Abstract
Owing to its diverse sex types, the cucumber plant has been studied widely as a model for sex determination. In addition to environmental factors and plant hormones, three major genes—F/f, M/m, and A/a—regulate the sex types in the cucumber plant. By combining the bulked segregant analysis (BSA) and the sequence-related amplified polymorphism (SRAP) technology, we identified eight markers linking to the M/m locus. Among them, the two closely linked SRAP markers flanking the M/m locus were the co-dominant marker ME1EM26 and the dominant marker ME1EM23. Further, the co-dominant marker ME8SA7 co-segregated with the M/m locus. With the chromosome walking method using the cucumber genomic bacterial artificial chromosome (BAC) library, we successfully developed a co-dominant SCAR marker S_ME1EM23 from the ME1EM23 sequence. Along with the other two co-dominant SCAR markers S_ME1EM26 and S_ME8SA7 (developed from ME1EM26 and ME8SA7, respectively) in a larger segregating population (900 individuals), the M/m locus was mapped between S_ME1EM26 (5.4 cM) and S_ME1EM23 (0.7 cM), and S_ME8SA7 co-segregated with it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucumber (Cucumis sativus L.; 2n = 2x = 14)—belonging to the family Cucurbitaceae—is a very important vegetable crop worldwide. Owing to its diverse sex types and the extensive physiological and genetic studies conducted on it, cucumber is becoming a model plant for sex determination research (Atsmon 1968; Tsao 1988; Perl-Treves 1999; Tanurdzic and Banks 2004). Cucumber plants generally produce male and female flowers separately in the same individual; however, certain lines also produce bisexual flowers. In the early stage of development, all floral buds contain staminate and pistillate primordia. The selective arrest of either the staminate or pistillate part results in female or male flowers, respectively. Further, if the arrest does not happen, hermaphrodite flowers are formed. (Atsmon and Galun 1960; Goffinet 1990; Perl-Treves 1999).
Depending on the ratio of the three types of flowers produced in the plant, the cucumber lines are classified into five phenotypes: monoecious, gynoecious, andromonoecious, hermaphrodite, and androecious. The monoecious lines—the most common cucumbers—bear male and female flowers separately. The gynoecious lines produce only female flowers, while the androecious cucumber plants bear only male flowers. Male and bisexual flowers are produced in the andromonoecious cucumber plants, while the hermaphrodite lines produce only bisexual flowers (Galun 1961; Shifriss 1961; Kubicki 1969; Malepszy and Niemirowicz-Szczytt 1991). Early genetic studies by Galun (1961), Kubicki (1969), and Robinson et al. (1976) have indicated that three major genes are responsible for sex expression and segregation in the cucumber plant—F/f, M/m, and A/a. The F gene may promote femaleness, while the M gene is responsible for the unisexuality of the whole plant. Further, along with the homozygous recessive F/f gene, the recessive a gene can intensify the androecious nature.
In addition to the effects of the three major genes, sex expression can be easily regulated by many environmental factors such as photoperiod and temperature and plant hormones such as IAA, GA, and ethylene (Atsmon 1968; Flankel and Galun 1977; Flankel and Galun 1977, 1983; Perl-Treves 1999; Yamasaki et al. 2005). Base on the “One-hormone hypothesis” (Yin and Quinn 1995) and the study by Yamasaki et al. (2001), it can be proposed that the F locus regulates the ethylene levels promoting the development of the pistillate primordial state. Further, M locus production mediates the inhibition of stamen development by ethylene. The ethylene signal is not transmitted if the genotype is m homozygous. As a result of this, stamen development is not inhibited and the plant produces hermaphroditic flowers.
A series of studies (Kamachi et al. 1997; Trebitsh et al. 1997; Kamachi et al. 2000; Yamasaki et al. 2003; Mibus and Tatlioglu 2004; Knopf and Trebitsh 2006; Saito et al. 2007) have been conducted to investigate the F/f gene. These studies have shown that CsACS1G, which encodes a key enzyme of ethylene synthesis, is the candidate gene for the F/f locus. However, studies on the M/m gene are not as advanced as those on the F/f gene. Kater et al. (2001) have provided evidence proving that sex determination in cucumber is restricted to specific whorls, thereby suggesting that the active point of the M-allele product may be in the third whorl of the flowers. Hao et al. (2003) and Terefe and Tatlioglu (2005) identified an anther-specific DNase and a putative nucleotide sugar epimerase, respectively; both of these are considered to be the downstream products of the M-allele.
The genetic base of cucumber is rather narrow (Dijkhuizen et al. 1996), and polymorphism in cucumber varieties is lesser than that in other vegetables such as tomatoes. In this study, by combining the bulked segregant analysis [(BSA) Michelmore et al. 1991] and the sequence-related amplified polymorphism (SRAP) technology developed by Li and Quiros (2001), we identified eight markers linked to the M/m locus in our plant population. Among them, the two closely linked SRAP markers flanking the M/m locus were co-dominant marker ME1EM26 and dominant marker ME1EM23. Further, the co-dominant marker ME8SA7 co-segregated with the M/m locus. With the chromosome walking method using the cucumber genomic bacterial artificial chromosome (BAC) library, we successfully developed a co-dominant SCAR marker S_ME1EM23 from the ME1EM23 sequence. Along with the other two co-dominant SCAR markers S_ME1EM26 and S_ME8SA7 (developed from ME1EM26 and ME8SA7, respectively) in a larger segregating population (900 individuals), we located the M/m locus between S_ME1EM26 (5.4 cM) and S_ME1EM23 (0.7 cM) and S_ME8SA7 co-segregated with it.
Materials and methods
Plant materials
Cucumber monoecious, indeterminate line S52 (inbred line derived from a southern Chinese local cultivar, ffMM) was crossed with the hermaphrodite, indeterminate inbred line H34 (European greenhouse type, FFmm), and F1 was self-pollinated to obtain 167 individuals of F2 plants in 2005. The following year, we used the same parental lines to obtain a larger F2 population (670 plants) and a BC1 population (230 plants, F1 was backcrossed with H34). The parental plants, F1, F2, and BC1 were grown under natural sunlight in a greenhouse at Shanghai Jiaotong University. From the time of initiation of flowering, two parents (ten plants each), F1 (15 plants), and individuals of the F2 and BC1 segregating populations were scored for the sex expression of the flowers at every node up to 30 or 35 nodes on the main stem and the primary lateral branch. A plant with normal bisexual flowers was considered to be a bisexual plant (hermaphrodite or andromonoecious). If this was not the case, it was considered to be a unisexual plant (monoecious or gynoecious).
DNA extraction and BSA design
The young leaves of individual cucumber plants were harvested, rapidly frozen in liquid nitrogen, and stored at −80°C. For the BSA design, ten unisexual (MM or Mm) and ten bisexual (mm) plants were randomly selected from the F2 population (167 individuals) in 2005, and were mixed in equal fractions to construct two DNA bulks. The genomic DNA of all materials (S52, H34, F1, F2, BC1, and two bulks) was extracted by the CTAB method (Clark 1997), dissolved in water, visualized after electrophoresis in 0.8% agarose gel in 1× TAE, diluted to 30 ng/μl by comparing with DNA standards (Promega, Shanghai, China), and maintained at −20°C until use for the PCR reactions.
SRAP analysis
The SRAP primers were according to the studies by Li and Quiros (2001), Ferriol et al. (2003), Li et al. (2003), and Wang et al. (2005) and also by Vos et al. (1995), and Xu et al. (2000), where they were used as AFLP markers. These AFLP primers were used in the SRAP method in the present study, and the resulting polymorphic bands were considered to be SRAP markers. Table 1 indicates the polymorphic SRAP primers defining the molecular markers used in the study. The primers were synthesized by Sangon Biological Engineering Technology & Service Co. (Shanghai). The PCR reaction for SRAP was started at 94°C for 3 min, followed by eight cycles at 94°C for 30 s, 35°C for 30 s, and 72°C for 1 min; 35 cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and ended by extension at 72°C for 5 min. The products were separated on 4% denatured polyacrylamide gel with 1× TBE buffer and 8 mol/l urea. Electrophoresis was performed at a constant power of 50 W for 1.5–2 h. After electrophoresis, the gel was stained with AgNO3 solution (Bassam et al. 1991).
Conversion of SRAP markers
The DNA bands corresponding to the SRAP markers linked closely to the M/m locus in the F2 population (167 individuals) were excised from the gel, washed three times with 20 μl ddH2O, and incubated at 95°C for 15 min. After slight centrifugation, the upper phase was used for re-amplification that was performed in a volume of 50 μl. The PCR reaction was started at 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min and ended by extension at 72°C for 5 min. The resulting PCR products were purified using the EZ-10 Spin Column DNA Gel Extraction Kit (Bio Basic Inc.) according to the manufacturer’s instructions. The reclaimed DNA fragments were cloned in the T/A-cloning vector pUCm-T (Fermentas) and transformed into the Escherichia coli strain DH5α. Clones of the expected size were sequenced using the T7/M13 primer manufactured by Sangon Biological Engineering Technology & Service Co. (Shanghai). The sequences obtained from an ABI 3700 Sequencer (ABI, China) were used to design the SCAR primers. Based on the co-dominant features of the SRAP markers ME1EM26 and ME8SA7, the primer pairs were designed to amplify the region containing the polymorphism regions between the two parents, and the new SCAR markers derived from them were named S_ME1EM26 and S_ME8SA7, respectively. With regard to the dominant SRAP marker ME1EM23, we first designed two specific oligonucleotides—P1F and P1R—from both ends of the SRAP fragment. The amplifications for all the SCAR markers were same as those for the SRAP analysis, with the exception of the cycling condition: 94°C for 3 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s and ended by extension at 72°C for 5 min. The products were analyzed in 3% agarose gel in 1× TAE for S_ME8SA7 and 4% denatured polyacrylamide gel in 1× TBE for S_ME1EM26.
Chromosome walking
To develop a co-dominant SCAR marker from the ME1EM23 marker, a cucumber genomic BAC library was used to determine the DNA sequence flanking the known fragment. The library which containing approximately 19,200 Hind III clones was constructed with an inbred line derived from a northern Chinese monoecious cultivar (ffMM) by a previously reported procedure (Guan et al. 2008). After screening with the PCR method, one of the clones containing the specific amplification fragment derived from the primer pair P1F and P1R was sequenced by the Beijing Genomics Institute (Beijing). P1F and P1R were also used as the sequencing primers. Therefore, both the resulting sequences contained the same specific amplification fragment as the SRAP marker ME1EM23. From both ends of the enlarged region, another primer pair—P2F and P2R—was designed to amplify the corresponding fragment in the two parental plants. With the method described in the subsection “Conversion of SRAP markers”, we developed a co-dominant SCAR marker S_ME1EM23 from the SRAP marker ME1EM23. The detection was the same as that for S_ME1EM26. Table 2 indicates all the SCAR primers defining the molecular markers used in the study.
Linkage analysis and marker nomenclature
MAPMAKER/EXP3.0 (Lander et al. 1987) was used for linkage analysis with a LOD threshold of 3.0 or more. The recombination percentages were converted to genetic distance by using Kosambi mapping function (Kosambi 1944). The polymorphic markers were named according to their corresponding primer or primer combination codes.
Results
Genetic analysis of sex expression in the cucumber plant
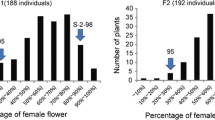
All the 15 plants of F1 obtained by crossing between S52 with H34 were gynoecious (unisexual plants), thus indicating that the development of unisexual flowers was dominant. Further, all the three (F2:2 and BC1:1) segregating populations were used to analyze the inheritance of the sex expression; the results are shown in Table 3. All these results confirmed the monogenic dominant inheritance of the unisexual (monoecious or gynoecious)/bisexual (hermaphrodite or andromonoecious) trait in the cucumber plant, which is generally defined as the effect of the M/m locus.
SRAP markers linked to the M/m locus
Of the 736 SRAP primer pairs tested, 360 primer combinations generated polymorphic bands (48.9%) between the two parental plants. On further testing with the DNA bulks, eight SRAP primer combinations—ME8SA7, ME1EM23, ME1EM24, ME1EM26, DC1EM9, ME42OD17, ME23SA4, and ME67 (with polymorphic band amplified from a single primer)—exhibited polymorphic bands between the unisexual and bisexual bulks. All the markers were mapped in the F2 population (167 individuals, 2005). The preliminary linkage analysis performed using MAPMAKER/EXP3.0 revealed that the two closely linked SRAP markers flanking the M/m locus were ME1EM26 (5.4 cM) and ME1EM23 (1.0 cM). The SRAP marker ME8SA7 co-segregated with the locus. Since their relatively loose linkage values, others markers’ pattern without any development showed in Fig. 3.
Development of SCAR markers
The primer pair ME1EM26 and ME8SA7 designed to amplify the region containing the polymorphism between the two parents behaved like co-dominant markers. Therefore, their conversion to SCAR markers was easy. The new markers were named S_ME1EM26 (GenBank Accession Number EU716639, EU716640) and S_ME8SA7 (GenBank Accession Number EU716641, EU716642), respectively. Both of them maintained their co-dominant pattern between the two parental plants. For the S_ME1EM26 marker, the sequence of the unisexual allele was 116 bp—3 bp longer than the bisexual allele (Supplementary material S1). Further, for the S_ME8SA7 marker, the unisexual allele had a 149 bp fragment—16 bp shorter than the bisexual allele (Supplementary material S2 and Fig. 1).
The analysis of the S_ME1EM26 sequence from the unisexual allele against the translated database (tblastx, Altschul et al. 1997) revealed that it has 81% similarity with the putative acetyl-CoA synthetase in Arabidopsis thaliana (AK118300.1), and its nucleotide sequence has a homologous relationship (75%) with a Vitis vinifera sequence (AM455130.2) determined through the blastn analysis in NCBI (http://www.ncbi.nlm.nih.gov). These results indicated that the S_ME1EM26 fragment may be a part of a gene sequence, and it is conserved in planta. However, in case of the S_ME8SA7 marker, homology to a known sequence in the NCBI database was not found.
After the polymorphism test, the SRAP marker ME1EM23 showed a dominant pattern. Through the sequencing of the unisexual allele band, we designed the first pair of primer (P1F and P1R) from both ends of the fragment. However, the primary dominant SCAR marker derived from the first primer pair was not found to be very reliable during the linkage analysis for the validation of the marker with the same F2 population (167 individuals, 2005). Therefore, a chromosome walking approach was used to identify the flanking sequence adjacent to the marker. We successfully obtained more than 1.1 kb flanking sequence that showed a 1 bp and a 3 bp insertion/deletion between the two parents (GenBank Accession Number EU716643, EU716644), thereby enabling the development of the co-dominant SCAR marker S_ME1EM23. The S_ME1EM23 marker sequence of the unisexual allele was 227 bp—4 bp longer than the bisexual allele (Supplementary material S3 and Fig. 2). The homology analysis did not reveal any known sequence.
Fine mapping of the M/m locus
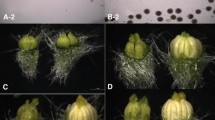
Three co-dominant SCAR markers were used to analyze the enlarged segregating population (670 F2 individuals and 230 BC1 plants) for fine mapping the M/m locus. The linkage pattern of S_ME1EM26 did not change. Further, no recombinant events were detected between the S_ME8SA7 marker and the M/m locus, thus indicating that S_ME8SA7 was still co-segregated with it. Owing to its co-dominant characteristic, S_ME1EM23 could detect more information than the previous dominant SRAP marker, and the linkage value was modified to 0.7 cM. The final result of the markers linked to the M/m locus is shown in Fig. 3. After mapping in the segregating population, we tested the co-segregation feature between the S_ME8SA7 marker and the M/m locus among several different cucumber plant lines. The co-segregation pattern was found to be conservative in at least five other cultivar lines (Fig. 4).
Discussion
As a relatively new marker system, SRAP has been used in genetic studies on plant species such as Brassica oleracea (Li et al. 2003), Cucurbita pepo (Ferriol et al. 2003), and Brassica rapa (Rahman et al. 2007). In these tested plant species, in comparison with other randomly amplified markers, the SRAP markers were found to be distributed more evenly throughout the whole genomes. This is suitable for gene localization, genetic map construction, and transcription mapping (Li et al. 2003). In the present study, we modified the SRAP analysis (5–8 cycles) according to Li and Quiros (2001) and obtained a good result for the cucumber plant with the silver straining method. Among the eight SRAP markers linked to the M/m locus, the marker ME1EM24 was first mapped to the same location as the marker ME1EM26 (data not shown), and further sequencing (GenBank Accession Number EU716645) confirmed that the two markers were derived from the same location of the cucumber genome (Supplementary material S4). Their “ME1” ends were the same; however, the “EM24” end was located in the interior of the ME1EM26 sequence. Therefore, we used the marker ME1EM26 to represent this polymorphic locus. On aligning the marker ME1EM26 and primer EM24 sequences, we found the genomic sequence at 11 nt from the 3′ end of the primer EM24. This incidental finding may explain why SRAP technology is more reliable than RAPD for identifying polymorphism. During the first 5 or 8 “lower” annealing cycles—considered to be “non-special” steps—the condition enables a “shorter” sequence (approximately 10 nt from the 3′ end) in the SRAP primer for promoting amplification, which is similar to RAPD. However, there are two different primers in SRAP analysis; therefore, the polymorphism may be twice or more than in RAPD technology. Interestingly, based on the combinations with other primers, we obtained the SRAP marker M67 whose polymorphic band was amplified by a single primer. The next 35 “higher” annealing cycles may “specially” amplify the products of the previous “non-special” cycles. With a moderate addition of the “lower” annealing cycles (5–8 cycles), we achieved a better polymorphism identification (48.9%) than the previous design derived from Li and Quiros (2001) in the cucumber plant with the silver straining method.
Since a dominant marker may supply less genetic information, the conversion from a dominant marker into a co-dominant marker is very valuable. Several researchers (Barret et al. 1998; Bradeen and Simon 1998; Negi et al. 2000; Noguera et al. 2005; Liu et al. 2006; Rahman et al. 2007) have successfully converted their dominant markers into co-dominant markers, such as RAPD markers, AFLP markers, and SRAP markers. A dominant marker sequence cannot supply sufficient information for a co-dominant conversion, which is based on the insertion or deletion fragment located in any of the two lines. Therefore, the additional flanking region needs to be sequenced. It has been confirmed that the adaptor ligation-based PCR-mediated walking (Mibus and Tatlioglu 2004) and inverse PCR method (Knopf and Trebitsh 2006) are efficient in isolating the flanking sequence in the cucumber plant. At the initial stage, based on the sequence of SRAP marker ME1EM23 obtained from S52, we designed several primer combinations for detecting polymorphism between the two parents. After optimizing the annealing temperature, we obtained the special primer pair P1F and P1R. However, when tested in the F2 population, the primary dominant SCAR marker derived from this primer pair was not very reliable. Next, by using the BAC library-mediated walking strategy, approximately 1.1 kb sequence containing the SRAP marker ME1EM23 was obtained, thus indicating that the polymorphism of the primary dominant SCAR marker derived from a nucleotide variance was located in the 3′ end of the primer P1R (Supplementary material S3). Based on the enlarged allele region of the two parental plants, we found insertion/deletion polymorphic sequences and successfully developed a co-dominant SCAR marker. Interestingly, the final marker did not contain any nucleotide derived from the primary SRAP marker.
The characteristic of one gene locus controlling the unisexual expression is unique in the Cucurbitaceae plants, particularly cucumber (Cucumis sativus L.) and melon (Cucumis melo L.). With the potential evolutionary course, the unisexual phenotype may be a gain-of-function mutant during the long-term breeding selection, which is not rare in the cucumber plant. Knopf and Trebitsh (2006) reported that the gynoecious-specific CsACS1G—mapped to the F locus—is a result of a relatively recent gene duplication and recombination between CsACS1 and CsBCAT. Therefore, clarifying the mechanism and function of unisexual expression has remarkable evolutionary significance.
Engelke et al. (1999) have reported an approach for isolating the M/m gene with differential display reverse transcription-polymerase chain reaction (DDRT-PCR). Przybecki et al. (2003) and Terefe and Tatlioglu (2005) attempted to isolate the M/m gene transcript by using similar methods; however, the results were not satisfactory. More recently, Saito et al. (2007) have reported that CsACS2 mRNA begins to accumulate just beneath the pistil primordia of flower buds at the bisexual stage, but not prior to the formation of the pistil primordia. Similarly, based on the study by Kater et al. (2001), transcription of the M/m gene may be transient or may have spatio-temporal localization. Therefore, the transcript may not be easily detected. On the other hand, the inheritance of the M/m locus is beyond dispute; therefore, cloning the M/m gene with the positional method appears to be feasible. In this study, we identified certain markers closely linked to the M/m locus. No recombinant events were detected between the marker S_ME8SA7 and the M/m locus, and the linkage value of the marker S_ME1EM23 was 0.7 cM. Further, the co-segregation feature between the marker S_ME8SA7 and the M/m locus was conservative in certain cultivar lines. These markers will be particularly useful for fine-mapping studies and ultimately for the cloning of the M/m gene.
References
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lippman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Atsmon D (1968) The interaction of genetic, environmental, and hormonal factors in stem elongation and floral development of cucumber plants. Ann Bot 32:877–882
Atsmon D, Galun E (1960) A morphogenetic study of staminate, pistillate and hermaphrodite flowers in Cucumis sativus L. Phytomorphology 10:110–115
Barret P, Delourme R, Foisset N, Renard M (1998) Development of a SCAR (sequence characterized amplified region) marker for molecular tagging of the dwarf BREIZH (Bzh) gene in Brassica napus L. Theor Appl Genet 97:828–833
Bassam BJ, Caetana-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Bradeen JM, Simon PW (1998) Conversion of an AFLP fragment linked to the carrot Y2 locus to a simple, co-dominant, PCR based marker form. Theor Appl Genet 97:960–967
Clark MS (1997) Plant molecular biology: a laboratory manual. Springer, Berlin
Dijkhuizen A, Kennard WC, Havey MJ, Staub JE (1996) RFLP variability and genetic relationships in cultivated cucumber. Euphytica 90:79–87
Engelke T, Mibus H, Tatlioglu T (1999) Approaches to isolating the gene M/m for femaleness in Cucumis sativus L. ISHS Acta Hort 492:355–361
Ferriol M, Pico B, Nuez F (2003) Genetic diversity of a germplasm collection of Cucurbita pepo using SRAP and AFLP markers. Theor Appl Genet 107:271–282
Flankel R, Galun E (1977) Pollination mechanisms: reproduction and plant breeding. Springer, Berlin Heidelberg New York
Galun E (1961) Study of the inheritance of sex expression in the cucumber: the interaction of major genes with modifying genetic and non-genetic factors. Genetica 32:134–163
Goffinet MC (1990) Comparative ontogeny of male and female flowers of Cucumis sativus. In: Bates EM, Robinson RW, Jeffrey C (eds) Biology and utilization of the Cucurbitaceae. Cormell University Press, New York, pp 288–304
Guan Y, Chen Q, Pan JS, Li Z, He HL, Wu AZ, Song RT, Cai R (2008) Construction of a BAC library from cucumber (Cucumis sativus L.) and identification of linkage group specific clones. Prog Nat Sci 18:143–147
Hao YJ, Wang DH, Peng YB, Bai SL, Xu LY, Li YQ, Xu ZH, Bai SN (2003) DNA damage in the early primordial anther is closely correlated with stamen arrest in the female flower of cucumber (Cucumis sativus L.). Planta 217:888–895
Kamachi S, Sekimoto H, Kondo N, Sakai S (1997) Cloning of a cDNA for a 1-aminocyclopropane-1-carboxylate synthase that is expressed during development of female flowers at the apices of Cucumis sativus L. Plant Cell Physiol 38:1197–1206
Kamachi S, Mizusawa H, Matsuura S, Sakai S (2000) Expression of two 1-aminocyclopropane-1-carboxylate synthase genes, CS-ACS1 and CS-ACS2, correlated with sex phenotypes in cucumis plants (Cucumis sativus L.). Plant Biotechnol 17:69–74
Kater MM, Franken J, Carney KJ, Colombo L, Angenent GC (2001) Sex determination in the monoecious species cucumber is confined to specific floral whorls. Plant Cell 13:481–493
Knopf RR, Trebitsh T (2006) The female-specific CS-ACS1G gene of cucumber. A case of gene duplication and recombination between the non-sex-specific 1-aminocyclopropane-1-carboxylate synthase gene and a branched-chain amino acid transaminase gene. Plant Cell Physiol 47:1217–1228
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kubicki B (1969) Investigation of sex determination in cucumber (Cucumis sativus L.). Genet Pol 10:5–143
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMARKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural population. Genomics 1:174–181
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system base on a simple PCR reaction: its application to mapping and gene tagging in Brassiac. Theor Appl Genet 103:455–461
Li G, Gao M, Yang B, Quiros CF (2003) Gene for gene alignment between the Brassica and Arabidopsis genomes by direct transcriptome mapping. Theor Appl Genet 107:168–180
Liu Z, Fu T, Wang Y, Tu J, Chen B, Zhou Y, Ma C, Shan L (2006) Development of SCAR and CAPS markers for a partially dominant yellow seed coat gene in Brassica Napus L. Euphytica 149:381–385
Malepszy S, Niemirowicz-Szczytt K (1991) Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci 80:39–47
Mibus H, Tatlioglu T (2004) Molecular characterization and isolation of the F/f gene of femaleness in cucumber (Cucumis sativus L.). Theor Appl Genet 109:1669–1676
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Negi MS, Devic M, Delseny M, Lakshmikumaran M (2000) Identification of AFLP fragments linked to seed coat colour in Brassica juncea and conversion to a SCAR marker for rapid selection. Theor Appl Genet 101:146–152
Noguera FJ, Capel J, Alvarez JI, Lozano R (2005) Development and mapping of a codominant SCAR marker linked to the andromonoecious gene of melon. Theor Appl Genet 110:714–720
Perl-Treves R (1999) Male to female conversion along the cucumber shoot: approaches to studying sex genes and floral development in Cucumis sativus. In: Ainsworth CC (ed) Sex determination in plants. BIOS, Oxford, pp 189–286
Przybecki Z, Kowalczyk ME, Siedlecka E, Urabanczyk-Wochniak E, Malepszy S (2003) The isolation of cDNA clones from cucumber (Cucumis sativus L.) floral buds coming from plants differing in sex. Cell Mol Biol Lett 8:421–438
Rahman M, McVetty PBE, Li G (2007) Develpoment of SRAP, SNP and multiplexed SCAR molecular markers for the major seed coat color gene in Brassica rapa L. Theor Appl Genet 115:1101–1107
Robinson RW, Munger HM, Whitaker TW, Bohn GM (1976) Genes of Cucubitaceae. HortSci 11:554–568
Saito S, Fujii N, Miyazawa Y, Yamasaki S, Matsuura S, Mizusawa H, Fujita Y, Takahashi H (2007) Correlation between development of female flower buds and expression of the CS-ACS2 gene in cucumber plants. J Exp Bot 58:2897–2907
Shifriss O (1961) Sex control in cucumber. J Hered 52:5–12
Takaheshi H, Saito T, Suge H (1983) Separation of effects of photoperiod and hormones on sex expression in cucumber. Plant Cell Physiol 24:142–154
Tanurdzic M, Banks JA (2004) Sex-determining mechanisms in land plants. Plant Cell 16:S61–S71
Terefe D, Tatlioglu T (2005) Isolation of a partial sequence of a putative nucleotide sugar epimerase, which may involve in stamen development in cucumber (Cucumis sativus L.). Theor Appl Genet 111:1300–1307
Trebitsh T, Staub JE, O’Neill SD (1997) Identification of a 1-aminocyclopropane-1-carboxylic acid synthase gene linked to the female (F) locus that enhance female sex expression in cucumber. Plant Physiol 113:987–995
Tsao TH (1988) Sex expression in flowering. Acta Phytophysiol Sin 14:203–207
Vos P, Hogers R, Bleeker M, Reijans M, Lee T, Hornes M, Friters A, Pot J, Paleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang G, Pan JS, Li XZ, He HL, Wu AZ, Cai R (2005) Construction of a cucumber genetic linkage map with SRAP markers and location of the genes for lateral branch traits. Sci China (Ser. C) 48:213–220
Xu M, Li X, Korban SS (2000) AFLP-based detection of DNA methylation. Plant Mol Biol Rep 18:361–368
Yamasaki S, Fujii N, Matsuura S, Mizusawa H, Takahashi H (2001) The M locus and ethylene-controlled sex determination in andromonoecious cucumber plants. Plant Cell Physiol 42:608–619
Yamasaki S, Fujii N, Takahashi H (2003) Photoperiodic regulation of CS-ACS2, CS-ACS4 and CS-ERS gene expression contributes to the femaleness of cucumber flowers through diurnal ethylene production under short-day conditions. Plant Cell Environ 26:537–546
Yamasaki S, Fujii N, Takahashi H (2005) Hormonal regulation of sex expression in plants. Vitam Horm 72:79–110
Yin T, Quinn JA (1995) Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am J Bot 82:1537–1546
Acknowledgments
The authors wish to thank Dr. Rentao Song (Shanghai University) for his technical assistance in BAC library. This work was supported by National Natural Science Foundation of China (No. 30500289), National “863” Project (No. 2008AA10Z150), and Shanghai Leading Academic Discipline Project (No. B209).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann.
Z. Li and J. Pan contribute equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Pan, J., Guan, Y. et al. Development and fine mapping of three co-dominant SCAR markers linked to the M/m gene in the cucumber plant (Cucumis sativus L.). Theor Appl Genet 117, 1253–1260 (2008). https://doi.org/10.1007/s00122-008-0859-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0859-3