Abstract
Section Arachis of the homonymous genus includes 29 wild diploid species and two allotetraploids (A. monticola and the domesticated peanut, A. hypogaea L.). Although, three different genomes (A, B and D) have been proposed for diploid species with x = 10, they are still not well characterized. Moreover, neither the relationships among species within each genome group nor between diploids and tetraploids (AABB) are completely resolved. To tackle these issues, particularly within the A genome, in this study the rRNA genes (5S and 18S–26S) and heterochromatic bands were physically mapped using fluorescent in situ hybridization (FISH) in 13 species of Arachis. These molecular cytogenetic landmarks have allowed individual identification of a set of chromosomes and were used to construct detailed FISH-based karyotypes for each species. The bulk of the chromosome markers mapped revealed that, although the A genome species have a common karyotype structure, the species can be arranged in three groups (La Plata River Basin, Chiquitano, and Pantanal) on the basis of the variability observed in the heterochromatin and 18S–26S rRNA loci. Notably, these groups are consistent with the geographical co-distribution of the species. This coincidence is discussed on the basis of the particular reproductive traits of the species such as autogamy and geocarpy. Combined with geographic distribution of the taxa, the cytogenetic data provide evidence that A. duranensis is the most probable A genome ancestor of tetraploid species. It is expected that the groups of diploid species established, and their relation with the cultigen, may aid to rationally select wild species with agronomic traits desirable for peanut breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachis is a South American native genus that comprises around 80 annual or perennial species arranged in nine taxonomic sections on the basis of morphology, cross-compatibility, viability of the hybrids (Krapovickas and Gregory 1994; Valls and Simpson 2005), and cytogenetics (Fernández and Krapovickas 1994). Among them, section Arachis is one of the most derived and diverse sections of the genus.
Section Arachis comprises 29 wild diploid species (2n = 2x = 20, 2n = 2x = 18) and two allopolyploids (2n = 4x = 40), the cultigen A. hypogaea (peanut) and A. monticola (Fernández and Krapovickas 1994; Lavia 1996, 1998, 2000; Peñaloza and Valls 2005). The natural distribution of these species extends in a east–west direction between the Chapada dos Parecis in the central west of Mato Grosso State (Brazil) and the northern edge of the Chacoan region. From this latitudinal central axis, in the east, the species extend toward the northeast along the Tocantins River (central Brazil) and southward along the Paraguay–Paraná and Uruguay River Basins (Paraguay, Argentina and Uruguay) reaching the northern shore of La Plata River. In the west, they are found toward the northwest along the Mamoré and Guaporé Rivers in north Bolivia and toward the southwest along the Parapetí, Pilcomayo, Bermejo, San Francisco and Juramento River Basins in southern Bolivia and northern Argentina.
Within section Arachis, three different genomes have been proposed for the diploid species with x = 10 according to their cross-compatibility and chromosome features (Smartt et al. 1978; Singh and Moss 1982, 1984; Singh 1986; Stalker 1991; Fernández and Krapovickas 1994; Singh and Smartt 1998). Most of these taxa have symmetric karyotypes, largely composed of metacentric chromosomes, and have been assigned to either the A or the B genomes. The A genome species are mostly perennials and karyotypically homogeneous, characterized by a small chromosome pair [the “A chromosome” after Husted (1933)]. The remaining species with symmetric karyotypes, but without the “A chromosome” pair, have been traditionally assigned to the B genome (or non A genome). These taxa are more diverse in karyotype formula and are annuals. The D genome, exclusively borne by the annual species, A. glandulifera (Stalker 1991), is characterized by an asymmetric karyotype with several submetacentric and subtelocentric chromosomes and the absence of the A pair (Robledo and Seijo 2008). Species with 2n = 40 are allotetraploid with an AABB genome constitution (Gregory and Gregory 1976, 1979; Seijo et al. 2007). Diploid species with x = 9 are not well characterized, and their genome constitutions still have to be determined.
The 15 wild diploid entities included within the A genome group (Fernández and Krapovickas 1994; Lavia 1996, 2000; Peñaloza and Valls 2005; Robledo et al. unpublished) are morphologically similar, cross compatible, and produce fertile hybrids with near normal chromosome pairing (Smartt et al. 1978; Stalker and Wynne 1979; Tallury et al. 2005). Based on these facts, the group has usually been considered highly homogeneous. However, the species inhabit extremely diverse ecological regions and are adapted to localities with very different selective pressures; that is, from sand dunes in xerophytic chacoan forest to the flooded plains of the Pantanal and from equatorial to temperate mid latitude climates. Moreover, as opposed to the modern peanut cultivars, that generally have a narrow genetic base (Isleib et al. 2001), the A genome species are genetically diverse (Kochert et al. 1991; Milla et al. 2005) and possess a number of genes valuable for peanut improvement, such as resistance/tolerance to early and late leaf spot, rust, nematodes and abiotic stresses (Burow et al. 2001; Simpson 2001; Dwivedi et al. 2007).
Concerning the origin of tetraploids, and in particular of peanut, a number of A genome species has been considered as putative genome donors on the basis of cytogenetic and molecular evidence (Gregory and Gregory 1976; Smartt et al. 1978; Singh 1986, 1988; Kochert et al. 1991; Fernández and Krapovickas 1994; Singh and Smartt 1998; Raina and Mukai 1999; Seijo et al. 2004, 2007; Milla et al. 2005). Four of the 15 species of the group have been proposed as parents of A. hypogaea (Krapovickas and Rigoni 1951, A. correntina; Gregory and Gregory 1976, A. cardenasii and A. duranensis; Smartt et al. 1978, A. cardenasii; Singh 1986, 1988, A. duranensis; Raina et al. 2001b, A. villosa), and others were revealed as closely related genetically to the tetraploids (Smartt and Gregory 1967 A. helodes; Milla et al. 2005, A. helodes and A simpsonii). Therefore, the relatedness of wild species to the cultigen is still unresolved, and the subject is a matter of intense research.
In phylogenetic approaches to the study of Arachis, the A genome species have always been treated in the context of the whole section or of the genus, and analyses have been principally focused on identifying peanut progenitors. Because of the high similarity of the species’ karyotypes, intragenomic species relatedness could not be established in pioneering cytogenetic studies (Fernández and Krapovickas 1994; Lavia 1996, 2000; Peñaloza and Valls 2005). In view of this concern, a wide spectrum of molecular markers have been developed and used toward constructing various phenetic and phylogenetic schemes (Kochert et al. 1991; Raina et al. 2001b; Gimenes et al. 2002; Moretzsohn et al. 2004; Milla et al. 2005; Tallury et al. 2005). All such analyses revealed the A genome species as a natural group, but because most of the studies included incomplete and different sets of species and because they were based on different methods, a comparative analysis of these reports revealed several inconsistencies. Therefore, a realistic framework of the species relationships within the A genome group has still been needed.
The rRNA genes are arranged in tandem repeats and occupy specific regions on one or several chromosomes. These clusters can change rapidly, both in number and in chromosomal location (Chung et al. 2008). Physical mapping by FISH of these gene clusters has proved to be a useful tool in the study of plant genome organization, the construction of reference physical maps, and for the analysis of species relationships (Jiang and Gill 1994; Moscone et al. 1999; Lim et al. 2000; Nakamura et al. 2001; Pires et al. 2004). This approach has been used for a few species of Arachis to demonstrate that the number of rDNA loci is variable (Raina and Mukai 1999). The subsequent mapping of these sites and heterochromatic bands allowed the discovery of chromosome homologies among some wild species and between the wild species and the tetraploids (Seijo et al. 2004).
Considering the aforementioned, in the present report we used rDNA FISH mapping and DAPI + heterochromatic bands in somatic metaphases of 13 species with the A genome to (1) investigate species relations by analyzing chromosome homologies among diploids and (2) to comprehensively re-evaluate the putative A genome donors of peanut by comparing the chromosome landmarks between diploid and tetraploid species. To determine interspecific homologies, we needed to build detailed cytogenetic maps for each species. The data provide reliable chromosome landmarks for FISH-based maps, improve our knowledge of the relations between the wild Arachis species and the cultigen, and contribute to the understanding of the variability harbored in the secondary gene pool of A. hypogaea.
Materials and methods
Plant material
Seeds of the Arachis species used in this study were obtained from the peanut germplasm collections maintained at the INTA Manfredi station in Córdoba, Argentina; at the Instituto de Botánica del Nordeste in Corrientes, Argentina; and at the Texas Agriculture Experimental Station, Stephenville, Texas, USA. The original provenances and voucher specimens of the samples studied are cited in Table 1. Arachis linearifolia and A. microsperma were not included in this analysis because either there is no material available in the seed banks or the countries where these species live restrict germplasm collection and exchange. Also, A. trinitensis was excluded from the study because it was reassigned to the B genome. Recent detailed karyotype analysis revealed that this species lacks the A chromosome pair (Robledo et al. unpublished). A. chiquitana (formerly of section Procumbentes) was included in this study since Lavia (2000) recommended its inclusion within section Arachis on the basis of the karyotype features (A chromosomes and SAT chromosomes type 3) found in analyzed specimens. The geographic distribution of the diploid species analyzed and the center of major diversity of A. hypogaea subsp. hypogaea, which is considered the most ancestral subspecies of peanut, are shown in Fig. 1.
Geographic distribution of Arachis species studied. 1 A. kempff-mercadoi; 2 A. cardenasii; 3 A. herzogii; 4 A. chiquitana; 5 A. simpsonii; 6 A. stenosperma; 7 A. helodes; 8 A. kuhlmannii; 9 A. diogoi; 10 A. schininii; 11 A. correntina; 12 A. villosa; 13 A. duranensis; 14 Major center of landraces with ancestral characters of A. hypogaea. Dark grey shaded Chiquitano group, light grey shaded Pantanal group and striped La Plata River Basin group. Dashed line indicates the geographic distribution of section Arachis
Chromosome preparations
Plants obtained from seeds were grown in pots under laboratory conditions. Healthy root tips (5–10 mm long) of seedlings were pretreated with 2 mM 8-hydroxyquinoline for 3 h at room temperature (Fernández and Krapovickas 1994) and, fixed in 3:1 absolute ethanol:glacial acetic acid for at least 12 h at 4°C. Somatic chromosome spreads were prepared according to Schwarzacher et al. (1980). Root apices were digested in 1% (w/v) cellulose (from Trichoderma viridae; Onozuka R-10, Serva, Heidelberg, Germany) plus 10% (v/v) pectinase dissolved in 40% glycerol (from Aspergillus niger, Sigma, St. Louis, Missouri, USA) in 0.01 M citrate buffer, pH 4.8, at 37°C for 2 h. Subsequently, the meristematic cells were removed from the root tip and squashed in 45% acetic acid. After removal of the coverslip with carbon dioxide, the slides were air dried, aged for 1–2 days at room temperature, and then kept at −20°C until use.
Probe labeling and fluorescent in situ hybridization
The 5S rDNA and 18S–26S rDNA loci were localized using probes isolated from genomic DNA of A. hypogaea subsp. fastigiata Waldron var. aequatoriana Krapov. and W. C. Gregory (race Zaruma; Ecuador, Quito, collection no.: Banks, Pietrarelli and Zurita 683) (Robledo and Seijo 2008). The 5S rDNA loci were mapped using the plasmid clone pA5S, which contains a 326-bp fragment corresponding to the 5S rRNA gene repeated unit, including the adjacent intergenic spacer. The 18S–26S rDNA loci were detected using an equimolar mixture of the plasmid clones pA18S and pA26S. The clone pA18S contains a 1,572-bp fragment corresponding to the coding region of the 18S rRNA gene; and the clone pA26S has a 3366-bp fragment corresponding to the coding region of the 26S rRNA gene. Probes were labeled by nick translation with digoxigenin-11-dUTP (Boehringer Mannheim, Mannheim, Germany) or biotin-11-dUTP (Sigma Aldrich, St. Louis, Missouri, USA).
Pretreatment of preparations, chromosome and probe denaturation, conditions for the in situ hybridization (hybridization mixes contained DNA probes at a concentration of 2.5–3.5 ng/μL, with a stringency to allow sequences with 80–85% identity to remain hybridized), posthybridization washing, blocking and indirect detection with fluorochrome-conjugated antibodies were performed according to Moscone et al. (1996). The first set of antibodies consisted of mouse anti-biotin (Dakopatts, Dako, Carpinteria, California, USA) and sheep anti-digoxigenin conjugated to fluorescein isothiocyanate (FITC) (Boehringer Mannheim). The second set of antibodies consisted of rabbit anti-mouse conjugated to tetramethyl-rodamine isothiocyanate (TRITC) (Dakopatts) and FITC-conjugated rabbit anti-sheep (Dakopatts). Preparations were counterstained and mounted with Vectashield medium (Vector Laboratories, Burlingame, California, USA) containing 2 mg/mL of 4′,6-diamidino-2-phenylindole (DAPI). The counterstaining with DAPI revealed a C-banding-like pattern, with major heterochromatic bands fluorescing more intensely (cf. Seijo et al. 2004).
Fluorescence microscopy and image acquisition
Chromosomes were analyzed and photographed with a Leica DMRX epifluorescence microscope (Leica, Heerbrugg, Switzerland) equipped with a computer-assisted Leica DC 350 digital camera system. Red, green and blue images were captured in black and white using appropriate filters for TRITC, FITC, and DAPI excitation, respectively. Digital images were combined using IM 1000 Leica software and then imported into Photoshop, version 7.0 (Adobe, San Jose, California, USA) to process color balance, brightness, and contrast uniformly across the image.
Karyotype analysis and loci mapping
At least three plants per species and five metaphase plates per individual were used for chromosome measurements using the free version of the MicroMeasure 3.3 program (http://www.colostate.edu/Depts/Biology/MicroMeasure/). The centromeric index (i = short arm length × 100/chromosome length) was used to classify the chromosomes according to Levan et al. (1964) as metacentric (m, i = 37.51–50), submetacentric (sm, i = 25.10–37.50) and subtelocentric (st, i = 12.51–25). Data from homologous chromosomes were combined to mean values—first among chromosomes in the same metaphase and subsequently between chromosomes in different metaphases of each species.
Mean karyotypic values for each species were represented as haploid complements in the ideograms. Chromosomes were ordered primarily by morphology and then by decreasing size. Subsequently, some chromosomes within each ideogram were rearranged using the nomenclature for the A genome of A. hypogaea and A. monticola in Seijo et al. (2004) so as to best fit the assumption that chromosomes fall into homologous groups with respect to the heterochromatic bands and the probes used.
Results
The general karyotype features, distribution and number of heterochromatic bands of the 5S and 18S–26S rDNA loci mapped for 13 Arachis species with the A genome are listed in Table 2. Representative somatic metaphases are shown in Fig. 2, and the consensus idiograms for each species are illustrated Fig. 3.
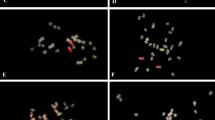
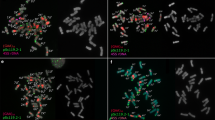
Somatic metaphases of Arachis species after double fluorescent in situ hybridization (FISH), showing the 5S rDNA loci (yellow-green signals), and 18S-26S rDNA loci (red signals). DAPI counterstaining (light blue) subsequent to the FISH procedure was used to highlight the heterochromatin bands and to stain euchromatin. a A. chiquitana; b A. kempff-mercadoi; c A. cardenasii; d A. herzogii; e A. diogoi; f A. kuhlmannii; g A. stenosperma; h A. helodes; i A. simpsonii; j A. schininii; k A. correntina; l A. villosa; m A. duranensis. Scale bar 5 μm (color figure online)
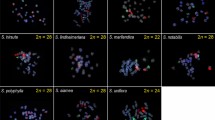
Idiograms of Arachis species showing the distribution of 5S (striped) and 18S–26S rDNA loci (black shaded), DAPI-enhanced heterochromatic bands (white shaded), DAPI-pale low condensed euchromatic regions (light grey shaded), and normally condensed euchromatin (dark grey shaded). Genes were mapped by FISH, and heterochromatic and differentially condensed euchromatic regions were distinguished by DAPI counterstaining of the FISH preparations. Asterisks in A4 pair of A. herzogii and A7 pair of A. diogoi and A. kulhmannii indicate 18S–26S rDNA sites observed in only one homolog. The ideogram of A. hypogaea/A. monticola was redrawn from Seijo et al. 2004. In the diploid species, the chromosomes were ordered by morphology, then according to decreasing size, except those with tentative homologies with chromosomes of the A. hypogaea. Chromosomes with similar morphology that lack any characteristic landmark were arranged in groups. Scale bar 3 μm
General karyotype features
The karyotypes of all species mainly consisted of metacentric chromosomes with a mean length between 2.81 μm (A. simpsonii) and 4.00 μm (A. kuhlmannii). Eleven entities had a haploid karyotype formula of 9 m + 1 sm, whereas A. correntina and A. schininii presented a formula of 8 m + 2 sm and 9 m + 1 st, respectively. The total haploid karyotype length varied between 28.19 μm in A. simpsonii and 39.77 μm in A. kuhlmannii (Table 2). The indexes of intrachromosomal asymmetry (A1) ranged from 0.19 to 0.24, while the interchromosomal asymmetry (A2) ranged from 0.13 to 0.17. Differences in asymmetry were mainly due to the presence of subtelocentric or submetacentric pairs in some species. As a whole, the karyotypes of A. cardenasii, A. correntina and A. schininii had higher asymmetry, while those of A. chiquitana and A. kempff-mercadoi were the least asymmetrical (Table 2).
Heterochromatin distribution
Nine species displayed conspicuous C-DAPI + centromeric bands in all the chromosome pairs; while A. cardenasii, A. chiquitana, A. herzogii and A. kempff-mercadoi presented one or two pairs (A7 and A4) with small and faint bands or without them (Fig. 2). However, the total amount of heterochromatin was similar in all the taxa and ranged from 10.28% (A. schininii) to 14.67% (A. simpsonii) of the karyotype length (Table 2).
In all the species analyzed, the size of the bands in relation to the chromosome length was homogeneous in most of the pairs, ranging from 9.33% (A. schininii) to 12.57% (A. duranensis). Exceptions were the A9 pair, in which the band ranged from 25.32% (A. schininii) to 46.28% (A. kempff-mercadoi), and the submetacentric A10 pair, in which the band ranged from 14.50% (A. correntina) to 20.12% (A. stenosperma). Arachis schininii was the only species in which the heterochromatic band of A10 pair was similar in relative size (9.08%) to that observed in the rest of chromosomes of the complement.
Chromosome mapping of the 5S and 18S–26S rRNA genes by FISH
All the diploid species have only one pair of 5S rDNA sites, localized in the interstitial position of the short arms of medium-sized metacentric chromosomes (A3), except for A. duranensis and A. correntina in which it is proximal (Fig. 3). These loci covered one fourth to one third of the length of the arms and had a strong fluorescent signal.
In contrast to the conserved 5S loci; the number, size and chromosomal localization of the 18S–26S rDNA sites varied among the diploids. The number of these gene clusters ranged from two (A. correntina, A. duranensis, A. schininii and A. villosa) to four pairs (A. cardenasii), with size variation between the loci of each karyotype (Fig. 2). From the detailed analysis of the 18S–26S rDNA loci several general features of the loci distribution within the complements of the A genome species (Fig. 3) can be discerned. Two pairs of loci were shared by all the species, one in the long arms of the A2 pair, and the other in the long arms of the submetacentric/subtelocentric A10 pair (Fig. 2). Both loci had a strong fluorescent signal and were localized in proximal position in almost all species. However, in the A10 pair of A. herzogii and A. villosa the loci were more interstitial. Nine of the 13 species had 18S–26S rDNA sites in the A7 chromosomes proximally localized on the long arms (usually in both homologous chromosomes, except in A. diogoi and A. kuhlmannii). In addition, A. cardenasii had a fourth pair of signals that mapped in proximal position on the long arms of A4 chromosomes, while in A. herzogii this locus was observed in only one of the homologous.
The size and intensity of the signals varied between species; however, the hybridization patterns of the 18S–26S rDNAs had some commonalities (Figs. 2, 3). In general, the largest loci were located in A10 pair, except for A. duranensis and A. correntina that were in the A2 chromosomes. In the other species, the A2 pair carried loci of intermediate size, while the smallest and faintest signals were borne by the A7 and A4 chromosomes.
According to the number, size, and distribution of the 18S–26S rDNA sites and to the pattern of heterochromatic bands three groups of species could be established (Fig. 3). The first one is the Chiquitano group, defined by having one or two chromosome pairs with small heterochromatic bands or without heterochromatin, and by three to four pairs of 18S–26S rDNA sites. This group includes A. cardenasii, A. chiquitana, A. herzogii, and A. kempff-mercadoi. The remaining species, which characteristically had heterochromatic bands in all the chromosomes, could be arranged in two different groups according to the number of 18S–26S rDNA sites. One of them, the Pantanal group, includes the species with three pairs of sites (A. diogoi, A. helodes, A. kuhlmannii, A. simpsonii, A. stenosperma), and the other, La Plata River Basin group, is composed of the taxa with two pairs of sites (A. correntina, A. duranensis, A. schininii, A. villosa).
The comparative analysis of the karyotype formula, asymmetry indices and morphology of chromosomes with rDNAs sites showed that the species included in the Chiquitano and Pantanal groups are very similar in their gross karyotype structure, while each of the taxa included in the third group had its own particular karyotype characteristics.
Chromosomes with nucleolar organizer regions (NORs)
The number of chromosomes with active 18S–26S rDNA clusters (that is, those clusters that remain uncondensed at metaphase and show negative DAPI staining) was variable between species, individuals or even between cells of one plant, ranging from two to six per plate. In most species, the two clusters of the A10 homologous were always active. For the species included in the Pantanal and La Plata River Basin groups, when additional clusters were active, the third corresponded to one borne by an A2 chromosome and the fourth to one located on an A7 chromosome. On the contrary, for the species within the Chiquitano group, the third and fourth active clusters corresponded to those located on the A7 chromosomes, the fifth to one on an A2 chromosome, while the sixth to one on an A4 chromosome. Arachis duranensis and A. correntina are exceptions to the pattern described because the most frequently active 18S–26S rDNA sites corresponded to those on the A2 pair, and additional active sites were located on the A10 pair. Arachis cardenasii is particular among the Chiquitano group because the third and fourth active sites corresponded to those on the A4 pair and the fifth site to one located on an A2 chromosome.
Discussion
The identification of genomic relations through chromosomal analysis is based on the idea that closely related species may have karyotypes that are more similar than those of species that are more distantly related. However, the cytological data previously published for species with the A genome (Fernández and Krapovickas 1994; Lavia 1996, 2000; Peñaloza and Valls 2005) contributed little information toward this purpose because of the high uniformity of the karyotypes as revealed by Feulgen staining. Our morphometric analysis on metaphase chromosomes counterstained with DAPI (after FISH treatment) is congruent with previous reports that all the species have very similar karyotype formulas and that no significant differences could be detected for their total chromosome length and karyotype asymmetry indices. Therefore, we exploited the ability to physically map DNA sequences using FISH combined with C-DAPI banding to generate additional chromosomal markers and to investigate species relations within the A genome group of Arachis.
Chromosomal patterns of heterochromatin and 5S and 18S–26S rDNA loci
The present report gives the first description of heterochromatic bands and rDNA loci localization for seven species, confirms the mapping of these loci in three, and gives additional information for clarifying the exact number and position of the rDNA loci in two taxa for which the published data were inconsistent. Here, we demonstrate that all the species included in the A genome group, besides having the small A chromosomes, have heterochromatic bands in all or almost all the chromosomes of the complement.
The centromeric bands of heterochromatin observed in all the species corresponded to AT-rich regions, as demonstrated by direct staining with DAPI (G. Seijo et al. unpublished). The exclusive position of the bands in the pericentromeric regions agrees with the patterns previously reported for a few species of the group (Raina and Mukai 1999; Seijo et al. 2004). According to the criteria used for other plant groups (Guerra 2000), the common patterns of heterochromatin distribution and composition observed between these species suggest a similar genomic organization and reflects that the species within the A genome are phylogenetically closely related. At the chromosome organization level in the interphasic nucleus, the equilocalization of the AT-rich heterochromatic bands in the complements of these species is congruent with the predictions of Schweizer and Loidl’s model (1987) for karyotypes composed of small chromosomes having a Rabl orientation.
The analysis of the 5S rDNA showed that the number, chromosome location and size were highly conserved among the complements of all the species. Our findings coincide with the four previously analyzed species except for A. stenosperma, in which we observed only one pair instead of the two reported by Raina and Mukai (1999). In this species, polytypic differences in karyotype formula and in type of SAT chromosomes were found between Brazilian plants from the Pantanal (VSSaGdW 7762, analyzed in this work) and those from the Atlantic coast (accession HLK 410, analyzed by Raina and Mukai 1999) (Lavia 1996; Custodio et al. 2005). Therefore, differences in 5S rDNA loci may be attributed to true interpopulation variation rather than to the lack of detecting one pair of loci.
As opposed to the high homogeneity of the heterochromatic patterns and the 5S rDNA loci, the number and distribution of the 18S–26S rDNA loci varied significantly among the 13 species analyzed. Our results are consistent with most of those reported previously for a few species (Raina and Mukai 1999, Seijo et al. 2004). For those species in which a discrepant number of loci were reported, our results always revealed the highest; that is four sites for A. villosa (according to Seijo et al. 2004) and eight sites for A. cardenasii (according to Raina and Mukai 1999). In both cases, the additional sites we detected corresponded to small loci. The differences observed may correspond to the better sensitivity of our technique, attributable to the probes used in each experiment, although interpopulation differences cannot be ruled out.
Even though the pattern of hybridization of rDNA loci showed a high degree of homomorphism in the number, intensity and size among the homologous chromosomes, the results showed heteromorphism for the 18S–26S rDNA in the A7 pair (A. diogoi and A. kuhlmannii) and in the A4 pair (A. herzogii). This heteromorphism could be attributed to either a true absence of the locus, or alternatively, to the presence of an undetected small cluster. The latter hypothesis is supported because in FISH analyses the signal strength of a particular locus is positively related to the number of copies of the repeated sequences present in the cluster (Moscone et al. 1999) and are generally only visualized when the target DNA is larger that 10 kb.
Nucleolar organizer region
So far, only one pair of SAT chromosomes has been reported for diploid Arachis [except for one accession of A. kulhmanii with two (Fernández and Krapovickas 1994)] based on classical techniques and molecular cytogenetic experiments. A detailed analysis of metaphases showed that some species may have two to six chromosomes with extended DAPI-negative chromatin regions (secondary constriction), that hybridized with the 18S–26S rDNA probe. Decondensation of nucleolar organizer region sequences during the interphase stage has been correlated with transcriptional activity (Appels et al. 1986). Moreover, it has been proposed that the appearance of stretched DAPI-negative secondary constrictions at metaphase reflects the remnants of decondensed ribosomal chromatin that was transcriptionally active in the preceding cell cycle at NORs (Suja et al. 1997; Pontes et al. 2004; Ansari et al. 2008). In the interphasic cells of Arachis species, the 18S–26S rDNA loci were visualized in the form of stretched or diffuse chromatin, indicating that the extended secondary constrictions observed in metaphasic chromosomes correspond to loci with transcriptional activity in the previous interphase. Variation in the number of active loci per cell of any individual in Arachis may be related to the epigenetic control of any particular region and to the number of copies that can be activated in each locus to accomplish particular demands of rDNA genes transcription.
Congruence between karyotype homologies and species geographic distribution
This study has shown that within the A genome species of Arachis, the number and positions of major 18S–26S rDNA sites varied among species. These variations in rDNA loci distribution may have phylogenetic implications because the closeness of taxa is usually correlated with the similarity of their rDNA FISH patterns (Hizume et al. 2002; Liu et al. 2003). Our results showed that three groups of species could be established on the basis of the karyotype homeologies. More interesting is the fact that these groups are supported by the geographic distribution of the entities. Species within each group tend to be more closely distributed than those belonging to different groups. The first group (Chiquitano) comprised the species that grow in the southern and western portion of the Chiquitanía biogeographic region in Santa Cruz Department of Bolivia. Some of these species (A. cardenasii, A. herzogii, A. chiquitana) have overlapping distribution around the San José and Santiago de Chiquitos ranges (S of the department); while A. kempff-mercadoi grows in an area located at the NW of the department (Krapovickas and Gregory 1994). Even though these areas appear slightly disjunct (separated by around 100 km), the exact boundaries of the species ranges are still unknown and may probably overlap to some extent. The second group (Pantanal) includes the species distributed in the Pantanal biogeographic region, in western Brazil, northern Paraguay and eastern Bolivia. Arachis diogoi, although having few known populations, has the broader natural distribution of the group growing from the nascent of the Paraguay River in the frontier between Mato Grosso do Sul State (Brazil) and in the center of the Santa Cruz department in Bolivia to Puerto Casado in northern Paraguay. Arachis kuhlmannii lives along the northeastern and southeastern limits of the Pantanal region, while A. helodes and A. simpsonii are only distributed in the northeast and northwest edges of this region, respectively. The last species of the group, A. stenosperma, is unique because it has a disjunct distribution with one set of populations in the southeast of the Pantanal and the other set on the Atlantic coast of Paraná State (Brazil). However, it has been proposed that the latter populations originated as a consequence of long-distance dispersal by ancient people through what it is called the Piauí road (Krapovickas and Gregory 1994; Custodio et al. 2005; Valls personal communication). The third group (La Plata River Basin) corresponds to the species distributed along the La Plata River basin (except for the region that comprises the upper stream of the Paraguay River in the Pantanal). These species have a disjunct distribution separated by the Chacoan region. One of them (A. duranensis) lives in the eastern edge of Los Andes Mountains and the adjacent lowlands in the south of Bolivia and the northwest of Argentina. The other three species grow in an area limited by the Paraná, Paraguay, and Uruguay Rivers, from North Paraguay toward the La Plata River in Uruguay. Arachis schininii grows in the western lowlands of the Amambay ranges of northern Paraguay, A. correntina in northwestern Corrientes province (Argentina) and in a region of lateritic soils close to Asunción city in Paraguay, and A. villosa on the sandy riversides of the Uruguay River (Krapovickas and Gregory 1994; Valls and Simpson 2005). Both areas, although separated by around 1,000 km are linked by the Pilcomayo, Bermejo and Salado Rivers that cross throughout the Chacoan region in a NW–SE direction. These rivers serve as corridors for many plant groups distributed on both sides of the Chacoan regions (Spichiger et al. 2004; Popolizio 1982) and probably have also acted as dispersal pathways for Arachis species.
Of the several molecular analyses of Arachis so far, most were focused mainly on determining the putative ancestors of A. hypogaea and usually included few diploids species with the A genome. Therefore, it is still difficult to integrate molecular data with our chromosome results. However, support for the Pantanal group is found in most of those reports (Kochert et al. 1991; Raina et al. 2001b; Milla et al. 2005). The Chiquitano group is the least represented in previous analyses, but whenever A. herzogii and A. kempff-mercadoi have been included, they always clustered together (Milla et al. 2005; Tallury et al. 2005). Our finding that the La Plata River group is the most diverse agrees with the greater genetic distance found for these species in dendrograms constructed using molecular markers (Milla et al. 2005). This fact may be due to their disjunct distribution and to the broader ecological regimes to which the species are adapted—from the xerophytic subtropical sand dunes in the western dry Chacoan region in Bolivia (A. duranensis) to the mild to cold temperatures of La Plata River (A. villosa).
It is important to note that despite the overlapping geographic range of many of the A genome species within each group, it is very rare to find individuals of different species living sympatrically (Seijo personal observation). This fact may be explained by the heterogeneity that each biogeographical region has at a local scale, where different soil and vegetation types are arranged as an intermingled set of patches with particular ecological regimes. Therefore, the usual allopatric distribution of the Arachis species at a local scale may be a consequence of the particular ecological preferences of each species. This local isolation, together with the predominance of autogamous fertilization, may also explain the fact that, even though many species of the A genome can be artificially crossed to produce hybrids of variable fertility, to our knowledge not a single natural hybrid has so far been identified.
Evolution of the 45S and 5S rDNA
Conservation of the 5S rDNA loci is not a common feature in legumes, for which variability in number, location, or both has been reported in the well-studied papilionaceous genera Vicia (Raina et al. 2001a) and Phaseolus (Moscone et al. 1999). The conserved distribution pattern of these loci in Arachis may be a consequence of their paracentromeric localization in the chromosomes (Martins and Galetti 2001), as opposed to other genera in which they are interstitial or distal. This hypothesis is supported by the facts that proximal regions of small chromosomes (such as those of Arachis) are rarely involved in structural rearrangements (Schweizer and Loidl 1987) and that most chiasmata in wild Arachis occurred distally (Lavia et al. 2001; Rodriguez et al. 2004).
Variation in the size and intensity of the hybridization signal has been inferred to reflect a difference in copy number of the tandemly repeated units (Li et al., 1997; Cerbah et al. 1998; Murata et al. 1997). Copy number of rDNA has not been determined in Arachis; however, the uniform size of the 5S rDNA sites suggests a similar number of copies of these genes in the clusters of all the analyzed species. By contrast, the diverse number of sites and intensity observed in the 45S rDNA loci suggests a variable number of gene copies both among loci of each complement and between species.
On the other hand, the variation in number, position, and size of the 45S rDNA sites observed between Arachis species suggest an active mechanism of loci re-patterning. The general structural similarity among the karyotypes of all the species studied suggested that major chromosome rearrangements are not frequent in this group. Therefore, mechanisms such as transposon mobility, amplification of cryptic minor rDNA sites or reduction of larger loci by unequal crossing-over rather than chromosomal rearrangements may be involved in the origin of the variation of the 45S rDNA (Schubert and Wobus 1985). Geographically, the Amambay ranges in Mato Grosso do Sul State of Brazil and northeast Paraguay is considered the center of origin of the genus. From this area, the genus extended toward the north, west and south. Within section Arachis, the species around the Pantanal would be the ancestral, while those distributed south- and westward may be derived. Considering this hypothesis, a reduction in the number of 45S rDNA in southern species and an increase in sites in western species would probably have occurred.
Genome donors of A. hypogaea
Several of the A genome species have been proposed as the genome donors of A. hypogaea. According to the features of the A genome of the cultigen, that has all of its chromosomes with conspicuous pericentromeric bands and just two pairs of 18S–26S rDNA loci (Fig. 3), the species included in the Chiquitano group can be excluded because they have one or two chromosome pairs without centromeric bands and a high number of 18S–26S rDNA sites. Similarly, the species of the Pantanal group are improbable genome donors of peanut because they have more 18S–26S rDNA loci than those observed in A. hypogaea. Within the species included in the La Plata River Basin, A. schininii can be easily excluded because of the presence of a subtelocentric chromosome pair, and A. correntina by the presence of an additional submetacentric pair than that borne by the A complement of A. hypogaea. Considering the remaining two species (A. duranensis and A. villosa), even though they are very similar in their chromosome markers, they are the most distantly distributed taxa within the group. Arachis duranensis lives close to B genome species like A. ipaensis and A. batizocoi, and in the area of A. monticola. In addition, according to the variability of the landraces of A. hypogaea, an area comprising south Bolivia to north Argentina is traditionally considered the primary candidate location for the domestication of peanut, as shown in Fig. 1 (Krapovickas and Gregory 1994). Arachis villosa, however, lives on the opposite side of the Chacoan region, very far from any B genome species and downstream of the rivers that cross it (which makes improbable long-distance dispersal by fluvialtile hydrocory toward the area of the B genome species). Therefore, our analysis of most A genome species of Arachis supports A. duranensis as the most probable genome donor of A. hypogaea. These results are in agreement with a GISH analysis in which the genome DNA of A. duranensis hybridized more strongly with the A genome chromosomes of A. hypogaea than with those of A. villosa (Seijo et al. 2007). Moreover, results on amphidiploid resynthesis showed that when A. duranensis and A. ipaensis are crossed and the chromosomes doubled by colchicine, the resultant tetraploids are fertile, morphologically similar to A. monticola, and compatible with the cultigen (Fávero et al. 2006).
In summary, we have provided detailed karyotypes of most of the A genome species, which will contribute to the ongoing construction of physical maps and to their correlation with the genetic maps. The heterochromatic and 18S–26S rDNA loci patterns provide the first comprehensive analysis of the species relationship within the A genome group, in which chromosome variability was geographically structured, and may reflect the unique genetic system of the Arachis species, that is autogamy and geocarpy. Additionally, the results provide strong support for A. duranensis as the most probable A genome donor of A. hypogaea.
References
Ansari HA, Ellison NW, Williams WM (2008) Molecular and cytogenetic evidence for an allotetraploid origin of Trifolium dubium (Leguminosae). Chromosoma 117:159–167
Appels R, Moran LB, Gustafson JP (1986) The structure of DNA from rye (Secale cereale) NOR R1 locus and its behaviour in wheat backgrounds. Can J Genet Cytol 28:673–685
Burow MD, Simpson CE, Starr JL, Paterson A (2001) Transmission genetics of chromatin from a synthetic amphidiploid to cultivated peanut (Arachis hypogaea L.): Broadening the gene pool of a monophyletic polyploid species. Genetics 159:823–837
Cerbah M, Souza-Chies T, Jubier MF, Lejeune B, Siljak-Yakovlev S (1998) Molecular phylogeny of the genus Hypochaeris using internal transcribed spacers of nuclear rDNA: inference for chromosomal evolution. Mol Biol Evol 15(3):345–354
Chung MC, Lee YI, Cheng YY, Chou YJ, Lu CF (2008) Chromosomal polymorphism of ribosomal genes in the genus Oryza. Theor Appl Genet 116:745–753
Custodio AR, Peñaloza APS, Valls JFM (2005) Further cytogenetic information on Arachis stenosperma (Leguminosae). Cytologia 70:331–335
Dwivedi SI, Bertioli DJ, Crouch JH, Valls JFM, Upadhyaya HD, Favero A, Moretzsohn M, Paterson AH (2007) Peanut genetics and genomics: toward marker-assisted genetic enhancement in peanut (Arachis hypogaea L). In: Kole C (ed) Oilseeds series: genome mapping, molecular breeding in plants oilseeds series: genome mapping and molecular breeding in plants, vol 2. Springer, Berlin, pp 115–151
Fávero AP, Simpson CE, Valls JFM, Yuksel B (2006) Study of the evolution of cultivated peanut through crossability studies among Arachis ipaensis, A. duranensis, and A. hypogaea. Crop Sci 46:1546–1552
Fernández A, Krapovickas A (1994) Cromosomas y evolución en Arachis (Leguminosae). Bonplandia 8:187–220
Gimenes MA, Lopes CR, Galgaro ML, Valls JFM, Kochert G (2002) RFLP analysis of genetic variation in species of section Arachis, genus Arachis (Leguminosae). Euphytica 123:421–429
Gregory WC, Gregory MP (1976) Groundnut. Arachis hypogaea (Leguminosae-Papilionatae). In: Simmonds NW (ed) Evolution of crop plants. Longman Group Ltd, London, pp 151–154
Gregory MP, Gregory WC (1979) Exotic germoplasm of Arachis L. interspecific hybrids. J Hered 70:185–193
Guerra M (2000) Patterns of heterochromatin distribution in plant chromosomes. Genet Mol Biol 23:1029–1041
Hizume M, Shibata F, Matsusaki Y, Garajova Z (2002) Chromosome identification and comparative karyotypic analyses of four Pinus species. Theor Appl Genet 105:491–497
Husted L (1933) Cytological studies on the peanut, Arachis I. Chromosome number and morphology. Cytologia 5:109–117
Isleib TG, Holbrook CC, Gorbet DW (2001) Use of Arachis spp. plant introductions in peanut cultivar development. Peanut Sci 28:96–113
Jiang J, Gill BS (1994) New 18S–26S ribosomal gene loci: chromosomal landmarks for the evolution of polyploid wheats. Chromosoma 103:179–185
Kochert G, Halward T, Branch WD, Simpson CE (1991) RFLP variability in peanut (Arachis hypogaea L.) cultivars and wild species. Theor Appl Genet 81:565–570
Krapovickas A, Gregory WC (1994) Taxonomía del género Arachis (Leguminosae). Bonplandia 8:1–186
Krapovickas A, Rigoni VA (1951) Estudios citológicos en el genero Arachis. Rev Invest Agric 5:289–294
Lavia GI (1996) Estudios cromosómicos en Arachis (Leguminosae). Bonplandia 9:111–120
Lavia GI (1998) Karyotypes of Arachis palustris and A. praecox (section Arachis), two species with basic chromosome number x = 9. Cytologia 63:177–181
Lavia GI (2000) Chromosome studies of wild Arachis (Leguminosae). Caryologia 53:177–181
Lavia GI, Fernández A, Simpson CE, Seijo G (2001) Meiotic analysis in wild diploid Arachis species. Cytologia 66:293–298
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Li X, Guo R, Pedersen C, Hayman D, Langridge P (1997) Physical localization of rRNA genes by two-colour fluorescent in-situ hybridization and sequence analysis of the 5S rRNA gene in Phalaris coerulescens. Hereditas 126:289–294
Lim KY, Matyasek R, Lichtenstein CP, Leitch AR (2000) Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. Chromosoma 109:245–258
Liu ZL, Zhang D, Hong DY, Wang XR (2003) Chromosomal localization of 5S and 18S-5.8S-25S ribosomal DNA sites in five Asian pines using fluorescence in situ hybridization. Theor Appl Genet 106:198–204
Martins C, Galetti PM Jr (2001) Two 5S rDNA arrays in Neotropical fish species: is it a general rule for fishes? Genetica 111:439–446
Milla SR, Isleib TG, Stalker HT (2005) Taxonomic relationships among Arachis sect. Arachis species as revealed by AFLP markers. Genome 48:1–11
Moretzsohn MC, Hopkins MS, Mitchell SE, Kresovich S, Valls JFM, Ferreira ME (2004) Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol 4:11
Moscone EA, Matzke MA, Matzke AJM (1996) The use of combined FISH/GISH in conjunction with DAPI counterstaining to identify chromosomes containing transgene inserts in amphidiploid tobacco. Chromosoma 105:231–236
Moscone EA, Klein F, Lambrou M, Fuchs J, Schweizer D (1999) Quantitative karyotyping and dual-color FISH mapping of 5S and 18S–26S rDNA probes in the cultivated Phaseolus species (Leguminosae). Genome 42:1224–1233
Murata M, Heslop-Harrison J-S, Motoyoshi F (1997) Physical mapping of the 5S ribosomal RNA genes in Arabidopsis thaliana by multi-color fluorescence in situ hybridization with cosmid clones. Plant J 12:31–37
Nakamura R, Kitamura S, Inoue M, Ohmido N, Fukui K (2001) Karyotype analysis of Nicotiana kawakamii Y. Ohashi using DAPI banding and rDNA FISH. Theor Appl Genet 102:810–814
Peñaloza A, Valls JFM (2005) Chromosome number and satellited chromosome morphology of eleven species of Arachis (Leguminosae). Bonplandia 14:65–72
Pires JC, Lim KY, Kovarik A, Matyasek R, Boyd A, Leitch AR, Leitch IJ, Bennett MD, Soltis PS, Soltis DE (2004) Molecular cytogenetic analysis of recently evolved Tragopogon (Asteraceae) allopolyploids reveal a karyotype that is additive of the diploid progenitors. Am J Bot 91:1022–1035
Pontes O, Neves N, Silva M, Lewis MS, Madlung A, Comai L, Viegas W, Pikaard CS (2004) Chromosomal locus rearrangements are a rapid response to formation of the allotetraploid Arabidopsis suecica genome. Proc Natl Acad Sci USA 101:18240–18245
Popolizio E (1982) Geomorphology of the Argentine Northeast. Water Int 7:162–177
Raina SN, Mukai Y (1999) Detection of a variable number of 18S-5.8S-26S and 5S ribosomal DNA loci by fluorescent in situ hybridization in diploid and tetraploid Arachis species. Genome 42:52–59
Raina SN, Mukai Y, Kawaguchi K, Goel S, Jain A (2001a) Physical mapping of 18S-5.8S-26S and 5S ribosomal RNA gene families in three important vetches (Vicia species) and their allied taxa constituting three species complexes. Theor Appl Genet 103:839–845
Raina SN, Rani V, Kojima T, Ogihara Y, Singh KP, Devarumath RM (2001b) RAPD and ISSR fingerprints as useful genetic markers for analysis of genetic diversity, varietal identification, and phylogenetic relationships in peanut (Arachis hypogaea) cultivars and wild species. Genome 44:763–772
Robledo G, Seijo JG (2008) Characterization of Arachis D genome by FISH chromosome markers and total genome DNA hybridization. Genet Mol Biol 31:717–724
Rodríguez V, Seijo JG, Lavia GI, Fernández A, Simpson CE (2004) Meiotic behaviour in wild diploid Arachis (Leguminosae) species. Cytologia 69:209–214
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92:143–148
Schwarzacher T, Ambros P, Schweizer D (1980) Application of Giemsa banding to orchid karyotype analysis. Plant Syst Evol 134:293–297
Schweizer D, Loidl J (1987) A model for heterochromatin dispersion and the evolution of C-band patterns. Chromosomes Today 9:61–74
Seijo JG, Lavia GI, Fernández A, Krapovickas A, Ducasse D, Moscone EA (2004) Physical mapping of 5S and 18S–25S rRNA genes evidences that Arachis duranensis and A. ipaensis are the wild diploid species involved in the origin of A. hypogaea (Leguminosae). Am J Bot 91:1294–1303
Seijo JG, Lavia GI, Fernández A, Krapovickas A, Ducasse DA, Bertioli DJ, Moscone EA (2007) Genomic relationships between the cultivated peanut (Arachis hypogaea—Leguminosae) and its close relatives revealed by double GISH. Am J Bot 94:1963–1971
Simpson CE (2001) Use of wild Arachis species/introgression of genes into A. hypogaea L. Peanut Sci 28:114–116
Singh AK (1986) Utilization of wild relatives in the genetic improvement of Arachis hypogaea L. Part 8. Synthetic amphidiploids and their importance in interspecific breeding. Theor Appl Genet 72:433–439
Singh AK (1988) Putative genome donors of Arachis hypogaea (Fabaceae), evidence from crosses with synthetic amphidiploid. Plant Syst Evol 160:143–151
Singh AK, Moss JP (1982) Utilization of wild relatives in genetic improvement of Arachis hypogaea L. 2. Chromosome complements of species of section Arachis. Theor Appl Genet 61:305–314
Singh AK, Moss JP (1984) Utilization of wild relatives in genetic improvement of Arachis hypogaea L. 5. Genome analysis in section Arachis and its implications in gene transfer. Theor Appl Genet 68:355–364
Singh AK, Smartt J (1998) The genome donors of the groundnut/peanut (Arachis hypogaea L.) revisited. Genet Resour Crop Evol 45:113–118
Smartt J, Gregory WC (1967) Interspecific cross compatibility relationships between the cultivated peanut Arachis hypogaea L and other species of the genus Arachis. Oléaginuex 22:455–459
Smartt J, Gregory WC, Gregory MP (1978) The genomes of Arachis hypogaea. 1. Cytogenetic studies of putative genome donors. Euphytica 27:665–675
Spichiger R, Calenge C, Bise B (2004) Geographical zonation in the Neotropics of tree species characteristic of the ParaguayParaná Basin. J Biogeogr 31:1489–1501
Stalker HT (1991) A new species section Arachis of peanuts with D genome. Am J Bot 78:630–637
Stalker HT, Wynne JC (1979) Cytology of interspecific hybrids in section Arachis of peanuts. Peanut Sci 6:110–114
Suja JA, Gébrane-Younès J, Géraud G, Hernandez-Verdun D (1997) Relative distribution of rDNA and proteins of the RNA polymerase I transcription machinery at chromosomal NORs. Chromosoma 105:459–469
Tallury SP, Hilu KW, Milla SR, Friend SA, Alsaghir M, Stalker HT, Quandt D (2005) Genomic affinities in Arachis section Arachis (Fabaceae): molecular and cytogenetic evidence. Theor Appl Genet 111:1229–1237
Valls JFM, Simpson CE (2005) New species of Arachis from Brazil, Paraguay, and Bolivia. Bonplandia 14:35–64
Acknowledgments
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)PIP5807, and Agencia Nacional de Promoción Científica y Técnica, PICT 2005 Nº34458, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Heslop-Harrison.
Rights and permissions
About this article
Cite this article
Robledo, G., Lavia, G.I. & Seijo, G. Species relations among wild Arachis species with the A genome as revealed by FISH mapping of rDNA loci and heterochromatin detection. Theor Appl Genet 118, 1295–1307 (2009). https://doi.org/10.1007/s00122-009-0981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-0981-x