Abstract
Although pronounced heterosis in inter-subspecific hybrids was known in rice for a long time, its utilization for hybrid rice breeding has been limited due to their hybrid sterility (HS). For the last two decades, however, a few inter-subspecific hybrids have been developed by incorporating wide-compatibility genes (WCG) that resolve HS, into parental lines of these inter-subspecific hybrids. For effective use of WCG, it is necessary to find convenient markers linked to WCG of practical importance. In this paper, initially a set of simple sequence repeat (SSR) markers in the vicinity of known WCG loci identified based on comparative linkage maps have been surveyed in a population derived from the three-way cross- IR36/Dular//Akihikari, where a known donor of WCG Dular was crossed to a representative indica and japonica cultivar. Of the five parental polymorphic markers, RM253 and RM276 were found to be closely linked to the WCG locus S5 at a distance of 3.0 and 2.8 cM, respectively. Later, loci for HS were examined in three F2 populations derived from inter-subspecific crosses, with same set of SSR markers. The locus S8 was confirmed to have major influence on HS in the F2 population derived from CHMRF-1/Taichung65 since two SSR markers in its vicinity, RM412 and RM141, co-segregated with HS at a map distance of 7.6 and 4.8 cM, respectively. In the F2 population derived from the cross BPT5204/Taipei309, three SSR markers in the vicinity of S5, RM50, RM276 and RM136 co-segregated with HS at a map distance of 4.2, 3.2 and 7.8 cM, respectively. In the third F2 population derived from Swarna/Taipei309, the SSR markers in the vicinity of S5, RM225, RM253, RM50, RM276 and RM136 were identified to co-segregate with HS at a map distance of 3.2, 2.6, 3.4, 2.6 and 6.6 cM, respectively. These results indicated a clear picture of WCG in Dular as well as the predominant role of HS alleles at S5 locus. The identified SSR markers are expected to be used for incorporation of WCG into parental lines in hybrid rice breeding to solve HS in inter-subspecific hybrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exploitation of pronounced heterosis in crosses between indica and japonica subspecies of the Asian cultivated rice (Oryza sativa L.) has been limited by HS, which has been recognized as a major basis to separate the subspecies since an early study (Kato et al. 1928). Despite intensive studies on this problem for decades by several workers, no consensus had been achieved for the genetic basis of hybrid sterility (HS) until Ikehashi and Araki (1986) reported wide compatible varieties (WCVs) which could produce fertile hybrids in their crosses with both indica and japonica varieties and proposed “allelic interaction model” to explain the phenomenon of wide compatibility (WC). This model explains the HS and WC by three type of alleles at a particular locus (S-locus), namely a neutral (S n), an indica (S i) and a japonica (S j) allele. Genotypes of S n/S i and S n/S j will be fertile while S i /S j will be semi-sterile due to partial abortion of gamete carrying S j allele at most of the known S-loci. Identification and use of WC genes have been found effective in overcoming the HS problem. Inter-subspecific hybrids of rice have been developed since an early attempt in China (Yuan 1992). Later, when some cross combinations showed HS despite the presence of the WC allele at the major WC locus S5, existence of other HS loci governing the spikelet fertility or sterility were identified (Yanagihara et al. 1992; Wan et al. 1996). Of the 28 HS/WC genes (S1 to S28) known so far in rice, at least 14 loci i.e., S5 (Ikehashi and Araki 1986), S7 (Yanagihara et al. 1992), S8 (Wan et al. 1993), S9 (Wan et al. 1996), S10 (Sano et al. 1992), S11 (Sano 1993), S15 (Wan et al. 1996), S16 (Wan and Ikehashi 1995a), S17 (Wan and Ikehashi 1995b), S18 (Devanand et al. 2000), S19 (Taguchi et al. 1999), S24 (Kubo et al. 2000), S25 (Kubo et al. 2001) and S26 (Kubo and Yoshimura 2001) have been identified in inter-subspecific crosses. Initially, the gene symbol S was used only for HS expressed by female gamete abortion, which lead to spikelet semi-sterility in panicles. Meanwhile, some major loci causing HS in male gametes were designated as ga3, ga11 etc. (Lu et al. 2000). But many recent studies assign S symbol to HS in male gamete or pollen (Taguchi et al. 1999; Kubo et al. 2000; Sobrizal et al. 2002), which is mostly detected through segregation distortion of particular genotypes because attributing any male gamete abortion to a particular genotype is difficult in a population of male gametes. Until now screening and development of wide compatible genotypes is accomplished by measuring the level of spikelet fertility in the progeny derived from crosses with appropriate testers utilizing morphological markers linked to a few WC genes. Initial genetic analysis, which resulted in the identification of S5 locus, was conducted with morphological markers, such as chromogen (C) and waxy gene (wx) (Ikehashi and Araki 1986). As these approaches based on morphological markers are difficult, time consuming and often inconclusive, there is a need for more efficient and robust techniques to identify and select specific WC gene/genotype(s). Use of PCR based DNA markers, which are abundant, phenotypically neutral and not influenced by environment (Joshi et al. 1999), can help overcome many of the limitations being encountered in the morphological marker-based screening of wide compatible genotypes. Microsatellite markers are one of the most widely used DNA markers for mapping and tagging of genes controlling important agronomic traits (Roder et al. 1998). Microsatellite map of rice with over 4,500 markers covering almost the entire rice genome is now available in public databases (http://www.gramene.org) and these markers are one of the most preferred markers for breeding applications (McCouch et al. 2002) and the list is growing day by day. Recently, SSR markers have been used extensively to identify WC genes or HS loci through genome-wide analysis. (Heuer and Miézan 2003; Zhu et al. 2005). Through the present study we attempted to identify rice microsatellite (SSR) markers for major WC gene loci through analysis of four populations segregating for the trait of WC or HS, particularly since little is known so far about the HS loci in Indian breeding materials.
Materials and methods
Plant material and mapping population(s)
The plant materials used in this study consisted of two kinds of populations. (1) 245 plants derived from a three-way cross, IR36/Dular//Akihikari were tested, where IR36 is an indica, Dular is a wide compatible and Akihikari is a japonica variety. Dular has a set of neutral alleles at many HS loci (Lu et al. 2000). The F1 plants (IR36/Dular) were fertile while the three-way cross-derived plants are expected to segregate for spikelet fertility in the ratio 1:1, because the three-way crossed population is composed of two genotypes with respect to HS genes; one expected from IR36/Akihikari may show HS while another expected from Dular/Akihikari may show no HS. (2) three F2 populations derived from the crosses CHMRF-1/Taichung65, BPT5204/Taipei309 and Swarna/Taipei309 were tested with 182, 204 and 170 plants, respectively. The F1 of these crosses were semi-sterile, and were expected to segregate for semi-sterility versus fertility in the F2 generation in the ratio of 1:1, because in respect of HS genes in the F2 there are two homozygotes of parental genotypes each at the ratio of 1/4, which would not show HS and one heterozygote with a genotype similar to F1 at the ratio of 1/2, which would show HS. When a level of HS is shown in the panicles of F1 plants, the sterility is caused by abortion of most of the female gametes carrying one of the two alleles according to the genetic model of HS. Therefore, the ratio of genotypes for HS is expected to be significantly biased from the ratio of 1:1 in the F2. In the present experiment, the bias of HS alleles in the F2 was examined with SSR marker genotypes. Phenotyping was done as per the procedure described by Yanagihara et al. (1995), where 4–5 panicles per plant were analyzed.
Selection of microsatellite markers
Thirty-nine SSR markers with map positions near eight hybrid sterility (HS) gene loci located on six chromosomes were selected (Table 1). These markers were chosen based on their position in a comparative linkage map consisting of Rice Morphological Map (2000), Cornell Rice SSR Molecular Linkage Map (2001) and Cornell Rice RFLP Linkage Map (2001), which are available online at http://www.gramene.org. These SSR markers were expected to cover a map distance of approximately 20 cM on either side of each S-locus, which has been previously described with morphological, isozyme or RFLP markers.
DNA extraction and PCR
DNA was extracted from leaves collected from 45–50 day old plants as per the procedure described by Zheng et al. (1995). Polymerase chain reaction (PCR) was carried out using selected rice SSR markers as per the protocol of Chen et al. (1997). PCR amplified products were resolved in 3.5% ethidium bromide stained agarose gels (Amresco, USA) in 0.5 × TBE buffer as per procedure described in Sambrook and Russell (2001), and photographed under UV light and documented (Alphainnotech, USA). The fragment sizes of the amplicons were calculated using the software utility Alphaease® (Alphainnotech, USA) using 100 bp ladder molecular weight marker (MBI Fermentas, Lithuania) as size standard.
SSR marker data processing and statistical analysis
In SSR analysis of the WCG in IR36/Dular//Akihikari, the SSR data were analyzed according to the scheme of backcross population, where individuals with IR36/Akihikari were scored as heterozygotes and those with Dular/Akihikari genotypes were scored as homozygotes in the co-segregation analysis. Linkage analysis was conducted by counting the number of heterozygotes exhibiting spikelet semi-sterility and number of homozygotes showing fertile spikelets. For calculation of marker-trait linkage distances, only segregants showing extreme phenotype, i.e., those plants with spikelet fertility of higher than 80% and those with spikelet fertility lower than 50% were considered, since genetic and environmental factors are most likely to be confounded in the intermediate fertility range (Yanagihara et al. 1995). Linkage distances were computed using MAPMAKER software version 3.0 (Lander et al. 1987) at minimum LOD score of 3.0 and genetic linkage maps were drawn using Kosambi map function (Kosambi 1944). A similar approach was followed for the co-segregation analysis in the F2 population, wherein extent of linkage between marker heterozygosity with spikelet semi-sterility and marker homozygozity with normal spikelet fertility was analyzed.
Results
Analysis of wide compatibility in IR36/Dular//Akihikari
The 245 plants derived from the three-way cross IR36/Dular//Akihikari showed a continuous distribution for spikelet fertility percentage, which ranged from 8 to 96. Even though overall, the distribution was continuous, there was an indication of bi-modality with a distinct valley at around 65% level (Fig. 1). Considering 65% as the cut-off point between fertility and semi-sterility, the population segregated for fertility: semi-sterility at the ratio of 1:1 (χ2 = 0.41, P>0.5). Of the 245 plants, 124 were observed to exhibit extreme phenotype wherein 75 plants had spikelet fertility below 50% and 49 had spikelet fertility above 80%. The 1:1 ratio of fertile and sterile plants in this population is just as expected from the S-locus model, because there are two component genotypes expected from the cross- IR36/Akihikari (semi-sterile) and Dular/Akihikari (fertile).
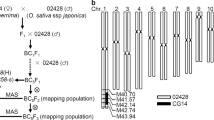
Among the 39 SSR markers analyzed, 5 markers i.e., RM253 & RM276 (S5), RM313 (S15), RM536 (S18) and RM422 (S19) amplified polymorphic alleles in this population. Only two markers, RM276 and RM253, which are in the vicinity of S5 locus exhibited a co-segregation between marker genotypes and spikelet fertility, where majority of plants with the genotype of IR36/Akihikari were semi-sterile with spikelet fertility < 65% and majority of plants with the Dular/Akihikari genotype were fertile with spikelet fertility ≥ 65%. The differences of two genotypes with regard to the ratio of sterile-fertile plants were highly significant by χ2 tests (Table 2). Linkage analysis using plants in the extremes (spikelet fertility above 80% and below 50%) revealed that RM276 was at a genetic distance of 2.8 cM and RM253 at 3.0 cM from the locus S5 and that the two markers were located on either side of the S5 locus (Fig. 2a).
Linkage maps of WC gene loci as inferred from co-segregation analysis of the three-way cross and F2 populations. Map distances are given in centimorgans. a Linkage map of a portion of chromosome 6 in the vicinity of the WC gene S5 as inferred from the co-segregation data of the three-way cross population IR36/Dular//Akihikari. Positions are shown for the markers RM253 and RM276 relative to S5. b Linkage map of a portion of chromosome 6 in the vicinity of the WC gene S8 as inferred from the co-segregation data of the F2 population –CHMRF/Taichung65. Positions are shown for the markers RM412 and RM141 relative to S8. c Linkage map of a portion of chromosome 6 in the vicinity of the WC gene S5 as inferred from the co-segregation data of the F2 population–BPT5204/Taipei309. Positions are shown for the markers RM276, RM50 and RM136 relative to S5. d Linkage map of a portion of chromosome 6 in the vicinity of the WC gene S5 as inferred from the co-segregation data of the F2 population –Swarna/Taipei309. Positions are shown for the markers RM225, RM253, RM276, RM50 and RM136 relative to S5. T Telomere, CEN Centromere
It is noteworthy that in the F1 of IR36/Dular there was no segregation distortion between Dular and IR36 allele because the frequency of Dular/Akihikari genotype and IR36/Akihikari genotype were found at the ratio of 1:1 (Table 2). This implied that Dular showed no HS in its cross with IR36 as well as to Akihikari.
Even though one marker each for the WC loci S15 (RM313), S18 (RM536) and S19 (RM422) exhibited parental polymorphism, none of these exhibited clear co-segregation with spikelet fertility.
Analysis of HS loci in three F2 populations
The F2 individuals from the cross CHMRF-1/Taichung65, BPT5204/Taipei309 and Swarna/Taipei309 also showed a continuous and bi-modal distribution for spikelet fertility percentage with a clear cut valley at 65% and segregated in 1:1 ratio (χ2 = 0.04, 0.18 and 0.01, P>0.5) for fertility and semi-sterility. This observation can also be explained on the basis of S-locus model, according to which the individuals of F2 are expected to segregate in the ratio of fertile homozygotes (1/2) and semi-sterile heterozygotes (1/2).
In the F2 population of CHMRF-1/Taichung65 out of the 39 SSR markers tested, 16 were polymorphic (Table 1). Two markers, RM412 and RM141 near S8 locus showed association with spikelet fertility. In the marker genotypes with respect to RM412 and RM141 for S8, plants with the genotype CHMRF-1/T-65 were mostly semi-sterile and the plants with the homozygous genotype of CHMRF-1 and the Taichung65 were mostly fertile (Table 3). The differences were confirmed with highly significant values of χ2. Exceptionally, as for RM141, plants with homozygous marker genotype of Taichung 65 showed nearly equal number of fertile and sterile plants. This was perhaps caused by the extremely reduced number of that genotype. Extreme segregation distortion was observed with respect to these two marker genotypes. This is because the gametes carrying the allele from Taichung65 were significantly aborted on the F1 plant. Linkage analysis was performed using plants exhibiting extreme phenotypes (spikelet fertility above 80% and below 50%) and the markers RM412 and RM141 were found to be linked to S8 at a genetic distance of 7.6 cM and 4.8 cM, respectively on either side of the gene locus (Fig. 2b). No clear pattern of co-segregation was noticed in this population for other polymorphic markers.
In the F2 population of BPT5204/Taipei309, out of the nine polymorphic markers the marker genotypes with respect to RM50, RM276 and RM136 near the S5 locus exhibited association with spikelet fertility. Majority of plants of the genotype BPT5204/Taipei309 were semi-sterile, while the parental genotype of BPT5204 exhibited mostly fertile plants. Another parental genotype of Taipei 309 did not differentiate the number of fertile and sterile plants probably due to the extreme decrease of that genotype (Table 3). Extreme segregation distortion from 1:2:1 ratio was noticed due to the significant decrease of Taipei 309 allele in the F1. The linkage analysis using plant of extreme phenotypes (as described earlier) revealed RM50, RM276 and RM136 to be linked with S5 at a genetic distance of 4.2, 3.2 and 7.8 cM, respectively (Fig. 2c). Though seven other markers RM505, RM234, RM412, RM202, RM536 and RM287 were polymorphic, they did not exhibit any clear pattern of co-segregation.
In the F2 population of Swarna/Taipei309, five of the 10 polymorphic SSR markers RM225, RM253, RM50, RM276 and RM136 near the S5 locus showed a significant association with spikelet fertility (Table 3). RM276 was observed to show a very tight co-segregation, where 81 out of 85 plants with genotype of Swarna/Taipei309 were in the class of semi-sterility, while 79 out of 80 plants with Swarna genotype were in the class of high fertility. As in the other two F2 populations, segregation distortion from 1:2:1 was noticed with highly significant χ2 values. This fact indicates that a majority of female gametes with japonica allele were aborted on the F1 plants. The markers RM225, RM253, RM50, RM276 and RM136 were identified to be linked to S5 locus at a distance of 3.2, 2.6, 3.4, 2.6 and 6.6 cM, respectively through a linkage analysis performed using plants showing extreme phenotype (Fig. 2d).
Discussion
In order to successfully exploit the higher yield heterosis in inter-subspecific hybrids, it is imperative to overcome the hybrid semi-sterility in such crosses. So far 28 loci for HS have been described each with several alleles for HS and WC (Nagato and Yoshimura 1998). Early workers have reported these loci to be linked with either morphological or biochemical (isozyme) markers. For instance, S5, the first ever discovered HS locus in indica/japonica crosses has been located on chromosome 6 using morphological (C and wx) and isozyme (Amp-3, Est-2, and Pgi-2) markers by Ikehashi and Araki (1986). Following this report other HS loci have been identified and linked to isozyme and RFLP markers by different workers as reviewed in the introduction.
In view of some obvious limitations of isozymes and RFLPs, PCR based markers, especially microsatellites, which are popularly called SSRs have been found more effective in practical rice breeding (McCouch et al. 2002). Recently, SSR markers have extensively been used to analyze HS loci in breeding works in China. But analyses of HS loci with SSR markers have not been reported for Indian breeding materials. For example, Williams et al. (1997) reported identification of a PCR based marker STS-213 linked to the S5 locus, but this marker did not show significant polymorphism between the WC varieties and the popular rice genotypes used in hybrid rice breeding in India (data not shown).
To solve HS problem in hybrid rice breeding, incorporation of WC alleles from known WC varieties is the initial step. Even though the distribution of percentage spikelet fertility was continuous, there was a distinct indication of bi-modality, based on which we considered the trait as a qualitative one and attempted mapping by considering only plants with extreme phenotypes. This approach successfully allowed us to map two WC loci, S5 and S8 with SSR markers on chromosome 6.
In the present study, first a set of SSR markers was utilized to tag the WCG in Dular, one of the well-known WCV, using a population derived from a three-way cross, Dular/IR36//Akihikari, where Dular was crossed to a representative indica and japonica cultivar. Of five polymorphic markers, RM253 and RM276 were identified to be closely linked to the WCG with a distance of 3.0 and 2.8 cM, respectively. In the present experiment Dular was confirmed to produce fertile hybrids in IR36 (indica)/Dular and Dular/Akihikari (japonica), while the genotype of IR36/Akihikari showed semi-sterility in the progeny of the three-way cross. Wang et al. (1998) have once analyzed the nature of wide compatibility in Dular using a three-way cross, similar to the one used in the present study. They reported that the trait of wide compatibility in Dular showed a complex genetic basis. But in the present study it was confirmed that a single WC locus S5 controls the trait of wide compatibility in Dular. In our experiment a bi-modal distribution of semi-sterility versus fertility in the three-way cross population was observed reflecting the semi-sterile genotype of IR36/Akihikari and fertile genotype of Dular/Akihikari, while Wang et al. (1998) could not observe a clear bi-modal distribution. Hence the observation of Wang et al. (1998) that WC in Dular might have a complex genetic basis was most probably due to some problems in phenotyping. In hybrid rice breeding, extensive incorporation of WC allele into parental materials is necessary. Although the agronomic traits of Dular are rather poor, incorporation of Dular’s WC allele into parental breeding lines is important as demonstrated by Heuer and Miézan (2003) and Lu et al. (2000).
Besides, in the present paper loci for hybrid sterility were examined with SSR markers in three F2 populations from inter-subspecific crosses. The locus S8 for HS was confirmed in the F2 derived from CHMRF-1/Taichung65 with the help of linked markers RM412 and RM141. In the F2 population of BPT5204/Taipei309, three SSR markers RM50, RM276 and RM136 were identified to cosegregate with S5 locus. In the F2 population of Swarna/Taipei309, five SSR markers, RM225, RM253, RM50, RM276 and RM136 were identified to co segregate with S5 locus. Although these markers are identified for HS locus, since WC allele is allelic to the HS alleles at S5 locus, these markers will also be useful to tag the WC alleles at S5 locus and use them in hybrid rice breeding.
Throughout the analyses of HS loci with SSR markers, it is noteworthy that S5 has emerged as a major locus for HS and WC. The effect of S8, another HS/WC locus was found only in a single cross. Therefore, incorporation of Dular’s WC allele with respect to S5 is expected to solve a major proportion of hybrid sterility problem in rice breeding.
One of the notable features of marker analyses in the F2 populations is the pronounced segregation distortion in all the three populations. The increased proportion of indica allele is considered to be caused by the abortion of female gametes carrying the japonica allele on F1 plant on the basis of the genetic model for HS.
The present study has added substantially to our understanding of the genetics of inter-subspecific HS. From practical breeding point of view, the identification of RM276, RM50, RM225, RM253 & RM136 (linked to S5) and RM141 & RM412 (linked to S8) through the present study would be useful as markers in the identification and deployment of WC genes for development of indica-japonica hybrids or varieties of promise, which are free from semi-sterility problem.
References
Chen X, Temnykh S, Xu Y, Cho YG, McCouch SR (1997) Development of microsatellite framework map providing genome wide coverage in rice (Oryza sativa L). Theor App Genet 95:553–567
Devanand PS, Rangaswamy M, Ikehashi H (2000) Identification of hybrid sterility gene loci in two cytoplasmic male sterile lines in rice. Crop Sci 40:640–646
Heuer S, Miézan KM (2003) Assessing hybrid sterility in Oryza glaberrima( O sativa hybrid progenies by PCR marker analysis and crossing with wide compatibility varieties. Theor Appl Genet 107:902–909
Ikehashi H, Araki H (1986) Genetics of F1 sterility in remote crosses of rice. In Rice Genetics IRRI:119–130
Joshi SP, Ranjekar PK, Gupta VS (1999) Molecular markers in plant genome analysis. Curr Sci 77:230–240
Kato S, Kosaka H, Hara S (1928) On the affinity of rice varieties as shown by the fertility of hybrid plants. Bull Sci Fakult Terkult Kyushu Imp Univ 3:132–147
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Kubo T, Yoshimura A (2001) Linkage analysis of a gene controlling F2 sterility in japonica/ indica backcross progenies of rice. Rice Genet Newsl 18:52–53
Kubo T, Eguchi M, Yoshimura A (2000) A new gene for F1 pollen sterility in japonica/ indica cross of rice. Rice Genet Newsl 17:63–64
Kubo T, Eguchi M, Yoshimura A (2001) A new gene for F1 pollen sterility on chromosome12 in japonica/ indica cross of rice. Rice Genet Newsl 18:54–55
Lander ES, Green P, Abrahmson J, Barloe A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary linkage maps of experimental and natural populations. Genomics 1:174–181
Lu CG, Takabatake K, Ikehashi H (2000) Identification of segregation distortion neutral alleles to improve pollen fertility of indica-japonica hybrids in rice. Euphytica 113:101–107
McCouch SR, Leonid T, Yunbi X, Katarzyna BL, Karen C (2002) Development and mapping of 2240 New SSR markers for Rice (Oryza sativa L.). Theor Appl Genet 109:199–207
Nagato Y, Yoshimura A (1998) Report of the committee on gene symbolization, nomenclature and linkage groups. Rice Genet Newsl 15:13–74
Roder MS, Plaschke J, Konig SU, Borner A, Sorrells ME, Tanksley SD, Ganal MW (1998) Abundance, variability and chromosomal location of microsatellites in wheat. Mol Genet 246:327–333
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual 3rd edn. Cold Spring Harbor, NY, USA pp 5.40–5.46
Sano Y (1993) Is an egg killer present in rice. Theor Appl Genet 86:1038–1042
Sano Y, Eiguchi M, Hirano HY (1992) Genetic elimination found in the indica-japonica hybrid of rice. Jpn J Breed 42:454–455
Sobrizal Y, Matsuzaki P, Yoshimura A (2002) Mapping of pollen semi-sterility gene S-28 (t) on rice chromosome 4. Rice Genet Newsl 19:34–37
Taguchi K, Doi K, Yoshimura A (1999) RFLP mapping of S-19, a gene for F1 pollen sterility found in back cross progeny of Oryza sativa and O.glaberrima. Rice Genet Newsl 16:70–71
Wan J, Ikehashi H (1995a) Identification of a new locus S-16 causing hybrid sterility in native rice varieties (Oryza sativa L.) from Tai-hu lake region and Yunnan Province, China. Breed Sci 45:461–470
Wan J, Ikehashi H (1995b) Loci for hybrid sterility in Basmati crosses. Intl Rice Res Notes 20:4
Wan J, Yanagihara S, Kato H, Ikehashi H (1993) Multiple alleles at a new locus causing hybrid sterility between Korean indica variety and a javanica variety in rice (Oryza sativa L.). Jap J Breed 43:507–516
Wan J, Yamaguchi Y, Kato H, Ikehashi H (1996) Two new loci for hybrid sterility in rice (Oryza sativa L.). Theor Appl Genet 92:183–190
Wang J, Liu KD, Xu CG, Li XH, Zhang Q (1998) The high level of wide compatibility of variety ‘Dular’ has a complex genetic basis. Theor Appl Genet 97:407–411
Williams CE, Yanagihara S, McCouch SR, Mackill D, Ronald PC (1997) Predicting success of indica/japonica crosses in rice based on a PCR marker for the S-5 n allele at a hybrid-sterility locus. Crop Sci 37:1910–1912
Yanagihara S, Kato H, Ikehashi H (1992) A new locus for multiple alleles causing hybrid sterility between an Aus variety and javanica varieties in rice (Oryza sativa L.). Jap J Breed 42:793–801
Yanagihara S, Couch SR, Ishikawa K, Ogi Y, Maruyama K, Ikehashi H (1995) Molecular analysis of the inheritance of the S-5 locus conferring wide compatibility in indica/japonica hybrids of rice (Oryza sativa L.). Theor Appl Genet 90:182–188
Yuan LP (1992) The strategy of the development of hybrid rice breeding. In: Yuan LP (ed) Current stratus of two line hybrid rice research 1–5, In Chinese, English summary. Agricultural Publishing Ltd, Beijing
Zheng K, Huang N, Bennet J, Khush GS (1995) PCR based marker-assisted selection in rice breeding. IRRI Discussion paper series No 12. International Rice Research Institute, Manila
Zhu S, Wang C, Zheng T, Zhao Z, Ikehashi H, Wan J. (2005) New gene located on chromosome 2 causing hybrid sterility in a remote cross of rice. Plant Breed 124:440–445
Acknowledgements
The authors profusely thank Dr. B. Mishra, Project Director, Directorate of Rice Research, Hyderabad for the kind help and assistance received and research facilities extended to pursue the present study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Sasaki
S.P. Singh , R.M. Sundaram contributed equally
Rights and permissions
About this article
Cite this article
Singh, S.P., Sundaram, R.M., Biradar, S.K. et al. Identification of simple sequence repeat markers for utilizing wide-compatibility genes in inter-subspecific hybrids in rice (Oryza sativa L.). Theor Appl Genet 113, 509–517 (2006). https://doi.org/10.1007/s00122-006-0316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-006-0316-0