C, Hirano H, Suzuki Y, Fujita M (2003) Detection of single nucleotide polymorphism (SNP) controlling the waxy character in wheat by using a derived cleaved amplified polymorphic sequence (dCAPS) marker. Theor Appl Genet 107:84–88
Žilić S, Serpen A, Akillioğlu G, Janković M, Gőkmen V (2012) Distributions of phenolic compounds, yellow pigments and oxidative enzymes in wheat grains and their relation to antioxidant capacity of bran and debranned flour. J Cereal Sci 56:652–658
Acknowledgments
The authors are grateful to Prof. R. A. McIntosh, Plant Breeding Institute, University of Sydney, for critical review of this manuscript. This study was supported by the National Natural Science Foundation of China (31260327, 31461143021), the National 863 Project (2012AA10A308), the Gene Transformation Projects (2011ZX08009-003, 2011ZX08002004-008), International Science & Technology Cooperation Program of China (2014DFG31690), and China Agriculture Research System (CARS-3-1-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest for this manuscript.
Ethical standards
We declare that these experiments comply with the ethical standards in China, where they were performed.
Additional information
Communicated by B. Hulke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, J., Geng, H., Zhang, Y. et al. Mapping quantitative trait loci for peroxidase activity and developing gene-specific markers for TaPod-A1 on wheat chromosome 3AL. Theor Appl Genet 128, 2067–2076 (2015). https://doi.org/10.1007/s00122-015-2567-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2567-0