Abstract
The rice low phytic acid 1 (lpa1) mutant was originally identified using a forward genetics approach. This mutant exhibits a 45% reduction in rice seed phytic acid with a molar-equivalent increase in inorganic phosphorus; however, it does not appear to differ significantly in productivity from its wild-type progenitor. A second lpa1 mutant was identified from additional screening for high seed inorganic phosphorus phenotypes. Using a positional cloning strategy, we identified a single candidate gene at the rice Lpa1 locus. Sequence analysis of the candidate gene from the lpa1 mutants revealed two independent mutations (a single base pair substitution and a single base pair deletion) that confirmed the identification of this candidate as the rice low phytic acid 1 gene, OsLpa1. The OsLpa1 gene has three splice variants. The location and nature of the two mutations suggests that these lesions only affect the translation of the predicted protein derived from the longest transcript. The proteins encoded by OsLpa1 do not have homology to any of the inositol phosphate metabolism genes recently characterized in plants, although there is homology to 2-phosphoglycerate kinase, an enzyme found in hyperthermophilic methanogens that catalyzes the formation of 2,3-bisphosphoglycerate from 2-phosphoglycerate. OsLpa1 represents a novel gene involved in phytic acid metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytic acid (myo-inositol-1,2,3,4,5,6-hexakisphosphate; InsP6) is the primary storage form of phosphorus (P) in seeds where it is found in the form of a mixed salt called phytate and accounts for 65–85% of the total seed P (Raboy 1997). During germination, seed phytases break down phytate into its constituents (myo-inositol, inorganic P, and mineral cations) which are then mobilized to support seedling growth and development (Lott et al. 1995). In yeast, InsP6 regulates of a number of nuclear processes including mRNA export, DNA repair, and RNA editing (York 2006). While InsP6 appears to act primarily as a nutrient reserve in seeds, its function in other plant cells has not been well characterized although recently it has been shown to be involved in calcium signaling in guard cells (Lemtiri-Chlieh et al. 2000, 2003).
Despite its very high concentration in seeds, relatively little is known about the biosynthesis of phytic acid in plants. During the past decade, however, several groups have reported the identification of mutants that exhibit low seed phytic acid (Raboy 2007). This work has been primarily driven by the interest in developing low phytate seed crops since phytic acid cannot be digested by non-ruminant livestock and humans. This property of phytic acid requires the costly supplementation of grain-based animal feeds with phytases and/or P (Brinch-Pedersen et al. 2002) and the phytic acid P that is excreted in animal waste can cause pollution of water resources (Sharpley et al. 1994; Raboy 2001). Another attribute of phytic acid that can negatively impact nutrition is its ability to strongly bind mineral cations which prevents their absorption and reduces the nutritional value of unprocessed cereal grains to consumers (Torre et al. 1991; Lopez et al. 2002).
Low phytic acid mutants have been identified in several crop species including maize (Raboy et al. 2000; Pilu et al. 2003; Shi et al. 2003, 2005), rice (Larson et al. 2000; Liu et al. 2007), wheat (Guttieri et al. 2004), barley (Larson et al. 1998; Rasmussen and Hatzack 1998; Dorsch et al. 2003; Bregitzer and Raboy 2006) and soybean (Wilcox et al. 2000; Hitz et al. 2002; Yuan et al. 2007). Although lpa mutants are characterized primarily by reduction in seed phytic acid and increases in inorganic phosphorus, some mutants have also exhibited significant accumulation of inositol phosphate intermediates (Shi et al. 2003) or myo-inositol (Shi et al. 2005).
In maize, genes corresponding to three low phytic acid mutants have been identified. The first of these to be cloned was the Lpa2 gene which encodes an inositol phosphate kinase belonging to the Ins(1,3,4)P3 5/6-kinase gene family (Shi et al. 2003). The maize Lpa3 gene encodes a myo-inositol kinase (Shi et al. 2005) and, like maize Lpa2, is directly involved in the inositol polyphosphate biosynthetic pathway. More recently, isolation of the maize Lpa1 gene was reported (Shi et al. 2007). Unlike the Lpa2 and Lpa3 genes, the Lpa1 gene encodes a multidrug resistance protein (MRP) ATP-binding cassette (ABC) transporter and appears to be involved in the transport or accumulation of phytic acid in the seed rather than its biosynthesis.
About 70% of the total seed P in rice is found in the form of phytic acid. Larson et al. (2000) identified the first rice lpa1 mutant by screening a population of gamma-irradiation induced mutants derived from the japonica cultivar Kaybonnet (KBNT). The homozygous mutant line, KBNT lpa1-1, exhibits a 45% reduction in seed phytic acid with a molar-equivalent increase in inorganic P and little change in total seed P, suggesting that the Lpa1 gene is involved in phytic acid biosynthesis. Despite a reduced amount of phytic acid, KBNT lpa1-1 appears to be comparable to KBNT under standard production conditions (Rutger et al. 2004) suggesting that the rice Lpa1 gene may be a good target for modifying seed phytic acid without affecting seed or plant performance. The mutation was initially mapped to the long arm of rice chromosome 2 (Larson et al. 2000) and, subsequently, fine mapped to a region of less than 150 kb using microsatellite and sequence tagged site markers (Andaya and Tai 2005).
We report here the isolation and characterization of a second rice lpa1 mutant (DR1331-2). Using a positional cloning approach, we have identified a single candidate at the OsLpa1 locus. Sequence analysis of the KBNT lpa1-1 and DR1331-2 mutants has revealed mutations in this candidate gene, indicating that the gene is OsLpa1. OsLpa1 encodes three expressed splice variants. Both mutations appear to affect the protein encoded by the longest transcript. The predicted proteins encoded by OsLpa1 do not have homology to any of the inositol phosphate metabolism genes recently characterized in plants (Shi et al. 2003, 2005, 2007; Stevenson-Paulik et al. 2005; Sun et al. 2007) although there is homology to 2-phosphoglycerate kinase, an enzyme found in hyperthermophilic methanogens (Lehmacher and Hensel 1994). Identification and characterization of the OsLpa1 gene will contribute to understanding phytic acid metabolism in rice and other plants and provide a foundation for the development low phytate crops.
Materials and methods
Plant materials, phenotyping, and DNA extraction
The Kaybonnet lpa1-1 (KBNT lpa1-1, also known as KB 1-1) mutant was crossed with the indica cultivar Zhe 733 (Lpa1/Lpa1) to generate an F2 mapping population (K/Z F2) consisting of 576 progeny. F2 seeds were cut in half and the embryo halves were surface sterilized and germinated on media [0.22% (w/v) Murashige-Skoog basal salts (Sigma-Aldrich, St. Louis, MO), 1% sucrose, and 0.25% Gelrite® (Sigma-Aldrich), pH 5.8]. Seedlings were transferred to the greenhouse for seed production and leaf tissue was collected for DNA extraction. F2 progeny were genotyped with the microsatellite markers RM3542 and RM482, which flank the OsLpa1 locus (Andaya and Tai 2005). The non-embryo half seeds of the recombinant F2 progeny were then phenotyped using the high inorganic phosphate (HIP) assay (Larson et al. 2000). F3 seeds were collected from the recombinants and used to confirm the F2 phenotyping. The K/Z F2 recombinants were used to fine map the OsLpa1 locus.
M2 seeds from 1,790 M1 plants derived from gamma-irradiation mutagenesis of the japonica cultivar Drew seeds (provided courtesy of J.N. Rutger) were screened using the HIP assay. Following the initial screening to identify putative mutant lines, the HIP assay was conducted using half-seeds to avoid destroying the embryos of seeds exhibiting the HIP phenotype. DR1331-2, a homozygous Drew mutant line exhibiting a HIP phenotype, was crossed with KBNT lpa1-1 (KBNT lpa1-1/DR-1331-2) to examine the relationship between the two mutants. The resulting F1 progeny were confirmed using microsatellite markers and F2 seeds were phenotyped using the HIP assay. Genomic DNA samples for use in mapping, sequencing, and gel blot analysis were extracted from leaf tissues as described previously except tissues were frozen and mechanically ground (Tai and Tanksley 1990). Chi-square (χ 2) tests were performed to examine the goodness-of-fit between the expected Mendelian ratios for the M2 and F2 populations and the segregation data for the lpa phenotype and the DNA markers.
Determination of phytic acid and inorganic P content
In order to ascertain if the HIP phenotype observed in DR1331-2 was indicative of a reduction in phytic acid, mature seeds (i.e. brown rice) of DR1331-2 and Drew were analyzed to determine the total phosphorus (total P), phytic acid phosphorus (phytic acid P), and inorganic P (Pi) contents as described by Raboy et al. (1984) with minor modification. Following wet-ashing of 100 mg of ground brown rice, total P was determined by colorimetric assay (Chen et al. 1956). For phytic acid P and Pi, single grains were weighed and ten volumes of 0.4 N HCl were added. Extraction was performed by homogenization for 15 min in a Minibeadbeater-96™ (Biospec Products, Bartlesville, OK) with stainless steel dowel pins (Small Parts, Inc., Miami Lakes, FL). Seed extracts were centrifuged and supernatants were transferred into fresh tubes for both phytic acid and Pi determinations. The total P, phytic acid-P, and Pi of each sample were expressed as P content on a dry weight basis. For seed dry weight, 32 mature seeds per line were weighed to determine the means and standard deviation. All assays were performed multiple times and data were expressed as an average with standard deviation. Statistical significance was evaluated using Student’s t test (P = 0.05). Seeds of KBNT lpa1-1 and KBNT were also analyzed for comparison.
High-performance liquid chromatography (HPLC) was performed using a Dionex DX-500 ion chromatography system (Dionex, Sunnyvale, CA) as described previously with minor modifications (Mitsuhashi et al. 2005). Supernatants from seed extracts were passed through 0.2 micron filters prior to loading 30 μL aliquots onto a Dionex IonPac AS11 analytical column (2 × 250 mm) and an IonPac AG11 guard column (2 × 50 mm). A Dionex IonPac ATC-1 column (4 × 35 mm) was used to remove carbon dioxide and carbonate. The inositol phosphates were eluted with a linear gradient from 0 to 60 mM NaOH using a flow rate of 1 ml min−1 at room temperature. A Dionex conductivity detector was used with an anion self-regenerating suppressor (ASRS-Ultra II) in an external water mode operating with a current of 300 mA. All standards were purchased from Sigma-Aldrich. The retention times for inorganic phosphate (K2HPO4) and the various inositol phosphates were: Ins(2)P1, 9.5 min; Pi, 15.5 min; Ins(1,4)P2, 21 min; Ins(1,3,4)P3, 32 min; Ins(13,4,5)P4, 33 min; Ins(1,3,4,5,6)P5, 35 min; and Ins(1,2,3,4,5,6)P6, 29 min.
Marker development and analysis of recombinants
Single nucleotide polymorphism (SNP) markers were identified by comparing sequence data from the Nipponbare (japonica) and 93-11 (indica) reference genomes (http://www.gramene.org). Regions (500 bp to 1 kb in length) containing potential SNPs were amplified from genomic DNA of parental lines (KBNT lpa1-1 and Zhe733) using primer designed from Nipponbare sequence. Sequence data were obtained by direct sequencing of PCR products that were amplified in 50 μL reactions consisting of 50 ng of genomic DNA template, 0.25 μM primer, 1X PCR buffer (20 mM Tri-HCl pH8.0, 100 mM KCl, 25 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol), 4 μL of 25 mM dNTPs, and 1 U of ExTaq polymerase (TaKaRa Bio USA, Madison, WI). PCR amplifications were performed using the following conditions: denaturation at 94°C for 5 min, 35 amplification cycles of 94°C for 20 s, 55°C for 30 s and 72°C for 1 min, with a final extension at 72°C for 5 min. Four independent PCR reactions for each line were combined and then purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA) prior to sequencing. SNP genotyping of recombinants was performed in the same manner. The SNP-7 marker was amplified with the primers SNP-7F: 5′-ATTCATGGTCCCACCGATT-3′ and SNP-7R: 5′-TCGGACACTAAACACACTTTCC-3′. SNP-14 marker was amplified with the primers SNP-14F: 5′-GATTAGCTCGCCATGAATCACC-3′ and SNP-14R: 5′-CCCAATATTCGCG TACACATTAGC-3′.
DNA sequencing was performed by the UC Davis Sequencing Facility. Sequences were analyzed using the Contig Express program of Vector NTI Advance 10 (Invitrogen, Carlsbad, CA). All primers used in this study were designed manually or using the Primer 3 program (http://www.genome.wi.mit.edu/) and were purchased from Integrated DNA Technologies (Coralville, IA).
Sequence analysis of the OsLpa1 candidate gene
Alleles of the OsLpa1 candidate gene from both lpa1 mutants (KBNT lpa1-1 and DR1331-2) and the wild-type cultivars (KBNT and Drew) were analyzed by sequencing genomic DNA. For the analysis of genomic DNA, primers were designed to amplify overlapping, 1 kb fragments using Nipponbare sequence. The overlap between fragment ends was about 250 bp to ensure that any differences between Nipponbare sequences and those of the parental lines were not mistaken for polymorphisms. PCR amplifications were performed using the same conditions as described for SNP marker analysis except annealing temperatures were optimized for each primer pair. To analyze the segregation of the mutant alleles of the OsLpa1 gene in selected recombinants, two primers (forward 5′-TGCAAACTGTTGCCTAGCTGATGAGA-3′ and reverse 5′ GCATTCACTCATCCGGAATTGTATGG-3′) were designed to amplify a 1 kb fragment of OsLpa1 that spans both the mutations identified in KBNT lpa1-1 and DR1331-2. Genotyping of the recombinants was performed as described for the other SNP markers. All of the PCR products used for sequencing were purified using QIAquick PCR purification kit (Qiagen) and directly sequenced two to four times using the appropriate primers. Sequences were analyzed as described earlier.
Gene structure of OsLpa1
Gene structure of OsLpa1 (Os02g57400 locus) was obtained using the FGenesh and GeneMarkHMM prediction algorithms and data from the TIGR rice gene expression database (http://www.tigr.org/). The presence of alternative splicing variants was verified experimentally by reverse transcriptase (RT)-PCR using One-Step RT-PCR kit (Invitrogen) according to the manufacturer’s instruction and exon-intron boundaries were verified by sequencing. All the RT-PCR experiments were carried out using total RNA samples isolated from 7-day old seedlings of mutant and wild-type genotypes using TRIzol® reagent (Invitrogen). Specific primers were designed for OsLpa1.1 (forward: 5′-ATGGCGGAGGAGGCGCCGCCGC-3′ and reverse: 5′-CTATGCACACGGCAGTTCTGTG-3′; 2,058 bp product), OsLpa1.2 (forward: 5′-GTGCATTTTCCAATTGTTCTGTTCAGAA-3′ and reverse: 5′-CTATGCACACGGCAGTTCTGTG-3′; 1,927 bp product), and OsLpa1.3 (forward: 5′-GTGCATTTCCAATTGTTCTGTTCAGAA-3′ and reverse: 5′-TCATACTGTAAACACACCAATG-3′; 1,540 bp product).
The full-length cDNA sequence of OsLpa1.1 was obtained by carrying out the 5′ and 3′ rapid amplification of cDNA ends (RACE) experiments using 5′ and 3′ RACE kit (Gibco-BRL, Gaithersburg, MD). The first strand of the 5′ RACE was synthesized from total RNA by using a gene specific primer (5′-CTTGAAGGCATGGCGAGCC-3′) and a poly(dC) tail was added according to the manufacturer’s instructions. The RACE product was amplified by using a nested primer (5′-GCGGCGGCGGCGCCTCCTCCGCCAT-3′) and an anchor primer. The first strand of the 3′ RACE was synthesized by using total RNA with the oligo(dT) primer according to the manufacturer’s instructions. Two successive PCR were carried out to amplify the 3′ RACE products; the first amplification was based on the anchor primer to the poly(A) + tail and a gene specific primer (5′-CTCATGCTGCAGTTCACCAAAG-3′), and the second amplification was performed with a nested primer (5′-AGTCCGACGAGGAATACGACGATCT-3′). The PCR products of 5′ and 3′ RACE were purified from a gel and sequenced directly.
Sequence identity searches were performed using the Basic Local Alignment Search Tool (BLASTX) at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). Additional homologous proteins were identified from the Gramene database (http://www.gramene.org/). Multiple sequence alignments were generated using the ClustalW program (http://www.ebi.ac.uk/clustalw).
OsLpa1.1 gene expression analysis
For the analysis of expression pattern of OsLpa1.1, total RNA samples were prepared from the shoots (leaf blade and leaf sheath) and roots of 2 week old seedlings of the variety Nipponbare. Total RNA samples of reproductive tissues were prepared from panicle tissue harvested during grain filling. First-strand cDNA was synthesized by random priming using SuperScript™ III reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Specific primer pairs were designed for OsLpa1.1 (forward 5′-CTTGCTTGCAGAATTAAGGAGAG-3′ and reverse 5′-GATCAACTCCGTTCTAGGAGGCA-3′; 372 bp product). The rice myo-inositol phosphate synthase (MIPS) (forward 5′-GAGGCTGCCGAGGCCGAGCAGC-3′ and reverse 5′-GAATTGGTTATGTTACAAGGCGAGA-3′; 326 bp product) and actin (forward 5′-GAAGATCACTGCCTTGCTCC-3′ and reverse 5′-CGATAACAGCTCCTCTTGGC-3′; 249 bp product) genes were also analyzed. A sum of 5 μL of single strand cDNA was used in 50 μL PCR reactions as described above and amplifications were performed using the following conditions: denaturation at 94°C for 5 min, 35 cycles of 94°C for 20 s, 55°C for 20 s, and 72°C for 30 s, followed by a final extension of 72°C for 5 min. RT-PCR products were separated and visualized using 1% agarose/1X TAE gels containing ethidium bromide.
Accession numbers
Sequence data from this article can be found in the Genbank Database under the following accession numbers: OsLpa1.1 (EU366951), OsLpa1.2 (EU366953), and OsLpa1.3 (EU366954).
Results
Identification and characterization of a second lpa1 mutant
In order to identify additional lpa1 mutants, a forward genetic screen was carried out using the HIP assay. Eight M2 seeds from each of 1,790 gamma-irradiation induced M1 lines were initially tested. One line was identified as segregating for the HIP phenotype (three mutants to five wild-types). Additional M2 seeds of this line were tested using half-seeds to facilitate recovery of putative homozygous mutant plants. In total, 47 M2 seeds of this line, designated DR1331, were assayed and the ratio of mutant to wild-type was 10:37, indicating the HIP phenotype is consistent with a single, recessive gene mutation (χ 2 = 0.348, df = 1, P value = 0.5555). The embryo halves of the DR1331 M2 half-seeds that exhibited the HIP phenotype were germinated and one plant (DR1331-2) was selected for seed increase and genetic analysis.
Comparison of DR1331-2 seed dry weight, total P, phytic acid P, and inorganic P with KBNT lpa1-1 and the wild-type cultivars Drew and KBNT indicated that DR1331-2, which exhibits a reduction in seed phytic acid of about 50% compared to Drew, is a low phytic acid mutant (Table 1). The total P of DR1331-2 and KBNT lpa1-1 seeds were not significantly different compared to their respective wild types. In addition, HPLC analysis of DR1331-2 produced the same profile as KBNT lpa1-1 (Fig. 1). Differences in peaks corresponding to phytic acid and inorganic P were observed in comparison to the wild-type cultivars. No accumulation of inositol phosphate intermediates was observed under the conditions used.
To determine the genetic relationship between KBNT lpa1-1 and DR1331-2, a cross was made (KBNT lpa1-1/DR1331-2) resulting in three F1 plants, which were verified as hybrids using the microsatellite markers RM164, RM228, and RM333 (data not shown). Twenty-four F2 seeds from each of the F1 plants were tested using the HIP assay and all exhibited the HIP phenotype indicating that the mutant gene in DR1331-2 is another lpa1 allele.
Fine mapping of the OsLpa1 locus
Previously, we reported the fine mapping of the OsLpa1 locus using a recombinant inbred line mapping population derived from the cross KBNT lpa1-1/Zhe733 (Andaya and Tai 2005). The region was delimited to approximately 47 kb between KN2, a PCR-based marker, and HK3, an RFLP marker. Sequence analysis of the candidate genes in this interval from KBNT lpa1-1 did not reveal any mutations (S. Kim and C. Andaya, unpublished results). Given this result, the recombinants and markers used to generate the fine map were re-analyzed. Sequence analysis of the KBNT lpa1-1 allele of the KN2 marker did not completely match the genomic sequence. Examination of different annealing temperatures revealed that a higher annealing temperature (60 vs. 55°C) resulted in a shift in amplification of the polymorphic product to that of a monomorphic product that completely matched the genomic sequence at the locus (S. Kim and C. Andaya, data not shown). Based on these findings, the OsLpa1 locus resides in the 110 kb interval between microsatellite marker RM3542 and the RFLP marker HK3.
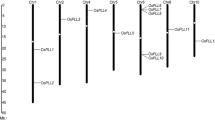
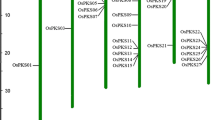
In order to further delimit the locus, recombinants were identified by screening the K/Z F2 mapping population with the flanking microsatellite markers RM482 and RM3542 (Andaya and Tai 2005) and new markers were developed between RM3542 and HK3. A total of 31 recombinants (20 between OsLpa1 and RM482 and 11 between OsLpa1 and RM3542) were identified from the population of 576 F2 individuals (Fig. 2). SNP markers were developed by comparing DNA sequences from KBNT lpa1-1 and Zhe733. Two markers, SNP-7 and SNP-14, were identified from this analysis. The SNP-7 marker amplifies a fragment containing one SNP whereas the SNP-14 marker amplifies a fragment with three SNPs. SNP genotyping of the 31 recombinants indicated that the OsLpa1 gene is located between SN-7 (one recombinant) and SNP-14 (four recombinants).
Identification and characterization of OsLpa1
Based on the Nipponbare genome, the region containing the OsLpa1 locus is about 8 kb (Fig. 2) and the available annotation indicates the presence of a single candidate gene, LOC_Os02g57400, which is predicted to have at least three splice variants that encode three different proteins (Fig. 3a). To determine if Os02g57400 is the OsLpa1 gene, a 5 kb genomic region spanning the candidate gene was sequenced from both lpa1 mutants and their wild-type progenitors. Comparison of KBNT and KBNT lpa1-1 sequences revealed a single base pair change (C/G to T/A). Sequencing of the DR1331-2 allele uncovered a single base pair deletion (T/A) compared to the wild-type Drew. Both mutations are located in the predicted coding region of the largest transcript designated OsLpa1.1 (Fig. 3a). The lpa1-1 mutation is located at position 409 (relative to the predicted ATG). This single base pair change results in a nonsense mutation. The DR1331-2 mutation is located at position 313 (relative to the predicted ATG), and therefore, is predicted to result in a frame shift that truncates the protein.
Gene structure of OsLpa1 and evidence for the presence of its transcript variants. a Gene structure of OsLpa1. Open boxes represent 5′ and 3′ UTR, filled boxes represent the coding regions, and the lines between boxes indicate represent introns. The Kaybonnet lpa1-1 (K) and DR1331-2 (D) mutations are in the second exon. Start and stop codons are as indicated. b RT-PCR analysis indicates that all three transcripts are expressed (1 = KBNT, 2 = KBNT lpa1-1, 3 = Drew, 4 = DR1331-2). RT-PCR products are indicated (double headed arrow) above in (a)
To verify the gene structure of OsLpa1.1, full-length cDNA sequence was obtained by sequencing an OsLpa1.1-specific RT-PCR product and the 5′ and 3′ RACE products. Sequence analysis confirmed the predicted gene structure. The 5′ UTR region of OsLpa1.1 is 410 bp long and a conserved poly A signal was detected in the 3′ UTR (200 bp) followed by a poly A tail (Fig. 4). The OsLpa1.1 open reading frame is predicted to encode a protein of 685 amino acids (aa). RT-PCR analysis using primers specific to each of the two shorter splice variants confirmed their expression in addition to OsLpa1.1 (Fig. 3b) and expressed sequence tags (EST) specific to each of the three transcripts were also identified from publicly available rice expression data (http://www.tigr.org/). No significant difference was observed in expression of each of the transcripts in seedlings of the lpa1 mutants and their respective wild-types (Fig. 3b). According to the annotation and gene prediction algorithms, the predicted start codon encoded by the two shorter splice variants, OsLpa1.2 and Os.Lpa1.3, is located at position 571 from the predicted OsLpa1.1 start codon and is in the same frame. Given the location of the lpa1 mutations, neither OsLPA1.2 nor OsLPA1.3 should be affected.
Nucleotide and amino acid sequence of full length cDNA of OsLpa1.1. Numbers on the left refer to nucleotides and numbers on the right refer to amino acid residues. Inverted triangle indicates the transcriptional start site. Nucleotides corresponding to the coding region are shown in uppercase. Wedge symbol indicates the exon-intron borders, Asterisk represents stop codon, and the 3′ Poly A signal is underlined
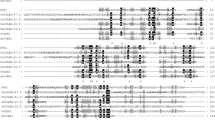
Sequence identity searches against the NCBI protein database revealed that OsLPA1.1 has homology to the P-loop kinase domain of 2-phosphoglycerate kinase (2-PGK) found in hyperthermophilic methanogens (Lehmacher and Hensel 1994; Aravind et al. 2000). The alignment of OsLPA1.1 and 2-PGK from Methanothermus fervidus is shown in Fig. 5. The homology to 2-PGK extends from aa 146 to aa 409 of OsLPA1.1, which contains both Walker A and B motifs. OsLPA1.2 and OsLPA1.3 are identical to OsLPA1.1 from aa 191 to aa 409 and neither has the Walker A motif. BLASTP analysis detected another rice protein, encoded by LOC_Os09g39870, which has 60% amino acid identity to OsLPA1.1. Os09g39870 is predicted to encode two OsLPA1.1-like proteins as a result of alternative splicing. OsLPA1.1 was also found to be a highly conserved protein among higher plants (Fig. 6). Two proteins in Arabidopsis exhibit significant homology to OsLPA1.1. The Arabidopsis protein encoded by the At3g45090 gene is 62% identical and the At5g60760 protein shows 60% identity. We recently obtained two Arabidopsis lines containing T-DNA insertions in the two homologues. One of these lines containing an insertion in the At5g60760 exhibits a low seed phytic acid phenotype as determined by the HIP assay and HPLC analysis, indicating that the function of OsLPA1.1 is important for seed phytic acid in dicots as well (S. Kim and T. Tai, manuscript in preparation).
Sequence alignment of the OsLPA1.1 with the 2-phosphoglycerate kinase (2-PGK) found in Methanothermus fervidus (CAA50058). The conserved sequences are found in the P-loop kinase domain of 2-PGK. The P-loop is an ATP/GTP binding site motif found in many nucleotide-binding proteins (Leipe et al. 2003). The characteristic Walker A [GxxxxGK(S/T)] and Walker B (hhhhEG) motifs are indicated with asterisks. Identical (highlighted) and similar (+) amino acid residues are as indicated. The first amino acid of OsLPA1.2 and OsLPA1.3 is marked (inverted triangle)
OsLPA1.1 is highly conserved in plants. The sequence alignment of OsLPA1.1 (EAY88043.1) with proteins from sorghum (Sbi_0.40583), maize_A (AC194424.2_FG002), maize_B (AC189706.4_FG007), Arabidopsis thaliana, AtLPA1a (At3g45090; NP_566873), AtLPA1b (At5g60760; NP_200884), Populus_A (fgenesh4_pg.C_LG_IX001462), Populus_B (fgenesh4_pg.C_scaffold_66000265), Vitis vinifera (CAN72472), and rice (LOC_Os09g39870, NP001063996) is shown. Sorghum, maize, and Populus accession numbers are from Gramene. Other accession numbers are from NCBI. Identical (asterisk) and similar (dot) amino acid residues are indicated
Analysis of OsLpa1.1 expression
In order to further examine the expression of OsLpa1.1, RT-PCR analysis was conducted using total RNA from various tissues including shoot, root and, panicle. Expression of OsLpa1.1 was compared to myo-inositol 3-phosphate synthase (MIPS) and actin, which served as a control (Fig. 7). OsLpa1.1 transcripts were detected in all the tissues examined, but expression was higher in reproductive tissue than in vegetative tissue. Expression of the MIPS gene, which catalyzes the first step of myo-inositol biosynthesis, was highest in the reproductive tissues followed by leaves. No expression was detected in root tissues.
Discussion
The rice low phytic acid 1 (lpa1) mutation results in a 45–50% reduction in seed phytic acid with a molar equivalent increase in inorganic P and little change in total seed P (Larson et al. 2000). We report here the cloning of the rice Lpa1 (OsLpa1) gene by fine mapping of the locus to a region of rice chromosome 2 containing a single candidate gene. Sequence analysis of two independent lpa1 mutant alleles confirmed the identification of this gene as OsLpa1. The OsLpa1 gene is predicted to encode three different proteins (OsLPA1.1, OsLPA1.2, and OsLPA1.3). Based on the location and nature of the mutations found in the two lpa1 mutant alleles, the largest of these proteins, OsLPA1.1, appears to be necessary for wild-type levels of seed phytic acid in rice. The existence of OsLPA1.2 and OsLPA1.3 and their possible involvement in phytic acid metabolism remains to be determined.
OsLPA1.1 has homology to a 2-phosphoglycerate kinase (2-PGK) found in hyperthermophilic methanogens (Lehmacher and Hensel 1994). This archaeal 2-PGK catalyzes the ATP-dependent phosphorylation of 2-phosphoglycerate to form 2,3-diphosphoglycerate (also known as 2,3-bisphosphoglycerate), which is in turn converted into cyclic 2,3-diphosphoglycerate (cDPG) by cDPG synthetase (Lehmacher et al. 1990). cDPG is the most abundant form of phosphate found in the hyperthermophilic methanogens M. fervidus, Methanothermus sociabilis, and Methanopyrus kandleri (Hensel and König 1988). The biological function of cDPG remains controversial, but the primary role of this compound may be as storage compound for carbohydrate and phosphate (Lehmacher et al. 1990). Another function of cDPG as a thermoadapter in response to environmental factors has also been proposed (Hensel and König 1988). The archaeal 2-PGK consists of an N-terminal ATP-cone regulatory domain and a P-loop-containing kinase domain (Aravind et al. 2000). OsLPA1.1 shares homology to the P-loop domain of 2-PGK, suggesting that this protein functions as a kinase.
OsLPA1.1. does not have homology to the proteins encoded by the maize Lpa genes or other genes involved in inositol phosphate metabolism (Shi et al. 2003, 2005, 2007; Stevenson-Paulik et al. 2005; Yoshida et al. 1999). Furthermore, no homologues of significance were found in yeast or humans. Within the rice genome, there is one gene, LOC_Os09g39870, on chromosome 9 that encodes a protein with homology to OsLPA1.1. The existence of this homologue suggests possible overlapping or redundant functions and could explain why the homozygous KBNT lpa1-1 mutant line appears to be as productive as its wild-type under standard production conditions (Rutger et al. 2004). Preliminary examination of OsLpa1 gene expression indicates that three splice variants are expressed in seedlings and the expression of each of these transcripts is similar in the two lpa1 mutants and their respective wild-types. Analysis of the expression of OsLpa1.1 indicates that this transcript can be detected in vegetative and reproductive tissues with stronger expression in the reproductive tissues as might be expected.
Two pathways, the inositol lipid-dependent and the lipid-independent, have been proposed for the biosynthesis of phytic acid (Raboy 2001). Recent studies involving the functional characterization of various plant inositol and inositol phosphate kinases have provided some evidence for both of these pathways (Shi et al. 2003, 2005, 2007; Stevenson-Paulik et al. 2002, 2005). However, some of the enzymes believed to be involved, particularly in the lipid-independent pathway, have yet to be identified. It is possible that OsLpa1.1 encodes a kinase involved in the early phosphorylation steps proposed in the lipid-independent pathway, but for which no genes have been cloned. Identification of this kinase activity would be definitive evidence of the lipid-independent phytic acid biosynthetic pathway in plants.
Another possibility is that the OsLPA1.1 protein indirectly impacts phytic acid biosynthesis and accumulation by catalyzing the formation of 2,3-bisphosphoglycerate (2,3-BPG). It has previously been shown that 2,3-BPG is a competitive inhibitor of inositol polyphosphate 5-phosphatases (Downes et al. 1982). These phosphatases breakdown the inositol polyphosphate intermediates leading to phytic acid. In this model, 2,3-BPG produced by OsLPA1.1 inhibits 5-phosphatases in the rice seed, thus allowing inositol polyphosphate intermediates to undergo further phosphorylation to form phytic acid. If this model is correct then rice lpa1 mutants should exhibit a reduction in 2,3-BPG and increased activity of 5-phosphatases in the developing rice seed.
Studies are currently underway to confirm the expression of OsLPA1.1 and to determine whether OsLPA1.1 is a kinase and its specific substrate(s). Functional characterization of OsLPA1.1 will contribute to our understanding of phytic acid biosynthesis in plants as well as the development of low phytate crops. The existence of alternative splice products as well as a homologue in the rice genome raises interesting questions regarding whether these predicted proteins are expressed and have any role in inositol phosphate metabolism.
References
Andaya CB, Tai TH (2005) Fine mapping of the rice low phytic acid (lpa1) locus. Theor Appl Genet 111:489–495
Aravind L, Wolf YI, Koonin EV (2000) The ATP-CONE: an evolutionarily mobile, ATP-binding regulatory domain. J Mol Microbiol Biotechnol 2:191–194
Bregitzer P, Raboy V (2006) Effects of four independent low-phytate mutations on barley agronomic performance. Crop Sci 46:1318–1322
Brinch-Pedersen H, Sorensen LD, Holm PB (2002) Engineering crop plants: getting a handle on phosphate. Trends Plant Sci 7:119–125
Chen PS, Toribara TY, Warner H (1956) Microdetermination of phosphorous. Anal Chem 28:1756–1758
Dorsch JA, Cook A, Young KA, Anderson JM, Bauman AT, Volkmann CJ, Murthy PPN, Raboy V (2003) Seed phosphorus and inositol phosphate phenotype of barley low phytic acid genotypes. Phytochem 62:691–706
Downes CP, Mussat MC, Michell RH (1982) The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J 203:169–177
Guttieri M, Bowen D, Dorsch JA, Raboy V, Souza E (2004) Identification and characterization of a low phytic acid wheat. Crop Sci 44:418–424
Hensel R, König H (1988) Thermoadaptation of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett 49:75–79
Hitz WD, Carlson TJ, Kerr PS, Sebastian SA (2002) Biochemical and molecular characterization of a mutation that confer decreased raffinosaccharide and phytic acid phenotype. Plant Physiol 128:650–660
Larson SR, Young KA, Cook A, Blake TK, Raboy V (1998) Linkage mapping 2 mutations that reduce phytic acid content of barley grain. Theor Appl Genet 97:141–146
Larson SR, Rutger JN, Young KA, Raboy V (2000) Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci 40:1397–1405
Lehmacher A, Hensel R (1994) Cloning, sequencing and expression of the gene encoding 2-phosphoglycerate kinase from Methanothermus fervidus. Mol Gen Genet 242:163–168
Lehmacher A, Vogt AB, Hensel R (1990) Biosynthesis of cyclic 2, 3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and cyclic 2, 3-diphosphoglycerate synthetase from Methanothermus fervidus. FEBS Lett 272:94–98
Leipe DD, Koonin EV, Aravind L (2003) Evolution and classification of P-loop kinases and related proteins. J Mol Biol 333:781–815
Lemtiri-Chlieh F, MacRobbie EAC, Brearley CA (2000) Inositol hexkisphosphate is a physiological signal regulating the K + -inward rectifying conductance in guard cells. Proc Natl Acad Sci USA 97:8687–8692
Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100:10091–10095
Liu QL, Xu XH, Ren XL, Fu HW, Wu DX, Shu QY (2007) Generation and characterization of low phytic acid germplasm in rice (Oryza sativa L.). Theor Appl Genet 114:803–814
Lopez HW, Leenhardt F, Coudray C, Remesy C (2002) Minerals and phytic acid interactions: is it a real problem for human nutrition? Intl J Food Sci Technol 37:727–739
Lott J, Greenwood J, Batten G (1995) Mechanisms and regulation of mineral nutrient storage during seed development. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 215–235
Mitsuhashi N, Ohnishi M, Sekiguchi Y, Kwon YU, Chang YT, Chung SK, Inoue Y, Reid RJ, Yagisawa H, Mimura T (2005) Phytic acid synthesis and vacuolar accumulation in suspension-cultured cells of Catharanthus roseus induced by high concentration of inorganic phosphate and cations. Plant Physiol 138:1607–1614
Pilu R, Panzeri D, Gavazzi G, Rasmussen SK, Consonni G, Nielsen E (2003) Phenotypic, genetic and molecular characterization of a maize low phytic acid mutant (lpa 241). Theor Appl Genet 107:980–987
Raboy V (1997) Accumulation and storage of phosphate and minerals. In: Larkin BA, Vasil IK (eds) Cellular and molecular biology of plant seed development. Kluwer , Netherlands, pp 441–477
Raboy V (2001) Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci 6:458–462
Raboy V (2007) The ABCs of low phytate crops. Nat Biotechnol 25:874–875
Raboy V, Dickinson DB, Below FE (1984) Variation in seed total phosphorus, phytic acid, zinc, calcium, magnesium, and protein among lines of Glycine max and G. soja. Crop Sci 24:431–434
Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PPN, Sheridan WF, Ertl DS (2000) Origin and seed phenotype of maize low phytic acid 1–1 and low phytic acid 2–1. Plant Physiol 124:355–368
Rasmussen SK, Hatzack F (1998) Identification of two low-phytate barley (Hordeum vulgare L.) grain mutants by TLC and genetic analysis. Hereditas 129:107–112
Rutger JN, Raboy V, Moldenhauer KAK, Bryant RJ, Lee FN, Gibbons JW (2004) Registration of KBNT lpa1–1 low phytic acid germplasm of rice. Crop Sci 44:363
Sharpley AN, Charpa SC, Wedepohl R, Sims JY, Daniel TC, Reddy KR (1994) Managing agricultural phosphorus for protection of surface waters: issues and options. J Environ Qual 23:437–451
Shi JR, Wang HY, Wu YS, Hazebroek J, Meeley RB, Ertl DS (2003) The maize low-phytic acid mutant lpa2 is caused by mutation in an inositol phosphate kinase gene. Plant Physiol 131:507–515
Shi JR, Wang HY, Hazebroek J, Ertl DS, Harp T (2005) The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant J 42:408–419
Shi JR, Wang HY, Schelin K, Li BL, Faller M, Stoop JM, Meeley RB, Ertl DS, Ranch JP, Glassman K (2007) Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol 25:930–937
Stevenson-Paulik J, Odom AR, York JD (2002) Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J Biol Chem 277:42711–42718
Stevenson-Paulik J, Bastidas RJ, Chiou AT, Frye RA, York JD (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci USA 102:12612–12617
Sun Y, Thompson M, Lin G, Butler H, Gao Z, Thornburgh S, Yau K, Smith DA, Shukla VK (2007) Inositol 1, 3, 4, 5, 6-pentakisphosphate kinase from maize: molecular and biochemical characterization. Plant Physiol 144:1278–1291
Tai T, Tanksley S (1990) A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol Biol Rep 8:297–303
Torre M, Rodrigues AR, Saura-Calixto F (1991) Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr 1:1–22
Wilcox JR, Premachandra GS, Young KA, Raboy V (2000) Isolation of high inorganic P, low-phytate soybean mutants. Crop Sci 40:1601–1605
York JD (2006) Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta 1761:552–559
Yoshida KT, Wada T, Koyama M, Mizobuchi-Fukuoka R, Naito S (1999) Temporal and spatial patterns of accumulation of the transcript of myo-inositol phosphate synthase and phytin containing particles during seed development in rice. Plant Physiol 119:65–72
Yuan FJ, Zhao HJ, Ren XL, Zhu SL, Fu XJ, Shu QY (2007) Generation and characterization of two novel low phytate mutations in soybean (Glycine max L. Merr.). Theor Appl Genet 115:945–957
Acknowledgments
This was supported by National Research Initiative Competitive Grant 2005-35301-15708 from the USDA Cooperative State Research, Education, and Extension Service (T.H.T). We gratefully acknowledge technical assistance from P.M. Colowit and D. Farzaneh. We are also thankful to J.N. Rutger and V. Raboy for critical reading of the manuscript and helpful suggestions for improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Xu.
S.I. Kim and C.B. Andaya contributed equally to this work. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Kim, S.I., Andaya, C.B., Goyal, S.S. et al. The rice OsLpa1 gene encodes a novel protein involved in phytic acid metabolism. Theor Appl Genet 117, 769–779 (2008). https://doi.org/10.1007/s00122-008-0818-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0818-z