Abstract
Seed oil production in oilseed rape is greatly affected by the temperature during seed maturation. However, the molecular mechanism of the interaction between genotype and temperature in seed maturation remains largely unknown. We developed two near-isogenic lines (NIL-9 and NIL-1), differing mainly at a QTL region influencing oil content on Brassica napus chromosome C2 (qOC.C2.2) under high temperature during seed maturation. The NILs were treated under different temperatures in a growth chamber after flowering. RNA from developing seeds was extracted on the 25th day after flowering (DAF), and transcriptomes were determined by microarray analysis. Statistical analysis indicated that genotype, temperature, and the interaction between genotype and temperature (G × T) all significantly affected the expression of the genes in the 25 DAF seeds, resulting in 4,982, 19,111, and 839 differentially expressed unisequences, respectively. NIL-9 had higher seed oil content than NIL-1 under all of the temperatures in the experiments, especially at high temperatures. A total of 39 genes, among which six are located at qOC.C2.2, were differentially expressed among the NILs regardless of temperature, indicating the core genetic divergence that was unaffected by temperature. Increasing the temperature caused a reduction in seed oil content that was accompanied by the downregulation of a number of genes associated with red light response, photosynthesis, response to gibberellic acid stimulus, and translational elongation, as well as several genes of importance in the lipid metabolism pathway. These results contribute to our knowledge of the molecular nature of QTLs and the interaction between genotype and temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oilseed rape (Brassica napus L.) is one of the most important sources of edible oil, and it can also be used in the production of biodiesel to serve as a replacement for fossil fuels. However, the seed weight and oil content of oilseed rape are greatly affected by environmental factors and cultivation practices, among which the temperature during the seed maturation stage plays a critical role (Canvin 1965; Edwards and Weinig 2011; Esteban et al. 2004; Piper and Boote 1999). Normally, hot temperatures (prevalent in some rapeseed production regions, such as the Yangtze Valley of China and Western Australia) during seed maturation result in a dramatic decline in yield. The degree of this negative effect largely depends on genotype.
Substantial genetic variation exists in seed development and fatty acid metabolism, involving a large number of genes responding to environmental and endogenous stimuli. Because of the large quantity of genes involved in seed oil formation, researchers and oilseed breeders experience difficulty identifying key genes related to seed oil formation. Many efforts have been made in mapping the quantitative trait loci (QTL) regulating seed oil formation. Previous studies have identified multiple QTLs associated with seed oil content (Delourme et al. 2006; Mahmood et al. 2006; Qiu et al. 2006; Shi et al. 2009). However, QTLs are environmentally sensitive, and the molecular mechanism underlying the interaction between QTLs and environmental factors remains poorly understood. Nevertheless, comparative mapping between the Brassica genomes and their close relations, the genomes of Arabidopsis, has provided useful information for inferring the likely presence of candidate genes for the control of oil content within QTL-containing regions of the genome (Parkin et al. 2005; Schranz et al. 2006). This genetic relationship means that data related to Brassica species substantially overlap that established for Arabidopsis (Hua et al. 2009; Lukens et al. 2003; Osborn et al. 1997; Parkin et al. 2003). In Arabidopsis, Beisson et al. (2003) searched the genome for the sequences of genes involved in lipid biosynthesis and classified over 600 genes according to their predicted function, subcellular location, and alternative splicing. According to their temporal and spatial expressions, about 70 genes have been identified as seed specific, and their expression levels exhibit a dramatic increase (up to 5,000-fold) at the beginning of the seed maturation phase (Beisson et al. 2003; Schmid et al. 2005). More recently, Shi et al. (2010) reported the screening of polymorphic markers at these loci putatively for seed oil formation between two geographically different genotypes, the Chinese cultivar Ningyou-7 (N) and the European cultivar Tapidor (T), used as two parents in generating the TN DH population for QTL mapping. Some seed-specific genes have been localized at the QTL regions contributing to seed oil content, and the allelic variations among a M2 population generated from Ningyou-7 have been analyzed and linked with seed qualitative traits like erucic acid content (Wang et al. 2008).
Seed development, a critical process in the life cycle of higher plants, consists of two stages: morphogenesis and maturation. Maturation begins with a transition phase when the switch from maternal to filial controls occurs. The seed then undergoes a period of embryo growth and seed filling. Maturation ends with a desiccation phase, after which the embryo enters a quiescent state, thereby permitting its maintenance and survival under a range of environmental conditions (Gutierrez et al. 2007). A number of genes play regulatory roles in this process. The mutations of TAG1 and WRI1 cause reduced seed oil content in Arabidopsis as well as B. napus (Cernac and Benning 2004; Zou et al. 1999). The identification of the master regulators ABI3, FUS3, LEC1, LEC2, and MYB30 in Arabidopsis have also augmented our knowledge regarding the control of transcription programs involved in seed maturation. Using a time-series gene expression dataset of seed development in B. napus and a well-selected dataset of interactions between transcription factors and their target genes, Tchagang et al. (2009) derived a dynamic regulatory map to uncover the activity of these regulatory genes. The transactional factors LEC2, WRIl, FUS3, MYB30, and ABI3 were active during early seed development, several of which, including LECI, LEC2, ABI3, and FUS3, remained active during seed maturation, indicating their major regulatory function during the maturation phase.
To date, little is known about the molecular nature of the QTL or the molecular mechanism underlying the interaction between genotype and temperature (G × T) in determining seed maturation and fatty acid metabolism. In this study, the two near-isogenic lines (NILs) were grown in pairs and treated under different temperatures during the seed maturation stage to compare the transcriptomes of the developing seeds 25 days after flowering (DAF). The genetic divergence of the NILs was narrowed to a region spanning a distance of approximately 9.0 cM, in which a QTL responding to the temperature during seed maturation was detected. The NILs differ from each other by 7.4% in the oil content of matured seeds. The objective of the study is to understand the effect of temperature on the global expression of the genes in developing oilseed rape seeds, the genetic architecture that leads to the difference in seed oil content between the NILs, and the molecular foundation underlying the interaction between temperature and genotype.
Materials and methods
QTL detection
QTL for seed oil content in Tapidor–Ningyou-7 (TN) DH population were analyzed using the composite interval mapping method (CIM) with WinQTL cartographer 2.5 software (http://statgen.ncsu.edu/qtlcart/WQTLCart.htm; Zeng 1994; Wang et al. 2006b). CIM was used to scan the genetic map and estimate the likelihood of a QTL and its corresponding effect at every 2 cM. The forward regression algorithm was used to get cofactors. The permutation test method was used to obtain the empirical thresholds of the experiment on the basis of 1,000 runs of randomly shuffling the trait values, which were expected to have a genome-wide type-I error of 0.05 (Churchill and Doerge 1994). Likelihood calculation results were exported with 10.0 cM distance to define two adjacent QTL, and confidence intervals of QTL were count as cM spanning regions of 2 LOD scores decrease both sides from the peak LOD value. Meta-analysis package of BioMercator 2.1 software (Arcade et al. 2004) was used to integrate redundant QTL information from multiple experiments. The Delete function of BioMercator 2.1 software was used to integrate QTL segment by segment on linkage group C2 as Chardon et al. (2004) described. Appropriate models of integration results were chosen with the AIC value minimal.
Development of NIL-9 and NIL-1
To develop the two near isogenic lines (NILs) NIL-9 and NIL-1, Ningyou-7 was used as recipient, and Tapidor was used as donor. The parents were selected primarily on the basis of contrasting trait characteristics and cultivation ranges (Meng et al. 1996). Tapidor is a European winter-type cultivar which has a strong vernalization requirement and with low seed erucic acid and glucosinolate content. Ningyou-7 is a Chinese semi-winter-type cultivar which has little vernalization requirement and with high seed erucic acid and glucosinolate content. Before development of the mapping population, doubled haploid (DH) lines were developed from both of the parental genotypes. A fourth-generation backcross (BC4) population of 927 lines was constructed through successive backcrossing Ningyou-7 with 146 lines from Tapidor × Ningyou-7 DH population. 89 PCR-based molecular markers, such as SSR, SRAP, and STS markers selected randomly from dense TN DH linkage map (Long et al. 2011) were used to survey the genetic similarity with the recurrent parent Ningyou-7. The amplification products were separated using 6% polyacrylamide gel electrophoresis (PAGE). A test locus showed recipient genotype (NN) was considered as 100% Ningyou-7 genotype and a locus showed donor genotype (TT) was considered as 0% Ningyou-7 genotype, whereas, a locus showed donor type and recipient type (TN) was considered as 50% Ningyou-7 type. The global genome similarity of the NILs was the mean value of all the test loci. A group of 32 molecular markers selected from linkage group C2 was used to determine the length of substituted segments in the NIL development (Supplemental Table S1). The substituted segment, in centimorgan, was determined based on its location on C2 linkage map. The length of substituted segments in NILs was estimated based on graphical genotypes (Young and Tanksley 1989). The NIL-9 and NIL-1 were selected from self-pollinated progenies of an individual plant ‘6F092-2’ of the BC4F3 population and showed 95.83% genetic background similarity with recurrent parent Ningyou-7 at the global genome level. The trait characteristics of the two lines are nearly the same as their recurrent parent Ningyou-7, except for the oil content and the fatty acid component in seed. They are genetically different in the confidence interval region of the seed oil content QTL, qOC.C2.2.
Alignment to Arabidopsis genome for the genes at qOC.C2.2 QTL
22 of 42 molecular markers on linkage group C2 are used for alignment to Arabidopsis genome (Supplemental Table S1). The principle of alignment was described in Long et al. (2007). The alignment of the genome segment flanking to the qOC.C2.2 region of B. napus to Arabidopsis genome and the genetic similarity of the NILs are elucidated in Fig. 1.
Diagram showing the alignment of the genome segment flanking the qOC.C2.2 region of B. napus to Arabidopsis genome (a) and the genetic similarity of the NILs (b). The black bar on the linkage group indicates the confidence interval of qOC.C2.2. The arrows show the conserved blocks of Arabidopsis genome (Parkin et al. 2005). The bars at the bottom (b) represent genome fragments from Tapidor (gray) and Ningyou-7 (black). The NIL-9 carries an introgression from Tapidor at the qOC.C2.2 region that spans a genetic distance of 9.0 cM, determined by the linkage map position of the markers for genotyping
Plant growth conditions
The B. napus NILs used in the study were grown in controlled growth chambers (Conviron, E8VH, Controlled Environments Ltd., Winnipeg, Manitoba, Canada) at Plant Biotechnology Institute of the National Research Council, Canada (PBI-NRC), in 2009. Seeds were germinated in pots and the seedlings were grown in champers in cycles of 16 h light (white fluorescent light at 500–600 μm m−2 s−1) at 25°C and 8 h dark at 18°C. At the six-leaf stage, plants of equal size were moved to a new growth chamber for a 6-week vernalization period in cycles of 16 h light at 10°C and 8 h dark at 4°C. Again, plants of similar size were selected and moved back to the growth chamber with cycles of 16 h light at 25°C and 8 h dark at 18°C. After the two selection processes, all of the plants flowered on the same day. On the day the first flowers opened, the plants were moved in pairs into different growth chambers under the following conditions: T1: 16 h light at 20°C and 8 h dark at 12°C; T2: 16 h light at 25°C and 8 h dark at 18°C; T3: 16 h light at 30°C and 8 h dark at 20°C. To harvest siliques of a certain defined stage, individual flowers were tagged and labeled with the date of flower opening. The siliques that were in the developmental stage 25 DAF were harvested and immediately frozen in liquid nitrogen. The developing seeds collected from the dissected siliques on dry ice were prepared for RNA extraction and the subsequent microarray experiment. The rest of the siliques that naturally matured were harvested for the seeds to be analyzed for total seed oil content and fatty acid composition.
Total RNA
Total RNA of seeds at 25 DAF was extracted using the RNeasy Plant Mini Kit (Qiagen, Mississauga, ON, Canada) and on-column DNase digestion with an RNase-free DNase set (Qiagen, Mississauga, ON, Canada) was performed for each sample according to the manufacturer’s protocols. The quantity and quality of total RNA were determined by both spectrophotometer (NanoDrop ND-1000, NanoDrop Technologies, Inc. Wilmington, USA) and gel.
Microarray analysis
Experimental design
The two NILs were employed for transcriptomic comparsion with each other for temperature effects (T1, T2, and T3) on developing seeds at 25 DAF. Triple biological duplicates and two technological repeats in each biological duplicate were designed for each G × T treatment.
Combimatrix Brassica 90K array
A total of 36 independent hybridizations were performed using the Combimatrix Brassica 90K microarray (http://www.combimatrix.com) produced at the PBI-NRC. Over 94,000 unique DNA probes were in situ synthesized using the Combimatrix patented virtual-flask technology. The semiconductor-based array consists of 29 row × 34 column spots.
RNA labeling, microarray hybridization, and washing
A single dye (Cy5) was used for RNA-labeling. Approximately 1 μg total RNA was reverse transcribed into aRNA using the Amino Allyl MessageAmpTM II aRNA Amplification Kit (Ambion, USA) according to the manufacturer’s protocols. The aRNA was selected as the template for fluorescent target preparation for microarray experiments. The quality of amplification was identified by agarose gel electrophoresis, and aRNA quantity was determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc. Wilmington, USA), followed by 1% agarose gel electrophoresis to check the quality of the aRNA. An aliquot of 5 μg aRNA was used to perform coupling using the Amino Allyl MessageAmpTM II aRNA Amplification Kit (Ambion, USA) according to the manufacturer’s protocols. RNA fragmentation was conducted using RNA fragmentation reagents (Ambion, AM 8740) following the manufacturer’s instructions. The pre-hybridization, hybridization, washing, and imaging procedures were performed according to the protocol of the Combimatrix manufacturer, and stripping procedures were performed using the CustomArrayTM Stripping Kit for 90K (Product Number 610049) following the manufacturer’s instructions.
Data collection
Hybridized arrays were scanned using an Axon 4000B scanner (Axon Instruments, CA, USA) at 5 μm resolution, 100% laser power, and different PMT values to obtain overall intensity. Raw spot fluorescence intensities were extracted with GenePix Pro 6.0 (Axon Instruments, CA, USA). The built-in quality control mechanism was used to flag questionable features on the array so that they could be removed from further analysis. Acuity 4.0 (Axon Instruments/Molecular Devices Corp.) was used to manage microarray datasets. In accordance with recommendations, a pre-processing step was performed before normalization. Flagged features that were not found, as well as bad and empty features, were removed (Flags ≥ 0). According to the GenePix 4000B datasheet, the signal-to-noise ratio of a feature should be above the detection limit of the device (SNR 635 > 3). To remove irregular features, a circularity metric was introduced into GenePix Pro (circularity >80). Saturated features were also swapped (F635% Sat. <2) from all arrays.
Normalization and data analysis
Microarray intensity values within datasets were normalized and standardized by Z-score transformation (Cheadle et al. 2003). The signal intensity (foreground median with background mean subtracted) of each gene/probe on the microarray was combined by averaging all of the duplicates in the same array. After the normalization and standardization process, the standardized signal intensities (hereafter referred to as signal intensities) of each gene/probe were obtained. Differentially expressed genes (DEGs) under different treatment levels were detected by a mixed linear model approach, using the MIXED procedure in SAS (SAS Institute, Inc). If y ijkl is the logarithm of measurement from the i-th genotype, the j-th temperature, the k-th biological replicate, and the l-th technical replicate, then a full model used to interpret the experimental design can be expressed as
where G i is the effect of the i-th level of the genotype; T j is the effect of the j-th level of the temperature treatment; GT ij is the interaction effect between the i-th genotype and the j-th temperature treatment; B k is the effect of the k-th biological replicate, B k ~\( N\left( {0,\sigma_{B}^{2} } \right) \); S l is the effect of the l-th technical replicate, S l ~\( N\left( {0,\sigma_{S}^{2} } \right) \); and ε ijkl is the residual effect accounting for uncontrollable random errors, ε ijkl ~\( N\left( {0,\sigma_{e}^{2} } \right) \). Model 1 is a modified form of the model proposed by Yang et al. (2009). Considering the different number of genes in B. napus, the cutoff p value was adjusted to control the false discovery rate at 0.0001.
Annotation and GO functional categorization
The DNA sequences that were used to design each of the probes spotted on the array were searched for their homologues in the TAIR database (version 9) using BLASTX at an E value less than 10−5 (Altschul et al. 1997). GO term information for the homologs in the GO database sequences with the best hit was used in combination with assumptions of the amino acid percent identity ≥30% and High Scoring Pair (HSP) length ≥100 for each pair (Kabsch and Sander 1984; Rost 1999). A GO evidence code of IEA (inferred from electronic annotation) was automatically assigned to each homolog sequence in B. napus. DEGs were picked for GO slim counting using the TAIR GO slim category. p ≤ 0.01 was used to pick out the sequences whose biological processes were most likely to be associated with the target effect. In this analysis, a gene/probe may be placed in more than one GO slim term ID according to the biological characteristics of the product. No attempt was made to identify or resolve these cases.
Clustering of DEGs
Acuity 4.0 (Axon Instruments/Molecular Devices Corp.) was used to perform a cluster analysis of the DEGs. To determine the optimal size of clusters, gap statistical analysis was first applied to the log-transformed datasets; Euclidean squared was used as the similarity metric. The same datasets were then subjected to a self-organizing map (SOM) analysis of DEGs using the same metric used in gap analysis. For the convenience of illustration, DEGs were divided into three datasets according to the factors used in the mixed linear model analysis: G, T, and G × T. In this analysis, a gene/probe may appear in more than one dataset according to the biological characteristics of the product.
Quantitative RT-PCR analysis of transcription levels
The rules suggested by Udvardi et al. (2008) were followed for the standardization of a quantitative RT-PCR (real-time PCR) procedure. Based on their probable significance for influencing oil accumulation, 14 DEGs observed in the microarray analysis were selected for quantitative RT-PCR verification. They are the genes either regulating embryo development or encoding enzymes critical to fatty acid biosynthesis. The primer pairs for the quantitative real-time RT-PCR were specific to the genes in question and designed to cover an 80- 200-bp region (Table 1). For real-time PCR templates, first-strand cDNAs were synthesized in a 20-μL reaction solution containing approximately 2 μg RNA (the same RNA samples from microarray hybridization) using the PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Japan, Catalog No. D6110A) with oligo (dT) 12–18 as a primer. The specificity of all primers was checked via BLASTN searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The BnACTIN7 was applied as an endogenous control for standardization. The BnACTIN7 sense primer (5′-GGAGCTGAGAGATTCCGTTG-3′) and antisense primer (5′-GAACCACCACTGAGGACGAT-3′) were designed according to Genbank Accession Number AF111812. The real-time amplification reactions were preformed using the iCycler iQ thermocycler (Bio-Rad) and the SYBR Premix Ex Taq™ Kit (Takara, Japan, Catalog No. DRR041A) following the the manufacturers’ instructions.
Analysis of seed oil content and fatty acid composition
Mature seeds were harvested and air dried until their weight remained constant. All conditions for the isolation and GC analyses of seed lipids for total oil content and fatty acid composition (expressed as μg/mg of total seed weight) were as described previously (Katavic et al. 1995; Zou et al. 1997). Data were classified with Win-Excel and analyzed via one-way analysis of variance (ANOVA) using the statistical package SAS (SAS Institute, Inc). Comparisons between the treatment mean were made by Duncan’s Multiple Range Test at a level of p ≤ 0.05.
Results
Characterization of the qOC.C2.2 QTL and the NILs
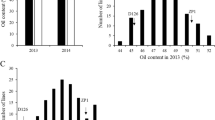
In previous studies, the construction and development of Tapidor–Ningyou-7 (TN) map were reported (Qiu et al. 2006). A higher marker resolution enabled the discovery of a QTL for seed oil content on linkage group C2 (abbreviated as qOC.C2.2) by analyzing the data achieved from multi-year and multi-site field experiments. Metrological records indicate that the qOC.C2.2 was only detectable when the seeds matured in a hot environment, as was present in Wuhan in spring 2003 and 2004, in Jingzhou in spring 2005, and in Hangzhou in spring 2007 (Fig. 2). This QTL was responsible for 6.13–13.00% of the total seed oil content variation among a population of 202 DH lines. In addition to the multi-year and multi-site field experiments, the major inflorescences of 196 DH lines were tagged to partition five sections (A to E) from the lower reaches to the upper parts during seed maturation. The average daily temperature aligned with pod maturation was recorded, and the seeds from each section were separately harvested in spring 2007 in Wuhan. The qOC.C2.2 was detected only by associating the molecular markers with the seed oil content of the D-section, which came into being during hot weather. NIL-9 and NIL-1 were developed using Ningyou-7 as the recurrent parental line. Both NILs were 95.83% identical to the recurrent parent Ningyou-7 at the global genome level; however, NIL-9 carries introgression from Tapidor at the qOC.C2.2 region that spans a genetic distance of 9.0 cM, as determined by the linkage map position of the SSR and SRAP markers for genotyping. On average, the seed oil content of NIL-9 was 7.4% higher than that of NIL-1 under Wuhan climate conditions, and the difference was significant at the 5% level.
The QTL for seed oil content at the qOC.C2.2 region detectable in hot environments. Arrows point to the QTL peaks detected in Wuhan in spring 2003 (arrow 1) and Spring 2004 (arrow 2), in Jingzhou in Spring 2005 (arrow 3), and in Hangzhou in spring 2007 (arrow 4). The bars represent genomic fragments from Tapidor and Ningyou-7. NIL-9 carries an introgression from Tapidor at the qOC.C2.2 region that spans a genetic distance of 9.0 cM, determined by the linkage map position of the SSR and SRAP markers for genotyping
Differences in seed qualitative traits under artificial conditions between the NILs
To investigate the effect of temperature on oil formation in the NILs, we grew the plants under artificial conditions in which temperature was strictly controlled. We analyzed and compared seed oil content and fatty acid composition in mature seeds. As shown in Fig. 3, temperature had a clear effect on seed oil content as well as fatty acid composition. Overall, the NIL plants responded to increasing temperature with decreasing levels of total seed oil, linoleic acid (C18:2), linolenic acid (C18:3), and erucic acid (C22:1). NIL-9 had higher seed oil content than did NIL-1 in all three growth chambers. However, the degree of surplus varied. The highest surplus was found in Chamber T3 and the lowest in Chamber T2. NIL-9 also had higher levels of docosanoic acid (C22:0) and erucic acid (C22:1) than NIL-1, but lower levels of stearic acid (C18:0), oleic acid (C18:1), and eicosenoic acid (C20:1) in all three chambers. NIL-9 surpassed NIL-1 in palmic acid (C16:0) and linoleic acid (C18:2) levels in Chamber T3, but not in Chambers T1 and T2, whereas NIL-9 surpassed NIL-1 in linolenic acid (C18:3) content in Chambers T2 and T3, but not in Chamber T1.
Comparison of seed qualitative traits between NIL-9 and NIL-1 grown in Chambers T1, T2, and T3. Error bars indicate SD values of three independent repeats. a seed oil content; b palmitic acid content; c stearic acid content; d oleic acid content; e linoleic acid content; f linolenic acid content; g arachidic acid content; h eicosenoic acid content; i docosanoic acid content, and j erucic acid content
General description of microarray data
To determine differentially expressed genes (DEGs), the mixed linear model approach was used for the analysis. Statistical calculations indicated that the effect of genotype, temperature, and the interaction between genotype and temperature on the expression of the genes in 25 DAF seeds were all significant at a 0.01% false discovery rate. A total of 19,111 DEGs were generated by temperature. A total of 4,982 DEGs resulted from global genotypic divergence, among which 791 DEGs, accounting for 15.9% of the global DEGs, are located at the qOC.C2.2 region as determined by alignment with the Arabidopsis genome. A total of 839 DEGs arose from the interaction effect between genotype and temperature. Detailed information on the sequence IDs is provided in Supplemental Table S2.
We classified the DEGs into three major clusters, resulting from temperature (Cluster-t, C-t), genotype (C-g), and the interaction between temperature and genotype (C-i), respectively. Furthermore, 11 sub-clusters were identified using the self-organizing map clustering technique (Wan et al. 2008), clusters C-t-1 to C-t-4, C-g-1 to C-g-4, and C-i-1 to C-i-3, respectively (Fig. 4).
C-t-3 and C-t-4 were two major sub-clusters within C-t, collectively accounting for 68.2% of the DEGs within C-t. The genes in these two clusters were expressed with the highest RNA transcript abundance under low temperature (T1) and with the lowest RNA transcript abundance under high temperature (T3). C-t-1 represented only 15.6% of the DEGs in C-t. The transcription level of the genes in this cluster increased from T1 to T2 as well as from T2 to T3. C-t-2 represented 16.1% of the DEGs in C-t. The genes in C-t-2 had a very low expression at T2. Both increasing and decreasing temperature to T1 or T3 resulted in higher expression of the genes in this cluster.
C-g-2 accounted for 43.6% of the DEGs in C-g. Relative to the genes of other clusters in the C-g, the genes in C-g-2 had higher RNA transcription levels. C-g-3 and C-g-4 totaled 35.8% of the DEGs in C-g. The transcription level in these two clusters was lower than that in C-g-2, but higher than that in C-g-1. The expression level of the genes in NIL-9 was lower than that in NIL-1 in the C-g clusters, except in C-g-1.
C-i-2 represented 53.5% of the DEGs in C-i. The expression of NIL-1 genes was very high, nearly 50% higher than that of NIL-9, under T1 and T3. However, the expression was lower than that of NIL-9 under T2. Clusters C-i-1 and C-i-3 collectively accounted for 46.5% of the DEGs in C-i. The general trend in C-i-1 was a decrease in transcription abundance in response to increasing temperatures. Moreover, the expression level of NIL-9 genes was lower than that of NIL-1 genes treated only with T2. The trend in C-i-3 was the opposite of that in C-i-1, demonstrating an increase in transcription level in response to higher temperatures. The transcription level of NIL-9 genes was higher than that of NIL-1 genes conditioned only with T2. The unisequence information for each cluster is provided in detail in Supplemental Table S3.
Expressional divergence of the NILs under different temperatures
To understand the expressional divergence of the NILs under different temperatures, we drew Venn diagrams (Fig. 5). The smallest expression differences were found under T2, under which NIL-1 differed from NIL-9 with the expression of 251 genes globally and 39 genes at the qOC.C2.2 region. More genetic divergences were observed under T1, under which the NILs differed from each other with 2 933 DEGs globally and 460 DEGs at the qOC.C2.2 region. The greatest difference was caused by T3, under which the NILs differed from each other with 3 499 DEGs globally and 558 DEGs at the qOC.C2.2 region. The number of DEGs at the qOC.C2.2 region accounted for about 11.7, 15.5, and 15.9% of the global DEGs under T1, T2, and T3, respectively. A total of 102 genes at the global level (or 15 genes at the QTL region; Fig. 5a, b) were always differentially expressed between the NILs under either T1 or T2. A total of 107 genes at the global level (or 15 genes at the QTL region) were always differentially expressed between the two NILs under either T2 or T3. A list of the DEG information is provided in Supplemental Table S4.
Globally, a total of 39 genes were differentially expressed between the NILs under all of the three temperatures, among which six were located in the qOC.C2.2 region (Table 2). 30 of these DEGs have orthologues in Arabidopsis, including the transcription factors VOZ1 (VASCULAR PLANT ONE ZINC FINGER PROTEIN), DCT2.1 (DICARBOXYLATE TRANSPORT 2.1), FLA7 (FASCICLIN-LIKE ARABINOOGALACTAN 7), SUF4 (suppressor of FRIGIDA4), other genes such as CAT3 (CATALASE 3), G6PD1 (GLUCOSE-6-PHOSPHATE DEHYDROGENASE 1), PROT1 (PROLINE TRANSPORTER 1), GDU3 (GLUTAMINE DUMPER 3). The six genes that were located in the qOC.C2.2 region are orthologous to Arabidopsis EMB3010 (AT5G10360), AT5G02910, AT1G70620, and AT1G80910, AT1G76200, AT1G70770 (Table 2). As described in Table 2, at least two of the six genes are the transcriptional factors that regulate reproductive organ development.
GO analysis of DEGs
To analyze the biological functions of the DEGs (in the QTL region), GO slim categories were assigned to DEGs arising from both genotype (Table 3) and temperature (Fig. 6). As shown in Table 3, 246 DEGs resulting from genotype can be grouped into various GO categories. Of these, a high proportion of GO slims were specifically related to embryo development (GO:0009793), DNA-dependant transcription regulations (GO:0006355), stress responses (such as GO:0055114 for oxidation reduction; GO:009651 for salt stress response; GO:0009408 for heat response; GO:0009611 for wound response; GO:0046686 for cadmium stress response), and photosynthesis (GO:0009853 for photorespiration; GO:008005 for photosystem stoichiometry adjustment; GO:0006096 for glycolysis; GO:0006108 for malate metabolism). These were overrepresented on the list of DEGs arising from genotype. A number of DEGs also belonging to the GO categories are involved in more general biological processes. Of these, some are associated with protein biosynthesis (GO:0006412), protein folding (GO:0006457) and protein intercellular transport (GO:0006886), translational initials (GO:0006413), jasmonic acid response (GO:0009753), and other functions. Detailed gene functions are presented in Supplemental Table S5.
Assuming that T2 represents the typical temperature during seed maturation, the change in temperature from T2 to T1 or from T2 to T3 generated 523 DEGs at the global level, classified according to GO slim categories (Fig. 6). Either increasing the temperature to T3 or decreasing the temperature to T1 resulted in the upregulation of the genes related to DNA-dependent transcription regulations (GO:0006355), embryo development (GO:0009793), stress response (GO:0042742 for bacterium defending response; GO:0046686 for cadmium stress response), protein biosynthesis (GO:0006412), amino acid phosphorylation (GO:0006468), and protein folding (GO:0006457). Increasing the temperature to T3 caused the upregulation of the genes involved in heat response (GO:0009408) and ubiquitin-dependent protein catabolism (GO:0006511), but the downregulation of several genes associated with fatty acid biosynthesis (GO:0006633), red light response (GO:0010114), photosynthesis (GO:0015979), gibberellic acid stimulus response (GO:0009739), and translational elongations (GO:0006414). Decreasing the temperature to T1 led to the downregulation of some genes regulating glycolysis (GO:0006096), malate metabolism (GO:0006108), abscisic acid stimulus response (GO:0009737), the tricarboxylic acid cycle (GO:0006099), and water deprivation response (GO:0009414). Either increasing temperature to T3 or decreasing temperature to T1 resulted in the downregulation of the genes of the cold response category (GO:0009409) and the up and downregulation of the genes of the oxidation reduction category (GO:0055114).
Quantitative RT-PCR confirmation
To assess the reproducibility and accuracy of microarray analysis, quantitative RT-PCR (qRT-PCR) was performed. The putative transcription factors, such as the Brassica orthologues to Arabidopsis LEAFY COTYLEDON1 (LEC1), WRINKLED1 (WRI1), ABA INSENSITIVE 3 (ABI3), and FUSCA3 (FUS3), are important in the regulation of embryo development and seed fatty acid metabolism (Tchagang et al. 2009). Other genes, such as the Brassica orthologues to Arabidopsis FATTY ACID DESATURASE2 (FAD2), FAD3, LATE ELONGATED HYPOCOTY 1 (LHY1), DIACYLGLYCEROL O-ACYLTRANSFERASE1 (TAG1), LYSOPHOSPHATIDIC ACID ACYLTRANSFERASES (LPAT2), AT4G25140 (encoding OLEOSIN), AT4G26740 (encoding CALEOSIN), AT2G43710 (encoding STEAROYL-ACP DESATURASE), AT2G47240 (encoding long-chain-fatty-acid-CoA ligase), and AT3G25110 (encoding FatA acyl-ACP thioesterase), play critical roles in various fatty acid biosynthesis pathways. These were selected and subjected to the experiment. Overall, a significant correlation was observed between the microarray and qRT-PCR results (Supplemental Table S6).
The genetic difference between NIL-9 and NIL-1 had a significant effect on the expression of the genes BnLACS1, BnOLEO1, BnCLO1, BnFatA, and BnLHY1 (Fig. 7). Relative to NIL-1, NIL-9 had a higher expression level of BnLACS1, BnCLO1, and BnLHY1, but a lower expression level of BnOLEO1 and BnFatA. The temperature significantly affected the expression level of the genes BnLEC1, BnWRI1, BnABI3, BnFUS3, BnFAD2, BnFAD3, BnTAG1, and BnLPAT2 as well as BnOLEO1, BnCLO1, BnFaTA, and BnLHY1. Increasing temperature led to a higher expression of BnABI3, BnFUS3, BnTAG1, BnOLEO1, BnCLO1, and BnFaTA, but a lower expression of BnLEC1, BnWRI1, BnFAD2, BnFAD3, and BnLPAT2. The G × T interaction significantly affected the expression of BnFAB2. Treated only under T1, NIL-9 had a higher expression of BnLPAT2 than NIL-1.
Quantitative RT-PCR confirms the microarray results showing the expression of the genes regulating seed development or the genes important in lipid metabolic pathways. Error bars indicate SD values of three independent repeats. The expression of BnLEC1, BnWRI1, BnABA3, BnFUS3, BnFAD2, BnFAD3, BnTAG1, and BnLPAT2 was significantly affected by temperature. The expression of BnLACS1 was significantly affected by genotype. The expression of BnOLEO1, BnCLO1, BnFatA, and BnLHY1 was significantly affected by both temperature and genotype. The expression of BnFAB2 was significantly affected by the interaction between genotype and temperature (p < 0.005)
Discussion
We developed two near-isogenic lines, NIL-9 and NIL-1, which were 95.83% identical to the recurrent parent Ningyou-7 at the global level. NIL-9 carries introgression from another parent (Tapidor) at the qOC.C2.2 region, which spans a genetic distance of 9.0 cM. A QTL could be detected within this region treated under a high environmental temperature during seed maturation. We grew the NILs in pairs and treated them under different temperatures at the seed maturation stage for the comparison of transcriptomes on the 25th DAF as well as the comparison of the qualitative traits of the seeds harvested. The 25 DAF seeds at low temperature might not be at the equivalent stage as 25 DAF seeds at high temperature. However, what important is that the developmental stage of the two NILs in a same growth chamber should be parallel. The analysis of microarray data indicate that the genotype, temperature, and interaction between genotype and temperature all significantly affected the expression pattern of the genes in the 25 DAF seeds and, as a result, also affected total seed oil content and fatty acid composition.
NIL-9 had higher seed oil content than did NIL-1 in field experiments (data not shown) as well as in growth chambers (Fig. 3). The highest surplus of seed oil content (9.56%) was detected under the highest temperature condition (T3), and the lowest surplus (1.38%) was found in the chamber with intermediate temperature (T2). The degree of the differences in seed oil content and fatty acid composition between the two NILs paralleled the number of DEGs in the 25 DAF seeds, which was 3,499, 251, and 2,933 for T3, T2, and T1, respectively (Fig. 5). The qOC.C2.2 region spans a genetic distance of 9.0 cM, accounting for less than 1.0% of the entire genome size of B. napus, yet this region accounted for 15.9, 15.5, and 11.7% of the global expression differences under T3, T2, and T1, respectively. Thus, the expressional divergence between the NILs, or among the DH lines for QTL mapping, would be magnified in a high-temperature environment. This may be the reason why the QTL (qOC.C2.2) was only detectable in hot environments during seed maturation. The overlap of DEGs between T2 and T3, which was not caused by changing temperature, can be excluded from the list of the genes that gave rise to the qOC.C2.2.
Parallel to almost every Arabidopsis gene, there exists orthologous copies in certain regions of Brassica genomes because the Arabidopsis and Brassica genomes originated from a common ancestor about 14.5–0.4 million years ago (Blanc and Wolfe 2004a, b). By aligning with the parallel region of Arabidopsis genome, the putative genes at the qOC.C2.2 region were deduced (Fig. 1). Six genes at the QTL region were differentially expressed between the NILs regardless of temperature changes, suggesting core genetic divergences at the QTL region that were unaffected by temperature (Table 2). The allelic divergence of these genes might directly cause the polymorphisms within the QTL. One of these genes that hit Arabidopsis AT5g10360 is a putative transcription factor (F-box family gene) regulating pollen germination and tube growth. One other gene hits Arabidopsis AT5G10360 (Creff et al. 2010; Mahfouz et al. 2006) that encodes a structural constituent of ribosome and regulates the development of embryo. The rest four genes all express in reproductive organs, leaf and vascular tissues. Their molecular and physiological functions are not clear to date. These six genes constitute the core genetic divergences between the two NILs. They might result in trans-regulatory effects giving rise to other 33 DEGs that locate out of the qOC.C2.2 region. Among these 33 genes, five were putative transcription factors orthologous to Arabidopsis VOZ1, SUF4, AT4G14410 (a bHLH protein), AT4G33940 (a C3HC4-type RING finger protein), and AT5G02910 (an F-box family protein). In Arabidopsis, VOZ1 binds to a 38-bp pollen-specific cis-acting region of the AVP1 gene relating to pollen development, and it is also expressed in the phloem tissue and root (Mitsuda et al. 2004). SUF4 is a putative zinc-finger-containing transcription factor and is required for the upregulation of FLC by FRI (Kim et al. 2006; Kim and Michaels 2006). The floral inhibitor FLC, a crucial regulator of flowering time, is positively regulated by the FRI gene in late-flowering winter-annual accessions (Andersson et al. 2008; Wang et al. 2006a). The bHLH genes constituting one of the largest families of transcription factors have a range of different roles in plant cell and tissue development (Toledo-Ortiz et al. 2003). The C3HC4-type RING finger protein functions in zinc ion binding and is involved in response to chitin, a well-established elicitor of plant defense responses (Libault et al. 2007). The F-box family proteins are responsible for substrate specificity in the ubiquitin–proteasome pathway and therefore play a pivotal role in many physiological activities such as cell-cycle progression, transcription regulation, programmed cell death, and cell signal transduction (Kipreos and Pagano 2000; Yang et al. 2008). The differences in these transcription factors may cause divergence in the global expressions of their target genes, thereby leading to differential expression of thousands of genes in response to temperature stimuli (Fig. 5).
GO analysis of DEGs between the two genotypes suggests that a high proportion of GO slims specifically related to transcription regulations, embryo development, stress responses, and photosynthesis affected the formation of seed oil. Furthermore, quantitative RT-PCR experiments confirmed the microarray results, demonstrating that NIL-9 had higher expression of BnLACS1, BnCLO1, and BnLHY1, but lower expression of BnOLEO1 and BnFatA (Fig. 7). In Arabidopsis, LACS1 encodes an acyl-CoA synthetase that acts on long-chain and very-long-chain fatty acids (Lu et al. 2009). CLO1 is expressed preferentially in the embryo and is found within seed lipid bodies (Hanano et al. 2006; Poxleitner et al. 2006). LHY1 is a myb-related putative transcription factor involved in circadian rhythms (Penfield and Hall 2009). FatA is Fatty acyl-ACP thioesterase (Cao et al. 2010; Kachroo et al. 2007), the major determinant of the level of saturated fatty acids found in most plant tissues. OLEO1 (Shimada and Hara-Nishimura 2010; Siloto et al. 2006) is found in oil bodies involved in seed lipid accumulation. The higher contents of the long-chain fatty acids, docosanoic acid (C22:0), and erucic acid (C22:1), in the matured NIL-9 seeds might have partly resulted from the higher expression of BnLACS1 during seed maturation (Fig. 3i, j). Conversely, the lower content of saturated fatty acids, such as palmic acid (C16:0), stearic acid (C18:0), and arachidic acid (C20:0), in the matured NIL-9 seeds could have been caused by the lower expression of BnFatA (Fig. 3b, c, g, k).
Notably, the erucic acid (C22:1) level was higher in the NIL-9 seeds, regardless of temperature conditions (Fig. 3j). FAE1 is a condensing enzyme that extends fatty acid chain length from C18 to C20 and C22 (Lassner et al. 1996). BnFAE1 was not found in the DEGs, because the QTL region (qOC.C2.2) paralleled the fragments of Arabidopsis chromosome 1 and 5, but AtFAE1 (AT4G34520) locates on chromosome 4. Nevertheless, five FAE1 orthologs namely CHLOROPLASTIC ACETYLCOENZYME A CARBOXYLASE 1, GAMMA-GLUTAMYL HYDROLASE 2, an IIF beta subunit (TFIIF-beta) family protein, STEROL METHYLTRANSFERASE 1, and DEOXYHYPUSINE SYNTHASE, which were clustered into one group due to similar expressional pattern, located at the QTL region and differentially expressed between the two NILs (Supplemental Table S3).
In general, temperature had a clear effect on seed oil content and fatty acid composition. The higher the temperature at seed maturation, the lower the seed oil content and yield of unsaturated fatty acid, such as that of linoleic acid (C18:3) (Fig. 3). High temperatures during the seed maturation stage are an important factor limiting the yield in many oilseed rape production regions, such as the middle and lower reach of the Yangtze Valley of China. Our experiment revealed that the increase in temperature caused the downregulation of several genes associated with red light response, photosynthesis, gibberellic acid stimulus response, and translational elongations (Fig. 6), as well as genes associated with lipid metabolism, such as BnLEC1, BnWRI1, BnFAD2, BnFAD3, and BnLPAT2 (Fig. 7). In contrast, the decrease in temperature led not only to the upregulation of the aforementioned lipid genes, but also to the upregulation of the genes associated with transcription regulation, embryonic development, protein biosynthesis, protein amino acid phosphorylation, and stress response. We noticed that BnABA3, an important regulatory factor in ABA signal transduction, was upregulated by higher temperatures. Since ABA plays important role in seed maturation and dormancy. This may contribute to the acceleration of seed maturation and less accumulation of seed storages under higher-temperature conditions. Tchagang et al. (2009) developed a dynamic regulatory map for understanding the interaction between transcription factors and their target genes and predicted the leading roles of LEC1, LEC2, WRI1, FUS3, MYB30, and ABI3 in controlling B. napus seed development and fatty acid metabolism. Our study indicates that temperature significantly affected the expression of BnLEC1, BnWRI1, BnABI3, and BnFUS3. However, neither the interaction between temperature and genotype nor genotype or temperature alone affected the expression of two other transcription factors that regulate seed development, MYB30 and LEC2.
We analyzed the expression profiles of two NILs in response to temperature during seed development and revealed the molecular nature of the genotypic divergence, temperature, and interaction effects between genotype and temperature that lead to seed qualitative trait differences. This work contributes to knowledge on the molecular nature underlying QTLs and the G × T effect. Our findings are also helpful for map-based cloning of the major genes contributing to oil QTLs, the genetic engineering of major regulatory genes for fatty acid accumulation, and improving crop cultivation practices for a higher and more stable seed oil production of oilseed rape.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Andersson CR, Helliwell CA, Bagnall DJ, Hughes TP, Finnegan EJ, Peacock WJ, Dennis ES (2008) The FLX gene of Arabidopsis is required for FRI-dependent activation of FLC expression. Plant Cell Physiol 49:191–200
Arcade A, Labourdette A, Falque M, Mangin B, Chardon F, Charcosset A, Joets J (2004) BioMercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics 20:2324–2326
Beisson F, Koo AJ, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, Mhaske VB, Cho Y, Ohlrogge JB (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132:681–697
Blanc G, Wolfe KH (2004a) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 16:1679–1691
Blanc G, Wolfe KH (2004b) Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 16:1667–1678
Canvin DT (1965) Effect of temperature on oil content and fatty acid composition of oils from several oil seed crops. Can J Botany 43:63–69
Cao Y, Yang J, Xian M, Xu X, Liu W (2010) Increasing unsaturated fatty acid contents in Escherichia coli by coexpression of three different genes. Appl Microbiol Biotechnol 87:271–280
Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Chardon F, Virlon B, Moreau L, Falque M, Joets J, Decousset L, Murigneux A, Charcosset A (2004) Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome. Genetics 168:2169–2185
Cheadle C, Cho-Chung YS, Becker KG, Vawter MP (2003) Application of z-score transformation to Affymetrix data. Appl Bioinformatics 2:209–217
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Creff A, Sormani R, Desnos T (2010) The two Arabidopsis RPS6 genes, encoding for cytoplasmic ribosomal proteins S6, are functionally equivalent. Plant Mol Biol 73:533–546
Delourme R, Falentin C, Huteau V, Clouet V, Horvais R, Gandon B, Specel S, Hanneton L, Dheu JE, Deschamps M, Margale E, Vincourt P, Renard M (2006) Genetic control of oil content in oilseed rape (Brassica napus L.). Theor Appl Genet 113:1331–1345
Edwards CE, Weinig C (2011) The quantitative-genetic and QTL architecture of trait integration and modularity in Brassica rapa across simulated seasonal settings. Heredity 106:661–677
Esteban AB, Sicardo MD, Mancha M, Martinez-Rivas JM (2004) Growth temperature control of the linoleic acid content in safflower (Carthamus tinctorius) seed oil. J Agric Food Chem 52:332–336
Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C (2007) Combined networks regulating seed maturation. Trends Plant Sci 12:294–300
Hanano A, Burcklen M, Flenet M, Ivancich A, Louwagie M, Garin J, Blee E (2006) Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J Biol Chem 281:33140–33151
Hua S, Shamsi IH, Guo Y, Pak H, Chen M, Shi C, Meng H, Jiang L (2009) Sequence, expression divergence, and complementation of homologous ALCATRAZ loci in Brassica napus. Planta 230:493–503
Kabsch W, Sander C (1984) On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci USA 81:1075–1078
Kachroo A, Shanklin J, Whittle E, Lapchyk L, Hildebrand D, Kachroo P (2007) The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol Biol 63:257–271
Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, Mackenzie SL, Covello PS, Kunst L (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108:399–409
Kim SY, Michaels SD (2006) SUPPRESSOR OF FRI 4 encodes a nuclear-localized protein that is required for delayed flowering in winter-annual Arabidopsis. Development 133:4699–4707
Kim S, Choi K, Park C, Hwang HJ, Lee I (2006) SUPPRESSOR OF FRIGIDA4, encoding a C2H2-Type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 18:2985–2998
Kipreos ET, Pagano M (2000) The F-box protein family. Genome Biol 1:REVIEWS3002
Lassner MW, Lardizabal K, Metz JG (1996) A jojoba beta-Ketoacyl-CoA synthase cDNA complements the canola fatty acid elongation mutation in transgenic plants. Plant Cell 8:281–292
Libault M, Wan J, Czechowski T, Udvardi M, Stacey G (2007) Identification of 118 Arabidopsis transcription factor and 30 ubiquitin–ligase genes responding to chitin, a plant-defense elicitor. Mol Plant Microbe Interact 20:900–911
Long Y, Shi J, Qiu D, Li R, Zhang C, Wang J, Hou J, Zhao J, Shi L, Park BS, Choi SR, Lim YP, Meng J (2007) Flowering time quantitative trait Loci analysis of oilseed brassica in multiple environments and genomewide alignment with Arabidopsis. Genetics 177:2433–2444
Long Y, Xia W, Li R, Wang J, Shao M, Feng J, King GJ, Meng J (2011) Epigenetic QTL Mapping in Brassica napus. Genetics. doi:10.1534/genetics.111.131615
Lu S, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009) Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J 59:553–564
Lukens L, Zou F, Lydiate D, Parkin I, Osborn T (2003) Comparison of a Brassica oleracea genetic map with the genome of Arabidopsis thaliana. Genetics 164:359–372
Mahfouz MM, Kim S, Delauney AJ, Verma DP (2006) Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18:477–490
Mahmood T, Rahman MH, Stringam GR, Yeh F, Good AG (2006) Identification of quantitative trait loci (QTL) for oil and protein contents and their relationships with other seed quality traits in Brassica juncea. Theor Appl Genet 113:1211–1220
Meng J, Sharpe A, Bowman C, Tian Z, Fu T, Qian X, Lydiate D (1996) Genetics diversity of Brassica napus detected with RFLP markers. J Genet Genomics 23(4):293–306
Mitsuda N, Hisabori T, Takeyasu K, Sato MH (2004) VOZ; isolation and characterization of novel vascular plant transcription factors with a one-zinc finger from Arabidopsis thaliana. Plant Cell Physiol 45:845–854
Osborn TC, Kole C, Parkin IA, Sharpe AG, Kuiper M, Lydiate DJ, Trick M (1997) Comparison of flowering time genes in Brassica rapa, B. napus and Arabidopsis thaliana. Genetics 146:1123–1129
Parkin IA, Sharpe AG, Lydiate DJ (2003) Patterns of genome duplication within the Brassica napus genome. Genome 46:291–303
Parkin IA, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, Lydiate DJ (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171:765–781
Penfield S, Hall A (2009) A role for multiple circadian clock genes in the response to signals that break seed dormancy in Arabidopsis. Plant Cell 21:1722–1732
Piper EL, Boote KJ (1999) Temperature and cultivar effects on soybean seed oil and protein concentrations. J Am Oil Chem Soc 76:1233–1241
Poxleitner M, Rogers SW, Lacey Samuels A, Browse J, Rogers JC (2006) A role for caleosin in degradation of oil-body storage lipid during seed germination. Plant J 47:917–933
Qiu D, Morgan C, Shi J, Long Y, Liu J, Li R, Zhuang X, Wang Y, Tan X, Dietrich E, Weihmann T, Everett C, Vanstraelen S, Beckett P, Fraser F, Trick M, Barnes S, Wilmer J, Schmidt R, Li J, Li D, Meng J, Bancroft I (2006) A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor Appl Genet 114:67–80
Rost B (1999) Twilight zone of protein sequence alignments. Protein Eng 12:85–94
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Schranz ME, Lysak MA, Mitchell-Olds T (2006) The ABC’s of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11:535–542
Shi J, Li R, Qiu D, Jiang C, Long Y, Morgan C, Bancroft I, Zhao J, Meng J (2009) Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics 182:851–861
Shi CG, Zhu YN, Li YL, Guo WL, Chen KM, Shamsi IH, Hua SJ, Zhong ZF, Zhou WJ, Jiang LX (2010) DNA allelic variations at the loci putatively implicated in seed oil formation among Brassica oilseed cultivars. Mol Breeding 26:51–64
Shimada TL, Hara-Nishimura I (2010) Oil-body-membrane proteins and their physiological functions in plants. Biol Pharm Bull 33:360–363
Siloto RM, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18:1961–1974
Tchagang AB, Bui KV, McGinnis T, Benos PV (2009) Extracting biologically significant patterns from short time series gene expression data. BMC Bioinformatics 10:255
Toledo-Ortiz G, Huq E, Quail PH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15:1749–1770
Udvardi MK, Czechowski T, Scheible WR (2008) Eleven golden rules of quantitative RT-PCR. Plant Cell 20:1736–1737
Wan Y, Poole RL, Huttly AK, Toscano-Underwood C, Feeney K, Welham S, Gooding MJ, Mills C, Edwards KJ, Shewry PR, Mitchell RA (2008) Transcriptome analysis of grain development in hexaploid wheat. BMC Genomics 9:121
Wang J, Tian L, Lee HS, Chen ZJ (2006a) Nonadditive regulation of FRI and FLC loci mediates flowering-time variation in Arabidopsis allopolyploids. Genetics 173:965–974
Wang T, Weir B, Zeng ZB (2006b) A population-based latent variable approach for association mapping of quantitative trait loci. Ann Hum Genet 70:506–523
Wang N, Wang YJ, Tian F, King GJ, Zhang CY, Long Y, Shi L, Meng JL (2008) A functional genomics resource for Brassica napus: development of an EMS mutagenized population and discovery of FAE1 point mutations by TILLING. New Phytol 180:751–765
Yang X, Kalluri UC, Jawdy S, Gunter LE, Yin T, Tschaplinski TJ, Weston DJ, Ranjan P, Tuskan GA (2008) The F-box gene family is expanded in herbaceous annual plants relative to woody perennial plants. Plant Physiol 148:1189–1200
Yang J, Zou Y, Zhu J (2009) Identifying differentially expressed genes in human acute leukemia and mouse brain microarray datasets utilizing QTModel. Funct Integr Genomics 9:59–66
Young ND, Tanksley SD (1989) Restriction fragment length polymorphism maps and the concept of graphical genotypes. Theor Appl Genet 77:95–101
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zou J, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC (1997) Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9:909–923
Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19:645–653
Acknowledgments
The work of LJ lab was supported by Natural Science Foundation of China (30971700 and 31171463) and of Zhejiang Province (Z3100130), Special Grand National Science and Technology Project (2009ZX08009-076B). Work at the JZ lab was supported in part by the National Research Council Canada-Genomic and Health Initiative Program. The authors are indebted to Dr. Faouzi Bekkaoui and Dr. Wenyun Shen for project management and technical support, to Dr. Jianyi Zhao of Zhejiang Academic of Agricultural Sciences for providing a part of the molecular markers for genotyping and sequence alignment.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by R. Visser.
Y. Zhu and Z. Cao contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, Y., Cao, Z., Xu, F. et al. Analysis of gene expression profiles of two near-isogenic lines differing at a QTL region affecting oil content at high temperatures during seed maturation in oilseed rape (Brassica napus L.). Theor Appl Genet 124, 515–531 (2012). https://doi.org/10.1007/s00122-011-1725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1725-2