Abstract
The Oryza sativa subsp. indica reference cultivar (cv.), 93-11 is completely resistant to many Chinese isolates of the rice blast fungus. Resistance segregated in a 3:1 (resistance/susceptible) ratio in an F2 population from the cross between 93-11 and the japonica reference cv. Nipponbare, when challenged with two independent blast isolates. The chromosomal location of this monogenic resistance was mapped to a region of the long arm of chromosome 12 by bulk segregant analysis, using 180 evenly distributed SSR markers. Five additional SSR loci and nine newly developed PCR-based markers allowed the target region to be reduced to ca. 1.8 cM, equivalent in Nipponbare to about 800 kb. In the reference sequence of Nipponbare, this region includes an NBS-LRR cluster of four genes. The known blast resistance gene Pi-GD-3 also maps in this region, but the 93-11 resistance was distinguishable from Pi-GD-3 on the basis of race specificity. We have therefore named the 93-11 resistance Pi41. Seven markers completely linked to Pi41 will facilitate both marker-assisted breeding and gene isolation cloning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blast is one of the most destructive diseases of rice worldwide (Ou 1985). The causative agent is the filamentous ascomycete Magnaporthe oryzae (Couch and Hohn 2002). Genetic resistance is the most economic, effective and environmentally responsible method for its control, but resistance genes (R genes) typically lose their effectiveness after a short period in commercial production. The combining of a spectrum of different R genes through marker-aided selection probably represents the best available means to achieve durable control (Hittalmani et al. 2000). For this approach, it is necessary to identify markers closely linked to each R gene being incorporated.
The rice-M. oryzae pathosystem has been developed as a model system for the study of the molecular events occurring during the host-fungal interaction (Valent 1990). This research has established the prevalence of race-specific resistance governed by gene-for-gene relationships (Silué et al. 1992; Jia et al. 2000). At least 50 major blast resistance genes have been identified to date (Chen et al. 2005; Liu et al. 2005; Deng et al. 2006; Gowda et al. 2006; Nguyen et al. 2006), seven of which (Pib, Pita, Pi9, Pi2, Piz-t, Pid2 and Pi36) have been positionally cloned and characterized (Wang et al. 1999; Bryan et al. 2000; Qu et al. 2006; Zhou et al. 2006; Chen et al. 2006; Liu et al. 2007). With the exception of Pi-d2, which encodes a B-lectin receptor kinase, all belong to the large NBS-LRR gene family (Martin et al. 2003; Monosi et al. 2004). The identification and isolation of additional host R genes and pathogen avirulence genes is now required to deepen our understanding of the molecular mechanisms involved in the host-pathogen interaction.
The finished genome sequence of the japonica reference cultivar (cv.) Nipponbare (International Rice Genome Sequencing Project 2005; http://dna.affrc.go.jp), and a whole-genome draft sequence of the indica cultivar, 93-11 (Yu et al. 2002; http://rise.genomics.org.cn) facilitate molecular mapping and positional cloning in rice (Sakaki et al. 2005; Xu et al. 2005). 93-11 was grown widely in China, and has been used extensively as a parent in a number of breeding programmes. For example, it acts as the restorer line for the popular hybrid cv. Liang-You-Pei-Jiu (Dai et al. 1997; Yu et al. 2002). Although 93-11 expresses a good level of blast resistance (Dai et al. 1997), the genetic basis of this resistance is poorly understood.
In this report, we describe the identification of Pi41, a major gene which contributes to the blast resistance of 93-11. The gene was located with the help of linkage analysis and its race specificity was assessed by pathotesting with a large collection of Chinese blast isolates.
Materials and methods
Plant materials and pathotesting
The genetic basis of the blast resistance carried by 93-11 was elucidated by segregation analysis in an F2 population derived from the cross between 93-11 (resistant) and Nipponbare (susceptible). The population was challenged with two blast isolates (CHL724 and CHL743) collected from Jilin province, China. Both isolates elicit a differential response on the parents of the cross. Seedling management, inoculum preparation, disease inoculation and evaluation were carried out in a greenhouse, as described elsewhere (Zhu et al. 2004).
Marker development and genetic map construction
Total DNA was extracted by the CTAB method (Murray and Thompson 1980) from frozen rice leaves. Bulk segregant analysis (BSA) (Michelmore et al. 1991) was employed to select markers putatively associated with the resistant phenotype. Two DNA pools were assembled by mixing equimolar amounts of DNA from either ten resistant or ten susceptible F2 individuals (based on their reaction to inoculation with isolate CHL724). The fine mapping of Pi41 was achieved by three rounds of linkage analysis (Table 1). Firstly, 180 SSR markers distributed evenly across all 12 rice chromosomes (McCouch et al. 2002; http://www.gramene.org) were used to identify those which produced a differential banding pattern from the resistant and susceptible pools. These markers were then genotyped in the whole mapping population. An additional set of SSR markers, located in the genomic region defined by the initial linkage analysis, was then applied to the set of recombinant progeny, along with some de novo generated sequence-tagged site (STS) and candidate R gene (CRG) markers developed from the alignment (using BLAST) within the critical region of the genomic sequences of 93-11 and Nipponbare. The STS markers were developed from InDel polymorphisms in non-genic sequence, while the CRG markers exploited InDel polymorphisms with NBS-LRR sequence open reading frames.
PCR amplification conditions consisted of a denaturing step of 94°C/3 min, followed by 35 cycles of 94°C/30 s, annealing temperature (see Table 1)/30 s, and 72°C/1 min, ending with an extension step of 72°C/7 min. Amplicons were separated by 6% polyacrylamide gel electrophoresis and visualised by silver staining. For STS40-5, the amplicon was digested with DraI, separated by 2% agarose gel electrophoresis and visualised by ethidium bromide staining. Primer sequences and other relevant properties of the marker assays are summarized in Table 1. The recombination frequency between adjacent loci was estimated as N r/2N T (N r being the number of recombinants, and N T the overall population size, Pan et al. 2003; Gu et al. 2004).
Physical map construction and candidate R gene characterization
The physical map in the critical region was based on the Nipponbare contig map (IRGSP 2005). The 93-11 contigs were anchored to this framework using the linked markers. Flanking markers were used to identify candidate NBS-LRR genes, with the help of GENSCAN (http://genes.mit.edu), FGENSH (http://sun1.softberry.com) and RiceGAAS (http://rgp.dna.affrc.go.jp) software. The sequence of each candidate gene was then compared with its Nipponbare homologue. To confirm the functionality of the 93-11 candidate R genes, 93-11 and 15 other genotypes harbouring identified major R genes were challenged with 543 blast isolates collected from three provinces within China.
Results
Genetic mapping of the R gene locus
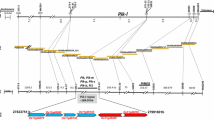
Altogether, 968 and 668 F2 individuals were inoculated with isolates CHL724 and CHL743, respectively. The segregation ratio between resistance and susceptibility in both cases was consistent with monogenic, fully dominant inheritance [resistant/susceptible: 735/233 (χ2 = 0.45) and 489/179 (χ2 = 1.15), respectively]. The linkage analysis indicated that the same R gene was detected by both fungal isolates. As a result, a combined F2 population, consisting of 341 resistant and 418 susceptible individuals, was taken forward as the mapping population for the genetic and physical mapping of the R gene locus. The BSA indicated that four SSR loci (RM247, RM101, RM7102 and RM519), all located on the long arm of chromosome 12, identified a polymorphism between the two parents and the two pools (Table 1). The recombinational distance from the R gene locus was 12.4, 2.7, 1.0 and 13.7 cM, respectively. As the recombinant progenies with respect to RM247, RM101 and RM7102 were different from those involving RM519, it was concluded that the R locus was flanked on the proximal side by RM247, RM101 and RM7102, and on the distal side by RM519 (Fig. 1a). This result allowed the subsequent fine mapping exercise to focus on the 33 recombinants with respect to RM7102 and the 215 recombinants with respect to RM519.
a An integrated genetic map of rice chromosome 12, including 14 blast resistance genes. Map positions were inferred from a: Yu et al. (1991); b: Yu et al. (1996); c: Zhuang et al. (2002); d: Liu et al. (2004); e: Hayashi et al. (1998); f: Sallaud et al. (2003); g: Naqvi and Chattoo (1996); h: Rybka et al. (1997), Bryan et al. (2000); i: Zheng et al. (1996); j: Ahn et al. (2000); k: Tabien et al. (2000); l: this study. *: recombinants/gametes; **: recombinants; CEN. centromere. Map distances in cM. b Nipponbare contig map around Pi41. The short horizontal lines represent BAC/PAC clones. The dashed lines denote marker positions. c 93-11 Contig map around Pi41. Short horizontal lines marked “?” refer to unanchored BAC/PAC clones. d Physical map of the Pi41 region. The numbers below the map are distances in kbp. The numbers in parentheses represent the number of recombinants between Pi41 and the marker locus. e Candidate genes for Pi41
Fine mapping of the R gene locus
The RM7102-RM519 interval includes five known SSR loci, which were polymorphic between 93-11 and Nipponbare, and these markers were genotyped in the 248 recombinants described above (Table 1). The respective number of recombination events at RM28059, RM28112, RM28130, RM1261 and RM28204 was 27, 25, 0, 5 and 28 (Fig. 1a). Thus the R locus co-segregates with RM28130, and is located at a distance of 1.8 and 1.6 cM, respectively, from RM28059 and RM28112 on the proximal side, and 0.3 and 1.8 cM from RM1261 and RM28204 on the distal side (Fig. 1a). This defines the position of the locus to a ca. 2.0 cM region, flanked by RM28112 and RM1261. The genotyping of the five new STS and four CRG markers in this interval defined 24 recombinant events at STS40-1 on the proximal side, and three at both STS40-3 and STS40-4 on the distal side. STS40-2, STS40-5 and all four CRG markers co-segregated with the resistance (Fig. 1a, d). Thus the R locus was located within a ca.1.8 cM interval flanked by STS40-1 and STS40-3, and co-segregates with STS40-2, CRG40-1, CRG40-2, CRG40-3, CRG40-4, RM28130 and STS40-5.
In silico physical mapping of the R gene locus
As 93-11 was sequenced using a “whole-genome shotgun” approach, the draft sequence contains several gaps in the target region (Fig. 1c). The physical map of the R gene locus region had therefore to be constructed from the Nipponbare sequence (Fig. 1b). Eight Nipponbare BAC/PAC clones were located within the region by a BLASTN analysis based on the sequence of the flanking and co-segregating markers, and the resulting physical map is shown in Fig. 1d. The distance between STS40-1 and STS40-3 is estimated to be about 800 kb (genomic position 16582733–17388355). On the basis of the 93-11 sequence, the distance between STS40-1 and STS40-3 is about 500 kb (genomic position 13208287–13708660).
The annotated Nipponbare chromosome 12 sequence suggests that the region flanked by STS40-1 and STS40-3 contains 122 predicted genes, including 16 known/putative genes, three expressed genes of unknown function, 31 hypothetical genes and 72 transposable element-related genes. A cluster of five NBS-LRR genes (Os12g28040, Os12g28050, Os12g28070, Os12g28100 and OS12g28250) lies between STS40-1 and RM28130 (The Rice Chromosomes 11 and 12 Sequencing Consortia 2005). GENSCAN predicts both Os12g28040 and Osg28050 to be intact NBS-LRR genes. Four NBS-LRR genes, each encoding an intact protein, were identified in the target region of 93-11 by RiceGAAS, GENSCAN and FGENSH. Sequence alignment of these four genes showed that they correspond to CRG40-1, CRG40-2, CRG40-3 and CRG40-4, at a homology level of 96.8, 99.3, 99.1 and 98.1% (data not shown), respectively.
Differential analysis of the R gene
Thirteen known major blast resistance genes [Pi4 (Yu et al. 1991), Pi6 (Yu et al. 1996), Pi12 (Zheng et al. 1996), Pi19 (Hayashi et al. 1998), Pi21 (Ahn et al. 2000), Pi24 (Zhuang et al. 2002), Pi31, Pi32 (Sallaud et al. 2003), Pi157 (Naqvi and Chattoo 1996), Pitq6 (Tabien et al. 2000), Pita/Pita-2 (Rybka et al. 1997; Bryan et al. 2000), Pi-GD-3 (Liu et al. 2004)] have been mapped to chromosome 12. With the exception of Pi-GD-3, all are located on the short arm of the chromosome (Fig. 1a). As to the location of the Pi-GD-3, it was roughly mapped based on the flanking markers RM179 (4.8 cM) and NLRinv-5 (23.8 cM) (Liu et al. 2004). Its position was, thus, inferred by the physical distance from the closest marker RM179, which is estimated to be about 1,500 kb based on the average physical/genetic distance ratio of rice, i.e., ~300 kb/cM. The R gene in 93-11 maps in the vicinity of the location of Pi-GD-3, so the specificities of these two genes were investigated. Since isolate CHL743 elicits a differential response, the 93-11 gene is likely not to be Pi-GD-3. Together, the R gene identified in 93-11 in the present study seems to be distinct from Pi-GD-3, and was designated Pi41.
The specificity of Pi41 was finally assessed against a panel of 15 R genes (Table 2). Pi41 conditions complementary reactions to these genes, and thus represents a useful component for R gene-stacking aimed at the breeding of durably blast resistant cvs of rice.
Discussion
Nipponbare and 93-11 are the reference cultivars for the japonica and indica types. The public availability of their whole-genome sequences has enabled the rapid and effective mapping and isolation of a growing number of functional genes (Gu et al. 2004; Chen et al. 2005; Liu et al. 2005; Xu et al. 2005; Deng et al. 2006; Chen et al. 2006; Liu et al. 2007). We have described here the identification and fine mapping of Pi41, delimiting it to a 1.8 cM or ca. 800 kb region. Although it maps to a similar location as Pi-GD-3 (Liu et al. 2004), it is a distinct gene, since the two genes react differentially when challenged with isolate CHL743. It is well established that most R genes are clustered (Michelmore and Meyers 1998; Monosi et al. 2004). Two-thirds of the >50 blast R genes identified to date map to chromosomes 6, 11 and 12. Of the 14 mapping to chromosome 12, 12 are either closely linked to the RFLP locus RG869 or are alleles of Pita, suggesting the presence of a major R gene cluster on the short arm of chromosome 12. Since R genes are typically identified in separate cultivars, it is difficult to carry out a classical allelism test between a new gene and others mapping within a cluster (Tabien et al. 2000; Sallaud et al. 2003; Deng et al. 2006). Thus some of the genes mapping to chromosome 12 may be identical to one another (Sallaud et al. 2003). The current alternatives to allelism testing are fine-scale mapping and differential pathotesting.
It has been well documented that the level of recombination frequency along a chromosome varies (Chen et al. 2002; Wu et al. 2003). Several R genes are located in regions of low recombination (Chauhan et al. 2002; Chen et al. 2005). In the 800 kb interval defined by STS40-1 and STS40-3, seven markers co-segregated with Pi41. This may reflect some localised suppression of recombination, which may be due to its pericentromeric location in the chromosome, where recombination is generally limited (Chen et al. 2002; Wu et al. 2003). An alternative scenario is that some chromosomal rearrangement has affected the region during the diversification of indica and japonica rice (Chauhan et al. 2002; Wu et al. 2003), resulting in a loss of sequence homology. At least 80% of the 93-11 STS40-1 to STS40-3 sequence is also present in Nipponbare. However, the estimated physical length of the interval in 93-11 is 300 kb less than that in Nipponbare, although much of this discrepancy is probably due to the gaps present in the sequence of 93-11. A third possibility relates to the observation that transposon-rich regions characteristically suffer from low levels of genetic recombination (Wu et al. 2003; Arabidopsis Genome Initiative 2000), given that the Pi41 region is composed of ca. 60% transposon sequence.
Recombination hotspots are commonly concentrated within genic sequence (Dooner and Martinez-Ferez 1997; Inukai et al. 2000; Yao et al. 2002), but recombination hotspots have been identified also in intergenic regions (Wulff et al. 2004; Yao et al. 2002). A potential hotspot is present proximal to Pi41, within a 2.5 kb, 1.6 cM interval defined by STS40-1 and STS40-2. In this interval, the physical/genetic ratio (P/G) is >160-fold less than the global mean in rice (Wu and Tanksley 1993). Recombination on both sides of the hotspot was strongly suppressed, suggesting that this hotspot may be specifically active for meiotic recombination, like the well characterized wx locus in rice (Inukai et al. 2000). The entire LRR domain, and part of the NBS domain of Os12g28040 is located within this hotspot. The LRR domains of R genes are known to play an important role in pathogen avirulence recognition (Martin et al. 2003; Chisholm et al. 2006), and novel resistance specificities generated by recombination have been documented at the flax rust resistance L loci (Elli et al. 2007). The Nipponbare and 93-11 sequences differ at 51 base pair positions in Os12g28040 (data not shown), so an intriguing possibility is that the recombination hotspot we have identified contributes to the rapid evolution of this region.
Although the delimiting region of Pi41 spans some 800 kb, only four genes with an intact NBS-LRR structure are present, and these all lie within a ca. 200 kb interval flanked by STS40-1 and RM28130 in Nipponbare. Thus these genes all represent good candidates for Pi41, and we are currently using a map-based in silico approach (Liu et al. 2007) as a strategy for gene isolation.
References
Ahn SN, Kim YK, Hong HC, Han SS, Kwon SJ, Choi HC, Moon HP, McCouch SR (2000) Molecular mapping of a new gene for resistance to rice blast (Pyricularia grisea Sacc.). Euphytica 116:17–22
Arabidopsis Genome initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Bryan GT, Wu KS, Farrall L, Jia YL, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12:2033–2045
Chauhan RS, Farman ML, Zhang HB, Leong SA (2002) Genetic and physical mapping of a new blast resistance locus, Pi-CO39(t), that corresponds to the avirulence gene AVR1-CO39 of Magnaporthe grisea. Mol Genet Genomics 267:603–612
Chen M, Presting G, Barbazuk WB (2002) An integrated physical and genetic map of the rice genome. Plant Cell 14:537–545
Chen S, Wang L, Que ZQ, Pan RQ, Pan QH (2005) Genetic and physical mapping of Pi37(t), a new gene conferring resistance to rice blast in the famous cultivar St. No. 1. Theor Appl Genet 111:1563–1570
Chen XW, Shang JJ, Chen DX, Lei CL, Zou Y, Zhai WX, Liu GZ, Xu JC, Ling ZZ, Cao G, Ma BT, Wang YP, Zhao XF, Li SG, Zhu LH (2006) A B-lectin receptor kinase gene conferring rice blast resistance. Plant J 46:794–804
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-Microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Couch BC, Hohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94:683–693
Dai ZY, Zhao BH, Liu XJ, Xia GH, Tan CL, Zhang BQ, Zhang HX (1997) A new middle-mature indica rice variety Yangdao 6 with high yielding, high quality and multiple disease resistance (in Chinese). Jiangsu Agric Sci 4:13–14
Deng YW, Zhu XD, Shen Y, He ZH (2006) Genetic characterization and fine mapping of the blast resistance locus Pigm(t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor Appl Genet 113:705–713
Dooner HK, Martinez-Ferez IM (1997) Recombination occurs uniformly with the bronze gene, a meiotic recombination hotspot in the maize genome. Plant Cell 9:1633–1646
Elli JG, Lawrence GJ, Dodds PN (2007) Further analysis of gene-for-gene disease resistance specificity in flax. Mol Plant Pathol 8:103–109
Gowda M, Barman-Roy S, Chattoo BB (2006) Molecular mapping of a novel blast resistance gene Pi38 in rice using SSLP and AFLP markers. Plant Breed 125:596–599
Gu K, Tian D, Yang F, Wu L, Sreekala C, Wang D, Wang G, Yin Z (2004) High-resolution genetic mapping of Xa27(t), a new bacterial blight resistance gene in rice, Oryza sativa L. Theor Appl Genet 108:800–807
Hayashi N, Ando I, Imbe T (1998) Identification of a new resistance gene to a Chinese blast fungus isolate in the Japanese rice cultivar Aichi Asahi. Phytopathology 88:822–827
Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N (2000) Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theor Appl Genet 100:1121–1128
International Rice Genome Sequencing Project (2005) The map-based sequence of the rice genome. Nature 436:793–800
Inukai T, Sako A, Hirano H, Sano Y (2000) Analysis of intragenic recombination at wx in rice: Correlation between the molecular and genetic maps with the locus. Genome 43:589–596
Jia YL, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014
Liu B, Zhang SH, Zhu XY, Yang QY, Wu SZ, Mei MT, Mauleon R, Leach J, Mew T, Leung H (2004) Candidate defense genes as predictors of quantitative blast resistance in rice. Mol Plant Microbe Interact 17:1146–1152
Liu XQ, Wang L, Chen S, Lin F, Pan QH (2005) Genetic and physical mapping of Pi36(t), a novel rice blast resistance gene located on rice chromosome 8. Mol Genet Genomics 274:394–401
Liu XQ, Lin F,Wang L, Pan QH (2007) The in silico map-based cloning of Pi36, a rice CC-NBS-LRR gene which confers race-specific resistance to the blast fungus. Genetics 176:2541–2549
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol 54:23–61
McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, Fu BY, Maghirang R, Li ZK, Xing YZ, Zhang QF, Kono I, Yano M, Fjellstrom R, Declerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and death process. Genome Res 8:1113–1130
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Monosi B, Wisser RJ, Pennill L, Hulbert SH (2004) Full-genome analysis of resistance gene homologues in rice. Theor Appl Genet 109:1434–1447
Murray MG, Thompson WK (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Naqvi NI, Chattoo BB (1996) Molecular genetic analysis and sequence characterized amplified region-assisted selection of blast resistance in rice. In: Khush GS (ed) Rice genetics III. IRRI, Manila, pp 570–576
Nguyen TTT, Koizumi S, La TN, Zenbayashi KS, Ashizaka T, Yasuda N, Imazaki I, Miyasaka A (2006) Pi35(t), a new gene conferring partial resistance to leaf blast in the rice cultivar Hokkai 188. Theor Appl Genet 113:697–704
Ou SH (1985) Rice diseases, 2nd edn. Commonwealth Mycological Institute, Kew, pp 109–201
Pan QH, Hu ZD, Tanisaka T, Wang L (2003) Fine mapping of the blast resistance gene Pi15, linked to Pii, on rice chromosome 9. Acta Bot Sin 45:871–877
Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172:1901–1914
Rybka K, Miyamoto M, Ando I, Saito A, Kawasaki S (1997) High resolution mapping of the indica-derived rice resistance genes II. Pita 2 and Pita and a consideration of their origin. Mol Plant Microbe Interact 10:517–524
Sakaki T, Matsumoto T, Antonio BA, Nagamura Y (2005) From mapping to sequencing, post-sequencing and beyond. Plant Cell Physiol 46:3–13
Sallaud C, Lorieux M, Roumen E, Tharreau D, Berruyer R, Svestasrani P, Garsmeur O, Ghesquiere A, Notteghem JL (2003) Identification of five new blast resistance genes in the highly blast-resistant rice variety IR64 using a QTL mapping strategy. Theor Appl Genet 106:794–803
Silué D, Notteghem JL, Tharreau D (1992) Evidence of a gene for-gene relationship in the Oryza sativa-Magnaporthe grisea pathosystem. Phytopathology 82:577–580
Tabien RE, Li Z, Paterson AH, Marchetti MA, Stansel JW, Pinson SRM (2000) Mapping of four major rice blast resistance genes from ‘Lemont’ and ‘Teqing’ and evaluation of their combinatorial effect for field resistance. Theor Appl Genet 101:1215–1225
The Rice Chromosomes 11 and 12 Sequencing Consortia (2005) The sequence of rice chromosomes 11 and 12, rich in disease resistance genes and recent gene duplications. BMC Biol 3:20
Valent B (1990) Rice blast as a model system for plant pathology. Phytopathology 80:33–36
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19:55–64
Wu KS, Tanksley SD (1993) PFGE analysis of the rice genome: estimation of the fragment sizes, organization of the repetitive sequences and relationships between genetic and physical distances. Plant Mol Biol 23:243–254
Wu JZ, Mizuno H, Hayshi-Tsugane M, Ito Y, Chiden Y, Fujisawa M, Katagiri S, Saji S, Yoshiki S, Karasawa W, Yoshihara R, Hayashi A, Hobayashi H, Ito K, Hamada M, Okamoto M, Ikeno M, Ichikawa Y, Katayose Y, Yano M, Matsumoto T, Sasaki T (2003) Physical maps and recombination frequence of six rice chromosomes. Plant J 36:720–730
Wulff BBH, Thomas CM, Parniske M, Jones JDG (2004) Genetic variation at the tomato Cf–4/Cf–9 locus induced by EMS mutagenesis and intralocus recombination. Genetics 167:459–470
Xu YB, McCouch SR, Zhang QF (2005) How can we use genomics to improve cereals with rice as a reference genome? Plant Mol Biol 59:7–26
Yao H, Zhou Q, Li J, Smith H, Yandeau M, Nikolau BJ, Schnable PS (2002) Molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc Natl Acad Sci USA 99:6157–6162
Yu ZH, Mackill DJ, Bonman JM, Tanksley SD (1991) Tagging genes for blast resistance in rice via linkage to RFLP markers. Theor Appl Genet 81:471–476
Yu ZH, Mackill DJ, Bonman JM, McCouch SR, Guiderdoni E, Notteghem JL, Tanksley SD (1996) Molecular mapping of genes for resistance to rice blast (Pyricularia grisea Sacc.). Theor Appl Genet 93:859–863
Yu J, Hu SN, Wang J et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296:79–92
Zheng KL, Zhuang JY, Lu J, Qian HR, Lin HX (1996) Identification of DNA markers tightly linked to blast resistance genes in rice. In: Khush GS (ed) Rice genetics III. IRRI, Manila, pp 565–569
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact 19:1216–1228
Zhu ML, Wang L, Pan QH (2004) Identification and characterization of a new blast resistance gene located on rice chromosome 1 through linkage and differential analyses. Phytopathology 94:515–519
Zhuang JY, Ma WB, Wu JL, Chai RY, Lu J, Fan YY, Jin MZ, Leung H, Zheng KL (2002) Mapping of leaf and neck blast resistance genes with resistance gene analog, RAPD and RFLP in rice. Euphytica 128:363–370
Acknowledgments
We thank Professors X.B. Zheng (Nanjing Agricultural University) and X.L. Guo (Jilin Academy of Agricultural Sciences) for the provision of blast isolates. This research was supported by grants from the National 973 project (2006CB/1002006), the National 863 project (2006AA100101; 2006AA10A103), the National Industry Science Research Project (200803008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Morgante.
Rights and permissions
About this article
Cite this article
Yang, Q., Lin, F., Wang, L. et al. Identification and mapping of Pi41, a major gene conferring resistance to rice blast in the Oryza sativa subsp. indica reference cultivar, 93-11. Theor Appl Genet 118, 1027–1034 (2009). https://doi.org/10.1007/s00122-008-0959-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0959-0